Fig. 3.1
Initial assessment of non-neurogenic patients with OAB: the minimum requirements for this diagnosis is history, physical examination, and urinalysis. American Urological Association (AUA) Guideline. Statement 1
In female patients, physical examination including abdominal, pelvic, and perineal evaluation with digital examination of the vagina should be performed to assess voluntary pelvic floor muscle contraction, estrogen status, and presence of urogenital prolapse (POP). The cough test should be performed in order to objectively assess presence and type of incontinence. In male patients, a rectal/genitourinary evaluation with digital rectal examination should be performed, in order to assess the urethral and prostate status.
Risk factors in men for the development of UI and OAB include increasing age, prostatectomy, UTIs, and cognitive and functional impairment. Presence of neurologic diseases or other genitourinary conditions (i.e., benign prostatic enlargement, bladder tumor, bladder lithiasis, congenital pathologies) should be adequately considered due to the impact of these conditions on bladder function. When a neurologic disease has been identified as responsible for the presence of bladder-sphincter dysfunction with OAB, a specialized assessment and management are required.
Urinalyses and culture are also useful to confirm or exclude the diagnosis of OAB (Fig. 3.2). In the presence of isolated hematuria, without signs of infection, patients should perform an in-depth urologic evaluation.
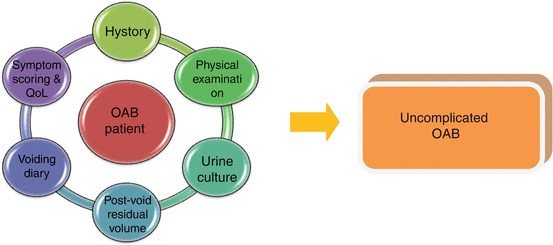

Fig. 3.2
Initial assessment of non-neurogenic patients with OAB: urine culture and/or postvoid residual assessment, bladder diaries, and/or symptom questionnaires are useful to make diagnosis. American Urological Association (AUA) Guideline. Statement 2
The assessment of residual urine by measuring the postvoid urinary volume with bladder scan or catheterization is useful particularly when patients present with symptoms suggestive of voiding dysfunction, POP, or recurrent UTIs (Fig. 3.2).
According to Nice and EAU Guidelines on urinary incontinence, in the initial assessment of patients with OAB, the use of micturition time charts, frequency volume charts, or voiding diaries should be strongly encouraged. Micturition time charts are used to record only the times of micturitions for a minimum of 24 h; frequency volume charts are used to record urinary volumes and times of each micturition for a minimum of 24 h. “Voiding” or “bladder” diary is used to report more detailed information on daily frequency of micturition, fluid intake, episodes of urgency and/or urgency incontinence, and pad usage. A minimum of 3 days voiding diary is usually required, and it should be completed during patients’ usual activities, such as both working and leisure days (Fig. 3.2).
The use of PAD test in the routine clinical assessment of patients with UI is not recommended by the Nice, EAU, and AUA Guidelines. Nevertheless, there is at least a good evidence to show that repeat pad testing can detect change following treatment for UI.
Symptom Scoring and Quality of Life Assessment
Although there is no information about whether using questionnaires to assess urinary symptoms and QoL improves outcomes in patients with UI or OAB, the use of validated symptom questionnaires is useful to quantify and characterize urinary symptoms and their impact on patient’s QoL (Fig. 3.2). Health-Related Quality Of Life questionnaires (HRQoLq) may be generic, as SF-36 or specific for a particular condition, as Incontinence Impact Questionnaire, the King’s Health Questionnaire, OAB-q, to study OAB. Additionally, questionnaires can help in evaluating the response to treatment along follow-up and to better monitoring patients’ condition along time. Questionnaires should have been validated for the language in which they are being used. Some of validated questionnaires mainly used for the assessment of OAB and urgency are showed in Table 3.1.
Table 3.1
Some of validated questionnaires used for the assessment of OAB and urgency
Assessment of severity and impact of OAB | Abbreviations |
|---|---|
Urinary Incontinence-Specific Quality of Life Instrument | I-QOL (ICIQ-Uqol) |
Overactive bladder questionnaire | ICIQ-OABqol |
King’s Health Questionnaire | KHQ |
OAB Satisfaction questionnaire | OAB-SAT |
Overactive Bladder Symptom Score | OAB-SS |
OAB-S = Overactive Bladder Satisfaction measure | OAB-S |
Assessment of the Impact of Urgency | |
Patient’s Perception of Intensity of Urgency | PPIUS |
Scale Urge Incontinence Impact Questionnaire | U-IIQ |
10-Item Scale to Measure Urinary Urgency | UU Scale |
Urge Urogenital Distress Inventory | U-UDI |
Urgency Severity and Intensity Questionnaire: Quality of Life | USIQ-S |
Urodynamic Testing
Multichannel cystometry, ambulatory urodynamics (UDS), or videourodynamics are diagnostic instruments including multiple tests to study lower urinary tract function during both bladder storage and emptying. While it is now recognized that UDS is not required to diagnose and manage OAB in non-neurogenic patients, information about the presence or absence of detrusor overactivity (DO) and the volume at which patients experiment OAB symptoms can be helpful in their management. In patients refractory to conservative treatment, UDS can help to identify DO, high pressure of uninhibited detrusor contractions, and a reduced compliance, which can all induce UUI and OAB. Importantly, it should be reminded that the diagnoses of UUI are not related to urodynamic presence of DO, and the presence of DO is neither sensitive nor specific for UUI. This is in agreement with the AUA Urodynamics Guidelines, which state that multichannel filling cystometry should be performed when it is important to determine whether “altered compliance, DO, or other urodynamic abnormalities are present or not in patients in whom invasive, potentially morbid, or irreversible treatments are considered.” This is the case when OAB patients who are refractory to conventional anticholinergics need to be treated with surgical intervention, such as sacral neurostimulation or intradetrusor onabotulinumtoxinA injections. The AUA, OAB Guidelines and the EAU Guidelines on UI support these statements and underline that UDS should not be used in the initial assessment of patients with uncomplicated OAB (Fig. 3.3). Also the Nice Guidelines do not recommend the use of UDS before starting conservative management. Only after a detailed history and physical examination, UDS should be performed before surgery in women who present with OAB symptoms suspected for DO and voiding dysfunction, or who had previous surgery for stress incontinence or POP.
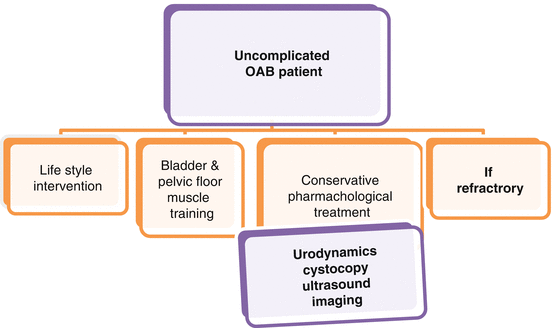

Fig. 3.3
Diagnostic investigations in patients with uncomplicated OAB
The use of videourodynamics or ambulatory urodynamics should be considered for OAB patients when the diagnosis remains unclear after standard UDS or for patients with OAB symptoms affected by neurogenic diseases.
Other Investigations
For the routine assessment of patients with isolated OAB syndrome, the use of cystoscopy and imaging (MRI, CT, X-ray) is not required.
With regard to the use of ultrasound in the evaluation of OAB patients, it has been suggested that lower urinary tract ultrasound should be useful to study detrusor and bladder wall thickness, as bladder hypertrophy can be related to the severity of detrusor contractions in OAB patients. Indeed, ultrasound measurement of detrusor and bladder wall thickness may be influenced by several factors, as the ability of the operator, type of ultrasound probes and image resolution, and degree of bladder filling. Actually, there is no demonstration that studying these measures could improve the management of patients affected by OAB. Thus, the use of ultrasound should be limited to the evaluation of postvoid residual urine volume.
Differential Diagnosis
Differential diagnoses should be made between OAB syndrome and some conditions that can mimic the same symptoms as polydipsia, nocturnal polyuria, interstitial cystitis, and/or bladder pain syndrome. In the latter case, the presence of bladder and/or urethral pain can help to perform a correct diagnosis. Additionally, OAB symptoms could be referred in the presence of the following conditions: neurogenic diseases, depression, erectile dysfunction, dementia, clinically benign pelvic masses, associated fecal incontinence, symptoms of voiding difficulty due to BPE or POP, previous continence surgery, previous pelvic cancer surgery, and previous pelvic radiation therapy. These conditions require a further, specialized assessment and management.
Conservative Treatment
(8)
Vanderbilt University Medical Center, Nashville, TN, USA
All patients with OAB should be provided with education and an explanation of normal lower urinary tract structure and function and what is known about OAB. According to the AUA panel, “explaining what is normal can help the patient understand their condition and give a comparator for establishing mutually identified and realistic goals for treatment. Education empowers the patient to participate in their treatment, an essential factor when interventions rely on behavioral change.” Patients with OAB will likely require long-term or lifelong therapy to control their symptoms, and as a result, a treatment plan should weigh the burden of symptoms on a patients’ QoL, as well as factor that potential benefit of a particular treatment against the treatment’s risk. Treatment initiation presumes a perceived improvement can be generated. The patient has to be a willing and engaged participant who understands that OAB has a variable and chronic course, and acceptable symptom control may require multiple therapeutic trials before it is achieved. It may not be possible to eliminate all symptoms. It must be stressed that no treatment is an acceptable choice made by some patients and caregivers.
First-Line Therapy
Lifestyle and behavioral modification should be offered to all patients with OAB. Lifestyle changes include cessation of smoking, weight reduction, elimination of known dietary bladder irritants such as alcohol or caffeine, adequate fluid intake, and regular bowel habits. Nuotio et al. showed a correlation between smoking and urinary urgency in a large population-based survey. In further support, the EUA gave a grade A to smoking cessation advice in line with good medical practice in patients with UI, but the level of evidence that it improves UI symptoms was only based of expert opinion and not randomized data (Lucas 2012). Obesity has been shown to be an independent risk factor for the development of SUI and mixed UI in women, and evidence exists that nonsurgical weight loss intervention has the potential to improve UI in overweight women. Indeed, a weight loss of 8 % in obese women reduced overall incontinence episodes per week and UUI episodes by 47 and 42 % vs. 28 and 26 % in controls. Reduced fluid intake is a widely used, inexpensive and noninvasive intervention but can predispose to UTI, dehydration, stone formation, and constipation. The data available is conflicting on whether fluid modification changes symptoms and QoL, but a recent trial showed a 25 % reduction in fluid intake improved symptoms with OAB but not UI. Caffeine has an excitatory effect on detrusor muscles, and randomized controlled data demonstrates significant improvements in urgency-frequency and UUI in those who reduced caffeine to <100 mg/day. Alcohol has a diuretic effect by reducing the release of antidiuretic hormone. Newman et al. reported a nearly uniform positive association between straining at stool and constipation and lower urinary tract symptoms which the authors surmised to be due to progressive neuropathy in the pelvic floor secondary to repeated straining efforts.
Behavioral treatments are a group of risk-free therapies which improve individual symptoms by changing patient behavior or the patient’s environment. They can be tailored individually and include bladder training, bladder control strategies, pelvic floor muscle training, and fluid management. This form of therapy relies on a patient with an intact nervous system to “relearn” to inhibit a detrusor contraction or a sensation of urgency. It generally consists of patient education, scheduling voiding with an aim to achieve an interval of 3–4 h between voids, and positive reinforcement that provides psychological support and encouragement. There is level 1 evidence in support of supervised bladder training but whose benefits are of short duration unless the program is practiced repeatedly. In the MOTIVE trial (Male overactive Bladder Treatment in Veterans), where Burgio et al. demonstrated equivalence between behavioral and antimuscarinic therapy, behavioral treatment involved verbal instruction followed by guided practice using verbal feedback based on anal palpation to teach participants how to contract and relax pelvic floor muscles while keeping abdominal muscles relaxed, as well as delayed voiding, monitoring with bladder diaries, and urge suppression techniques to inhibit or suppress detrusor contractions and thus reduce urgency-frequency and incontinence (Burgio 2011). Similar findings from randomized trials have been published that have demonstrated equivalence or superiority to medications in reducing symptoms, while more limited literature exists that indicates behavioral and drug therapy simultaneously may improve outcomes.
Pelvic floor muscle training (PFMT) is used frequently in conjunction with bladder training. The purpose is to increase the strength and durability of contraction of the pelvic floor muscles, which may help inhibit bladder contractions in patients with OAB. Biofeedback therapy can be used to augment PFMT and involves the use of monitoring equipment to detect, measure, and amplify internal physiological responses to provide patients with feedback concerning those responses. It increases patient awareness of the pelvic floor muscles, using visual, tactile, or auditory stimuli and is used to help teach patients to exercise their musculature more effectively. The most common modalities include electromyography (EMG) and manometry that measures the activity of the anal or urinary sphincter, the pelvic floor musculature, as well as the detrusor muscle with the aim to restore bladder control by teaching patients to modulate the mechanism of continence. There is level 1 evidence supporting both PFMT and the addition of biofeedback for added benefit. Berghmans et al. conducted a meta-analysis study assessing the efficacy of biofeedback in UUI and concluded that patients were able to achieve improvement after biofeedback. They stated that the actions were easy to perform in cognitively intact patients but took personal effort and time to perform the exercises.
Second-Line Treatment
Antimuscarinic agents act via competitive inhibition at the postganglionic receptor sites and, as a result, reduce bladder contractility and particularly exert their effects on the filling phase of the bladder, reduce urgency, increase bladder capacity, and minimize the effects of the parasympathetic system. While effective in reducing symptoms, they are commonly associated with side effects (e.g., dry mouth, constipation, dry eyes, dyspepsia, urinary retention, impaired cognitive function) as a result of the affinity of the drugs to muscarinic receptors widely distributed in the body. Additionally, for those patients with narrow angle glaucoma, impaired gastric emptying, or a history of urinary retention, consultation with each respective specialist should be sought prior to initiation of therapy. Clinicians must use caution in prescribing these agents in patients who are using other medications with anticholinergic properties. Agents available include darifenacin, fesoterodine, oxybutynin, solifenacin, tolterodine, or trospium. Both the EUA and AUA performed an extensive review of the randomized trials evaluating these agents for OAB and found no compelling evidence for differential efficacy across medications. Thus, the choice of medication is largely determined by patient factors that include age, side-effect profile, and out-of-pocket costs. However, if extended-release formulations are available, they should be preferentially prescribed because of the lower rates of side effects (Grade A recommendation, EUA). For likewise reasons, transdermal preparations may be offered, if available (Grade B recommendation, EUA). In those individuals who experience inadequate symptom relief and/or unacceptable adverse events, with one antimuscarinic agent, then a dose modification or a different antimuscarinic medication may be tried.
Special attention should be given to the frail elderly, a group where data on the use of antimuscarinic is not as robust and side effects may be more severe. Elderly people have been underrepresented in randomized controlled trials (RCTs) of antimuscarinic agents, despite having a higher prevalence. There is level 2 evidence that oxybutynin IR may worsen cognitive function and as a result received a grade A recommendation against its usage in those at risk of cognitive impairment (Lucas 2012). However, the effectiveness and risk of adverse events, including cognitive dysfunction, of solifenacin, tolterodine, and darifenacin, do not differ with patient age (level 1b evidence (Lucas 2012)). Nocturia, a symptom with a multifactorial etiology, is an another especially concerning issue in the elderly as it has been linked to a risk of falls, fractures, and even death. The role of antimuscarinics for the management of nocturia in the context of OAB is limited. Pooled analysis and/or post hoc analyses have demonstrated significant reduction of nocturnal voids per night, but the effect size was low and the clinical significance doubtful. To our knowledge, only one RCT that compared tolterodine with placebo focused on nocturia as a primary endpoint. In this study, micturitions were classified as either OAB related or not depending on the degree of urgency prior to that micturition. Eight hundred and fifty patients were randomized, and while tolterodine did significantly reduce OAB specific symptoms, there was no difference between the two groups for nocturia for the non-OAB micturitions and when both non-OAB and OAB micturitions were considered together. This clearly demonstrates the complex nature of nocturia resulting from its multiple contributing etiologies.
Nearly all studies for OAB either require UUI for study inclusion or include a mix of patients with both OAB dry and wet. For this latter case, rarely is subgroup analysis performed and thus depriving the ability to compare efficacy of treatment between OAB dry and wet. While it is believed, based on expert interviews, that OAB is represented by a spectrum of severity, from mild OAB with no leakage (OAB dry) to severe OAB with frequent leakage (OAB wet), there is no evidence to substantiate this. Lacking this, it is unknown whether each represents a unique clinical entity with differential treatment outcomes. This may largely be an academic exercise as urgency rather than incontinence has been cited as the most bothersome symptom and the one with the greatest impact on quality of life. Urgency data is available as an endpoint in most studies and is favorable (Table 3.2). Since OAB treatment is aimed at symptom relief, this may be more important than the dichotomy of OAB into wet or dry.
Table 3.2
Selected published series of randomized controlled trials of various anticholinergic agents
Author | Drug | Inclusion | Length of study | n | Results | Adverse events | Notes |
|---|---|---|---|---|---|---|---|
Rudy et al. (2006) | Trospium 20 mg BID | Females and males, >18 years old, OAB >6 months; urinary frequency ≥10/day, +urgency, ≥7 UUI/week | 12 weeks | 658 | Daily voids: −2.67 vs. −1.76, p < 0.0001 Daily UUI episodes: −1.86 vs. −1.29, p < 0.0001 Urgency score: −0.21 vs. −0.02, p < 0.001 Volume voided per void/24 h: +35.59 vs. +9.44, p < 0.0001 | Dry mouth: 19.8 % vs. 5.2 % Constipation: 10.9 % vs. 5.8 % Discontinuation rate: 7.3 % vs. 4.6 % | Quaternary amine and ionized at physiological pH; low lipophilicity and reduced CNS penetrance |
Dmochowski et al. (2008) | Trospium 60 mg extended release, daily | Females and males, >18 years old, OAB >6 months; urinary frequency ≥10/day, >1 severe urgency with void, mean >1 UUI/day | 12 weeks | 564 | Daily voids: −2.5 vs. −1.8, p < 0.001 Daily UUI episodes: −2.4 vs. −1.6, p < 0.001 Urgency score: −0.28 vs. −0.13, p < 0.001 Volume voided per void/24 h: +31.5 vs. +17.8, p < 0.01 | Dry mouth: 12.9 % vs. 4.6 % Constipation: 7.5 % vs. 1.8 % Discontinuation rate: 6.4 % vs. 2.8 % | |
Van Kerrebroeck et al. (2001) | Tolterodine ER 4 mg daily, tolterodine IR 2 mg BID | Females and males, >18 years old, OAB >6 months; urinary frequency ≥8/day, ≥5 UUI/week | 12 weeks | 1529 | Daily voids : ER −3.5 vs. −2.2, p < 0.001; IR −3.3 vs. −2.2, p = 0.0002 Weekly UUI episodes: ER −11.8 vs. −6.9, p < 0.0001; IR −10.6 vs. −6.9, p = 0.001 Volume voided per void/24 h: ER +34 vs. +14, p < 0.0001; IR +29 vs. +14, p < 0.0001 | Dry mouth: ER 23 %, IR 30 %, placebo 8 % Constipation: ER 6 % IR 7 %, placebo 4 % Discontinuation rate: ER 5 % IR 5 %, placebo 6 % | Prodrug and requires cytochrome P450 activation; ER formulation 18 % more effective than IR in UUI episodes; ER 23 % less dry mouth than IR |
Chapple et al. (2005) | Darifenacin 7.5 mg and 15 mg daily | Females and males, >18 years old, OAB >6 months; urinary frequency ≥8/day, 5–50 UI/week, mean of ≥1 urgency episode/24 h | 12 weeks | 1059 | Mean voids/day: 7.5 mg −1.6 vs. −0.9, p < 0.001; 15 mg: −1.9 vs. −1.0, p < 0.001 Mean UI episodes/week: 7.5 mg −4.0 vs. −2.0, p < 0.001; 15 mg −4.8 vs. −2.7, p < 0.001 Mean urgency episodes/day: 7.5 mg −2.0 vs. −1.0, p < 0.001; 15 mg −2.3 vs. −1.2, p < 0.001 Average void volume: 7.5 mg +15 vs. +8, p = 0.007; 15 mg +27 vs +6, p < 0.001 | Dry mouth: 7.5 mg 20.2 %, 15 mg 35.3 %, placebo 8.2 % Constipation: 7.5 mg 14.8 %, 15 mg 21.3 %, placebo 6.2 % Discontinuation rate: 7.5 mg 1.5 %, 15 mg 5.1 %, placebo 2.6 % | Selective muscarinic M3 receptor antagonist; randomized data available demonstrating no change in cognition or QTc intervals |
Cardozo et al. (2005) | Solifenacin 5 mg and 10 mg daily | Females and males, >18 years old, OAB >3 months; urinary frequency ≥8/day, >3 urgency episodes and/or >3 UI during 3 day diary | 12 weeks | 907 | Daily void: 5 mg −2.37 vs. −1.59, p = 0.002, 10 mg −2.81 vs. −1.59, p = 0.0001 Daily UI: 5 mg −1.63 vs −1.25, p = 0.002; 10 mg −1.57 vs. 1.25, p = 0.016 Urgency episodes: 5 mg −2.84 vs −1.98, p = .003; 10 mg −2.9 vs. −1.98, p = 0.002 Mean voided volume: 5 mg +30.75 vs. +10.75, p = 0.0001; 10 mg +36 vs. +10.67, p = 0.0001 | Dry mouth: 5 mg 7.7 %, 10 mg 23.1 %, placebo 2.3 % Constipation: 5 mg 3.7 %, 10 mg 9.1 %, placebo 2.0 % | |
Abrams et al. (2005) | Solifenacin 5 and 10 mg daily | Females and males, >18 years old, OAB >3 months; urinary frequency ≥8/day, ≥1 urgency episodes /day | 12 weeks | 975 | Daily voids: 5 mg −3.6 vs. −1.6, p < 0.001; 10 mg −2.8 vs. −1.6, p < 0.001 Urgency episodes/day: 5 mg −3.2 vs. −2.1, p < 0.001; 10 mg −3.2 vs. −2.1, p < 0.001 Mean voided volume 5 mg +25 vs +7, p < 0.001; 10 mg +33.9 vs. +7, p < 0.001 | Dry mouth: 5 mg 10.8 %, 10 mg 24.4 %, placebo 3.6 % Constipation: 5 mg 4 %, 10 mg 12.2 %, placebo 1.3 % Discontinuation rate: 5 mg 2.8 %, 10 mg 7.8 %, placebo 6.2 % | Pooled analysis of 4 RCTs with subgroup analysis solely for OAB dry |
Nitti et al. (2007) | Fesoterodine 4 mg and 8 mg daily | Females and males, >18 years old, OAB >6 months; urinary frequency ≥8/day and mean of >6 urgency episode or >3 UUI over 3 day diary | 12 weeks | 836 | Daily voids: 4 mg −1.61 vs −1.08, p < 0.001; 8 mg −2.09 vs. −1.08, p < 0.001 Daily UUI: 4 mg −1.65 vs. −0.96, p < 0.001; 8 mg −2.28 vs. 0.96, p < 0.001 Daily urgency episodes: 4 mg −1.91 vs. −.079, p < 0.001; 8 mg −2.3 vs. −0.79, p < 0.001 Voided volume: 8 mg +33.6 vs. +8.38, p < 0.001 | Dry mouth: 4 mg 16 %, 8 mg 36 %, placebo 7 % Constipation: 4 mg 5 %, 8 mg 8 %, placebo 3 % Discontinuation rate: 4 mg 6 %, 8 mg 9 %, placebo 4 % | Prodrug with same active metabolite as tolterodine; dual excretion pathways |
Versi et al. (2000) | Oxybutynin 5 mg/day IR vs ER, 5 mg dose increases q7 days to max of 20 mg/day | Females and males; >7 UUI/week; +prior response to oxybuytnin | 226 | IR group 76 % reduction in UUI ER 83 % reduction-no significant difference | Dose dependent significant difference in dry mouth between both groups | Has direct muscle relaxant effect as well | |
Dmochowski et al. (2003) | Oxybutynin transdermal patch 3.9 mg/day; tolterodine LA 4 mg/day | Females and males; +prior prestudy response; >4 UUI, >24 voids, <350 cc/void over 3 day diary | 12 weeks | 361 | Daily UUI: patch −3 vs. placebo −2, p = 0.01 Average void volume: patch +24 vs. placebo +5.5, p = 0.001 | Site erythema: patch 8.3 % vs placebo 1.7 % < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





