2Renal Dietitian, Logan Hospital, Brisbane, Australia
Introduction
This chapter aims to describe the main aspects of diet in chronic kidney disease (CKD) and to provide non-specialists with practical information on managing the dietary requirements of patients on renal replacement therapy (RRT) and post renal transplant. There is no single diet for people with kidney disease, but most people will need to adapt their diet according to the stage and effects of their condition. The diet also needs to be individualised for each patient according to factors such as socioeconomic background, religious and pre-existing dietary restrictions and other clinical conditions.
Dietary treatment can be grouped broadly into three main areas:
Patients with advanced disease will often be well versed in their dietary regimes, and in the first instance non-specialists can often establish important dietary principles from the patient and or their family. Where more detailed information is needed or the patient requires a specialist review, renal dietitians are easily accessible through the patient’s kidney unit.
Specialist dietitians
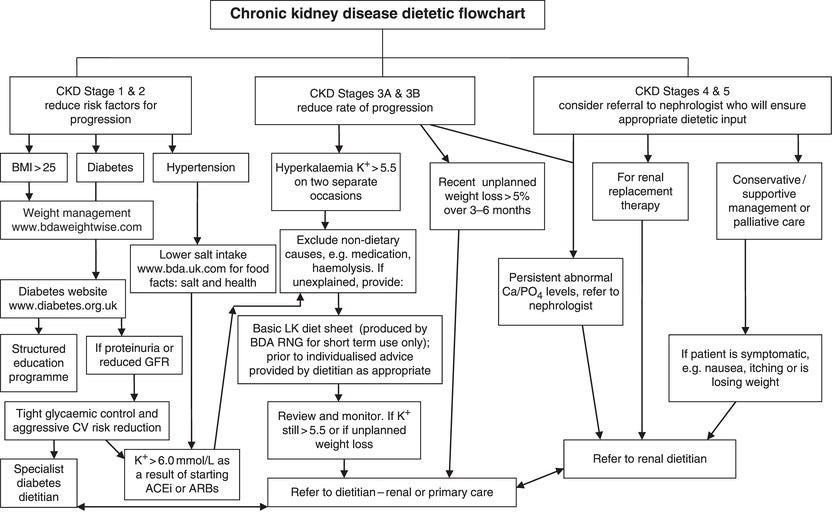
Individuals on dialysis or receiving a transplant will have renal dietitians assigned to their unit and should be seen promptly for dietetic treatment, with regular follow-up and review. Other outpatients with CKD stages 3–5 may have access to a renal dietitian at nephrology clinic, diabetes clinic or other specialist centre. However, many patients would welcome dietary advice at an earlier stage. The experienced clinician can safely and effectively provide initial advice, for example on healthy eating, low-salt and low-potassium diets (see Figure 9.2). However, a referral to a dietitian can be helpful if outcomes are not being achieved.
CKD patients who would benefit from a renal dietetic referral include:
- Patients with CKD stage 5, particularly those new to peritoneal dialysis or haemodialysis, and those choosing conservative management.
- Patients with CKD stage 4–5, with reduced appetite or intake, or any symptoms such as nausea or dysphagia that may compromise intake, as well as those with actual weight loss.
- CKD patients with chronic conditions such as cancer and gastrointestinal disorders that increase the risk of malnutrition.
- Patients following multiple dietary restrictions such as vegan, low-potassium and/or low-phosphate diets.
- Those following a low-phosphate diet and needing advice on the use of phosphate binders in relation to intake (some renal dietitians are prescribers of phosphate binders).
CKD and healthy eating
Prevention
Diet has an important role in reducing the risk of developing CKD and delaying its progression. This is primarily due to the contribution of diet to the management of chronic conditions such as hypertension, diabetes and obesity.
Healthy eating guidelines for CKD
In patients with existing CKD, diet and lifestyle remain important modifiable risk factors. National healthy eating guidelines are applicable in most cases and can be adapted for dialysis patients and others on restrictive therapeutic diets. There are specific guidelines for the role of diet in managing chronic conditions in patients with CKD (KDOQI 2007). UK national guidelines emphasise the need for a balance between energy needs and nutrient requirements so that energy intake should not exceed or fall short of requirements. People with CKD have similar energy requirements to the general population, but those on dialysis are at an increased risk of an energy deficit, and the guidelines reflect this (Ash et al. 2006).
General recommendations suggest eating a wide range of foods for a balanced intake of nutrients. This applies equally to patients with CKD on restricted diets. If this cannot be achieved they should be referred to a dietitian. Particular caution should be exercised with certain dietary approaches that result in an imbalance of particular nutrients such as:
- high-protein, low-carbohydrate diets
- very high intake of fruit or vegetables (risk of high serum potassium levels)
- high intake of dairy products (risk of excess phosphate/protein/potassium intake)
The healthy eating guidelines are summarised below, with comments on their applicability to individuals with CKD.
- Base meals on starchy foods (potatoes, cereals, pasta, rice and bread) – Where possible, wholegrain varieties and unsalted options should be chosen. Starchy foods should amount to a third of total food intake. People on a low-potassium diet will need to boil potatoes, yams and other starchy vegetables before eating or cooking further.
- Eat lots of fruit and vegetables – Five (80 g) portions of different types of fruit and vegetables a day are recommended. Even on a low-potassium diet one should maintain total fruit and vegetable intake as much as possible, and most patients can include at least four daily portions with the correct advice (see section on potassium, below).
- Eat more fish – At least two portions a week should be eaten, including one portion of oily fish. If possible, fish should be fresh, frozen or canned in spring water, as alternatives can be high in salt. Oily fish is high in omega-3 fats, which may help to prevent heart disease. Low-phosphate diets usually limit oily fish to no more than once or twice weekly and restrict fish with edible bones such as whitebait and tinned fish.
- Cut down on saturated fat and sugar – Saturated fat is associated with an increased risk of high cholesterol levels and cardiovascular disease. It is found in butter, lard, meat fat, cream, hard cheese, sausages, pies and many cakes and biscuits. Unsaturated vegetable oils and margarines are preferable. All fats have a high energy content, and limiting intakes will help to reduce the risk of becoming overweight. Sugary foods and drinks are often high in calories and low in other nutrients. However, In patients with poor appetites and an energy deficit, increasing dietary fat and sugar can be a practical and palatable way to optimise energy intake.
- Eat less salt – Eating less salt can reduce the risk of CKD, hypertension, stroke and heart disease in the general population and the progression in existing CKD. In more advanced CKD, a lower salt intake will help reduce the risk of fluid retention and is generally essential for patients requiring a fluid restriction.
- Get active and be a healthy weight – Overweight and obesity increase the risk of hypertension, diabetes, cardiovascular disease and CKD in the general population and are associated with more rapid progression in established CKD. Transplant candidates usually need to have a body mass index (BMI) below a maximum upper limit, typically 30–35 kg/m2, and aim for a healthy weight (BMI 20–25 kg/m2) post transplant. However, in dialysis patients there is evidence that heavier individuals have a lower mortality risk. Therefore advice on reducing weight needs to be individualised and balanced with other health considerations.
- Drink plenty of water – About six to eight glasses of water (or other low-calorie fluids) a day to prevent dehydration are recommended for the general population, more in warm weather or with increased activity. However, in advanced stages of CKD, fluid (and salt) restriction may be necessary to treat fluid retention in association with diuretics. Most people on dialysis require a fluid restriction (see section on fluid, below).
- Don’t skip breakfast – It is important to aim to eat regularly and not skip breakfast or other meals. This is good advice for everyone, with or without CKD, regardless of weight. In patients who are underweight or have problems eating enough, attempting more frequent meals or snacks will help to improve overall intake.
The eatwell plate
The eatwell plate (Figure 9.1) is a pictorial representation of the recommended balance of the diet as divided into five main food groups. It is intended to reflect the entire day’s intake but can also provide an indication of the balance of a single meal. It can be useful for people with CKD as well as those with other chronic conditions and those who want to reduce their risk of CKD. In more advanced CKD, the relative proportions of each food group may need to be adapted according to symptoms and any therapeutic dietary requirements such as energy supplementation or potassium restriction.
Diet for patients with CKD
The main principles of diet for people with CKD (Figure 9.2) are:
- There is no single diet for CKD – an individual approach is required.
- Optimise nutritional management of any underlying chronic condition such as hypertension, diabetes and obesity.
- Delay progression of CKD by preventing excessive protein intake, control of acidosis and prevention of muscle wasting with adequate energy intake.
- Manage complications of CKD such as hyperkalaemia, fluid retention, hyperphosphataemia and malnutrition.
- Achieve and maintain dietary adequacy and balance, including energy, fibre, macronutrients and micronutrients.
Figure 9.1 The ‘eatwell plate’. Crown copyright (Department of Health in association with the Welsh Government, the Scottish Government and the Food Standards Agency in Northern Ireland).
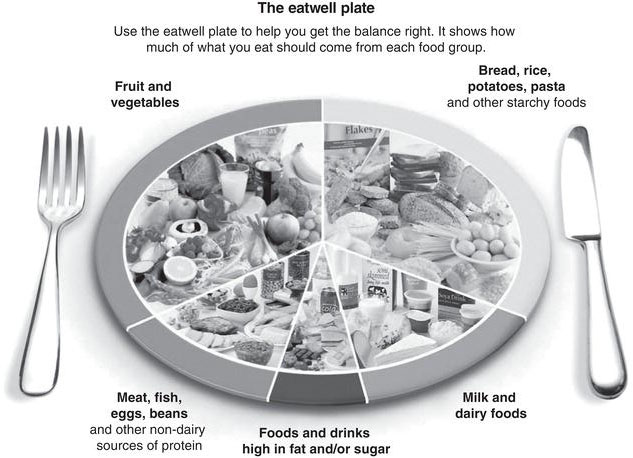
Figure 9.2 British Dietetic Association Renal Nutrition Group flowchart of dietetic intervention in CKD (Renal Nutrition Group).
Diabetes
Diabetes is a major cause of renal impairment in the UK, and dietary treatment of diabetes is essential in both the management and prevention of CKD. Together with appropriate medical management, people with diabetes should be encouraged to follow a healthy diet and lifestyle. The main strategies are summarised in the 2011 Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes (Dyson et al. 2011).
Glycemic index
Low-glycemic-index (GI) diets have been shown to improve both blood glucose and lipid levels in people with diabetes. The GI is a ranking of carbohydrates according to the extent to which they raise blood glucose levels after eating (Brand-Miller et al. 2003). Low-GI foods are more slowly digested and absorbed and cause a more gradual rise in blood glucose and insulin levels. Foods with a high GI are those which are rapidly digested and absorbed. It is also thought that foods with a lower GI are beneficial for weight management, because they help control appetite and delay hunger.
Strategies for managing diabetes include:
- regular carbohydrate-containing meals
- promote lower-GI choices
- maintain a healthy weight
- regular physical activity
- avoid foods high in added sugar
- encourage fruit and vegetables (within any potassium restriction if applicable)
- avoid saturated fat and choose unsaturated fats instead
Hypertension
Hypertension is both a cause and an effect of CKD. A low-salt diet, together with other lifestyle measures, will assist in reducing blood pressure and managing CKD (Sacks et al. 2001).
Lifestyle strategies for reducing hypertension include:
- aim for a healthy weight (body mass index 20–25 kg/m2)
- follow a low-salt diet – ideally less than 100 mmol/day
- limit alcohol consumption – less than three units per day for men and less than two units per day for women
- aim for regular exercise – 30 minutes of physical activity such as brisk walking at least three times each week
- include fruit and vegetables daily – aim for five portions per day (or maximum permitted on low-potassium diet if applicable)
- follow a diet low in saturated fat
Low-salt diet
Many patients find it difficult to follow a low-salt diet. Hidden salt contributes to the majority of a patient’s salt intake. In the UK people eat around 9 g of salt each day, and much of the excess salt comes from processed foods and ready-made meals. About a quarter comes from the salt we add to meals ourselves. Patients should be advised to avoid added salt and to choose lower salt options whenever possible. Note that potassium-containing salt substitutes such as LoSalt and PanSalt should be avoided by those with CKD.
Practical advice to reduce salt intake:
- It takes time to adjust to the taste of a lower salt diet – allow at least 6–8 weeks.
- Avoid adding salt to meals.
- Use other flavourings such as pepper, lemon, garlic, herbs and spices in place of salty items such as stock cubes, gravy granules and bottled sauces.
- Add only a little, if any, salt to the food when cooking.
- Eat plain or roast meats instead of processed and cured meats such as bacon, sausages and tinned meats.
- Avoid instant noodles and packet and tinned soups.
- Cook with fresh foods as often as possible rather than relying on ready-made or convenience foods.
- Choose low-salt or unsalted options when buying tinned and packaged foods.
- Avoid salty snacks such as crisps, salted crackers, popcorn or salted nuts.
- Read food labels. In sandwiches and packaged meals aim for less than 0.5 g sodium (1.25 g salt) per meal. To convert from sodium to salt multiply the amount by 2.5.
Obesity
Obesity is defined as BMI > 30 kg/m2. A weight loss of 5–10% can result in improved blood pressure and improved insulin sensitivity. A realistic weight-loss target is approximately 0.5–1.0kg (1-2lb) per week. Weight loss greater than this can lead to loss of muscle rather than fat, and unintentional weight loss should be avoided for the same reason. Physical activity should be encouraged, with discussion of safety and barriers to exercise as well as the choice, timing and frequency of exercise.
In general, patients with CKD should be advised to aim for a BMI within the healthy range of 20–25 kg/m2, with some variation according to ethnicity. For some groups of CKD patients, such as the more infirm, the elderly and those with more advanced CKD, the need for adequate nutritional intake may override any concerns about overweight. Malnutrition does occur in the obese and is often more difficult to diagnose, delaying treatment. Moreover, a higher BMI is associated with decreased mortality risk in individuals on haemodialysis and a BMI above 23 kg/m2 is recommended (Fouqueetal. 2007). Therefore advice on weight loss needs to be carefully considered in the context of other health priorities.
Nutrients
Protein
The requirement for protein in CKD has often been an area of controversy. Previous advice focused on very strict protein restrictions for the preservation of renal function. The Modification of Diet in Renal Disease trial (Klahr et al. 1994), although inconclusive, found that protein intake appeared to have only a minimal effect, if any, on decline in renal function. Furthermore, it is known that patients may spontaneously reduce their protein intake and therefore increase their risk of malnutrition with declining renal function. Current guidelines therefore suggest a moderate protein intake of 0.75–1.0 g of protein per kg (Wright & Jones 2010). This is in fact similar to the advice for the general population. Patients with anorexia, nausea, taste aversions and other barriers to adequate intake will require close monitoring and active encouragement to increase protein intake via food or nutritional supplements. An adequate energy intake is also essential.
Potassium
Declining renal excretion of potassium leads to adaptation by the gut to increase intestinal losses. However, as renal disease progresses, serum potassium levels will rise, depending on dietary intake and other factors. Where possible, the other causes of hyperkalaemia should be corrected to limit the need for dietary intervention.
Non-dietary causes of hyperkalaemia in CKD include:
- medications – including angiotensin converting enzyme inhibitors (ACE inhibitors), angiotensin receptor blockers (ARBs), spironolactone, beta-blockers, non-steroidal anti-inflammatory drugs (NSAIDs) and other contributing drug therapies
- catabolic state as a result of trauma or weight loss (muscle wasting)
- acidosis (check and correct bicarbonate levels)
- heavy exercise
- blood transfusions
- under-dialysis
- constipation (reduced gut losses)
Hyperkalaemia can lead to cardiac arrhythmias and even cardiac arrest. An overzealous approach to potassium restriction can be a problem due to an understandable fear of the worst possible outcome. However, potassium is present in a wide variety of foods including fruit, vegetables, meat and milk, and any intervention needs to be done so as to minimise the effect on choice, palatability and intake of micro- and macronutrients. The aim of the diet is to keep levels stable within safe limits, preventing any sudden increase in serum potassium levels. See Table 9.1 for reference ranges. Patients need to be aware of those foods that have particularly high potassium content. It is not usually necessary for patients to completely avoid all such foods to achieve adequate control, but they will require advice on levels of intake and suitable substitutions where possible.
Table 9.1 Reference ranges for blood potassium levels.
Renal Association Guidelines (UK).
| CKD | 3.5–5.3 mmol/L (or local normal values) |
| Haemodialysis | 4.0–6.0 mmol/L (level taken immediately before midweek dialysis session) |
There is no need to avoid all fruit and vegetables, as this directly contradicts healthy eating guidelines in removing many beneficial components of the diet. Instead, individuals can be advised to select lower-potassium fruit and vegetables and to adapt cooking methods (Jackson et al. 2006). Control of high-potassium drinks and snacks is important, as they may otherwise be taken in inconsistent amounts and cause unstable serum levels.
A low-potassium diet should be regularly reviewed to assess the appropriate level of restriction, particularly in response to medication changes or in situations such as inter-current illness. In the case of anorexia or poor intake for other reasons, a referral to the dietitian for a review of the potassium restriction will help to prioritise energy and other nutrients.
Treatment of hyperkalaemia
Suggested first-line potassium restriction for outpatients with hyperkalaemia includes:
- Avoid all salt substitutes which are potassium-based, such as Selora, LoSalt and PanSalt.
- Choose lower-potassium drinks such as water, fizzy drinks, flavoured water, squash (not high juice varieties), tea and herbal tea. Limit milk to half a pint (280 mL) daily. Limit coffee to 1–2 cups day. Avoid or limit these potassium-rich drinks: fruit and vegetable juices, chocolate and malted drinks.
- Choose lower-potassium snacks such as plain, jam, cream-filled biscuits and cakes, doughnuts, crumpets, crackers, croissants, boiled sweets and regular or sugar-free mints. Eat fewer high-potassium snacks such as crisps, nuts, Bombay mix, chocolate, toffee, fudge and liquorice.
- Have a moderate fruit and vegetable and salad intake of a total of 4–5 portions daily. A portion is about 80 g or about one handful of fruit or 2–3 tablespoons of cooked vegetables or salad. Some are particularly high in potassium, so avoid or eat less of the following: bananas, avocado, okra, spinach, all dried fruit and tomato puree.
- Adapt cooking methods to remove some of the potassium. Boil potatoes, yams and other vegetables in plenty of water and throw away the water before eating or cooking further. This includes before frying or roasting or before adding to stews, soups and curries. Avoid microwaving or steaming vegetables. There is no need to soak vegetables before boiling.
Low-potassium advice for hospitalised patients
Hospitalised patients are even more vulnerable to the side effects of a restricted diet as there are multiple barriers to adequate food intake. It is particularly important to consider non-dietary causes of hyperkalaemia and to avoid compromising energy and protein status. Excess potassium often derives from sources other than the hospital menu, such as chocolates, nuts, dried fruit, fresh fruit, juices, coffee and milky drinks. Patients can be advised on suitable alternatives such as lower-potassium fruit, plain, jam and cream biscuits and cakes, tea and fruit squashes. The hospital menu should provide lower-potassium vegetable and fruit choices and alternatives to potato such as rice, bread, pasta and noodles where possible. Potassium-based salt substitutes must not be given. For patients requiring nutrition support, a referral to the ward dietitian is recommended for specialist advice. Powdered milkshakes, soups and other products with high milk content are generally to be avoided.
Renal replacement and potassium
Haemodialysis (HD) is an effective method of potassium removal. However, there is a risk of excessive accumulation of potassium between dialysis sessions. Therefore, in many patients (but not all) some dietary control of potassium is necessary to limit the peak potassium level between haemodialysis sessions within safe limits, as defined by the Renal Association (Table 9.1). Lower pre-dialysis potassium levels, even those within the normal range, may indicate an over-restrictive diet regimen or inadequate dietary intake as a whole and an increased risk of malnutrition. Individualising and reviewing dietary advice remains essential in maintaining a balanced and adequate nutritional intake.
Peritoneal dialysis (PD) removes potassium effectively on a daily basis, so the need for potassium restriction is less common and often there is a risk of hypokalaemia in patients with an inadequate intake. On starting PD, patients can often relax dietary potassium restrictions and may even need specific advice on a high-potassium diet. This should be given in the context of the whole diet, including protein and energy and other nutrients, and optimising fruit and vegetables, rather than just considering potassium as an isolated nutrient.
Renal transplant patients may develop hyperkalaemia with delayed or inadequate graft function or as a result of immunosuppressive drug regimens and will need to follow a potassium restriction until these issues are resolved.
Phosphate
Phosphate retention is a consequence of a reduction in glomerular filtration rate (GFR) and can occur before any rise in serum phosphate levels is apparent. It has a detrimental effect on calcium homeostasis and parathyroid hormone (PTH) production, leading to the development of CKD mineral and bone disorder (CKD-MBD). This is characterised by a low serum calcium, high serum phosphate and high PTH levels in CKD stage 4 and 5. A reduction in the production of the active form of vitamin D (1,25-dihydroxycholecalciferol or calcitriol) in the kidneys contributes to hypocalcaemia and hyperparathyroidism.
Table 9.2 Reference ranges for blood phosphate levels.
Renal Association Guidelines (UK).
| CKD stage 3B–5 | 0.9–1.5 mmol/L |
| Dialysis | 1.1–1.7 mmol/L |
Dietary phosphate restriction is important in phosphate control, but the stage at which this should start is unclear. It is usually initiated once phosphate levels start to rise above normal. However, limits on phosphate intake at an earlier stage may be beneficial (Ash et al. 2006). Table 9.2 refers to reference ranges.
Low-phosphate diet
A low-phosphate diet is central to the management of CKD-MBD (KDIGO 2009). High-phosphate foods include meat, fish, eggs, nuts and protein-rich dairy foods such as milk, cheese and yoghurt. The challenge is to ensure that the low-phosphate diet remains balanced in terms of protein, energy and other nutrients and is compatible with chronic disease prevention and any other dietary regimens. To avoid compromising protein intake it is usual to limit foods with a higher phosphate content relative to their protein content such as dairy foods, offal, shellfish and oily fish (Fouque et al. 2007).
To address concerns about phosphate retention, patients in earlier stages of CKD and who have normal phosphate levels can be advised to follow national healthy eating guidelines. This provides a guide to moderate protein portions and can help to curb excess phosphate intakes while addressing chronic disease management and risk. High intakes of dairy foods should be avoided.
Phosphate-binding medication
Phosphate binders are used in combination with a low-phosphate diet to reduce the absorption of phosphate into the body (KDIGO 2009). They bind with dietary phosphate in the gut so that it is excreted in the stool. Calcium-based binders are the most commonly used, as they are cheap and effective, but there are some concerns about the effects of excess calcium absorption with larger doses. Alternatives include binders based on sevelamer (a polymer resin), lanthanum (a rare metal) and aluminium, although the last is now seldom used because of the risk of aluminium toxicity.
Patients and their carers need to be aware of the need to match administration of binders with the quantity and timing of phosphate intake. Binders should be taken with or before phosphate-containing meals or snacks. This will optimise their action and reduce the risk of side effects such as hypercalcaemia.
Low-phosphate diet: main principles
The main principles of a low-phosphate diet include:
- Dairy and eggs – relatively high phosphate-to-protein ratios – allowances set for milk, cheese, milk products and eggs. Avoid tinned and dried milk powder.
- Meat and fish – limit processed meat products, offal, shellfish, oily fish and fish with edible bones (such as whitebait, anchovies, tinned fish and dried or ground whole fish). Other meat and fish as usual but take a binder if prescribed.
- Drinks and snacks – limit milky drinks. Limit nuts including coconut. Limit chocolate and gram-flour-based snacks.
- Starchy staples (such as bread, pasta, rice, potatoes), fruit, vegetables, herbs and spices are mostly unrestricted.
- Sugar, jam, honey, fats, oils and cream are low in phosphate and can be taken as usual.
- Correct choice, dose and timing of phosphate binder in relation to intake.
Hypophosphataemia
In dialysis patients, low phosphate levels can be an indicator of low phosphate (and inevitably protein) intake as phosphate continues to be dialysed regardless. An excess dose of phosphate binders in relation to phosphate intake can also cause hypophosphataemia. A serum phosphate level below the recommended range should prompt a review of binders and adequacy of nutritional intake as a whole, particularly protein.
Fluid
Fluid management is important for cardiovascular health. Although multifactorial, fluid overload is known to be a significant precursor in the development of left ventricular hypertension and increased mortality in people with kidney disease. Patients at all stages of CKD find fluid management confusing and will benefit from clear explanations of the concepts involved. Most patients on dialysis are fluid restricted, and they often find this one of the most challenging aspects of the diet.
Establishing a fluid allowance
Patients need to be advised on an individualised fluid allowance, and this should be reviewed regularly. Patients on dialysis will usually experience a reduction in urine output over time. They should be aware of their 24-hour urine output volume and how to measure this on a regular basis, or as clinically indicated.
Helpful strategies for patients on fluid restrictions include:
- Know your fluid allowance.
- Know your dry (or target) weight.
- Know the guidelines for inter-dialytic weight gain (haemodialysis).
- Spread fluid over the day.
- Limit salt and salty foods to reduce thirst.
- Limit other foods that are found to increase thirst such as very spicy, astringent or powdery/dry foods.
- Try sucking an ice cube instead of a cold drink.
- Snacking on fruit (within any fruit allowance) can help to reduce thirst.
- Choose small cups or glasses.
- Try sucking on sugar-free boiled sweets, mints or chewing on gum between meals.
- Practise good dental hygiene – including regular brushing and use of suitable mouthwash.
- Sip drinks to make them last longer.
- Add lemon or lime wedges to drinks.
- Limit high-fluid foods such as soup, gravy, sauces and jelly.
- When having casseroles, curry, stews, main-course soups and other similar dishes, drain off the liquid before serving.
Table 9.3 shows the approximate volumes of a range of containers for fluids.
Table 9.3 Volume guide (approximate amounts).
| Mug | 250 mL |
| Teacup | 180 mL |
| Espresso cup | 80 mL |
| Medium wine glass | 250 mL |
| Water glass | 200–300 mL |
| Ice cube | 15–20 mL |
| 1 tablespoon | 15 mL |
Vitamins and minerals
Vitamin supplementation in renal patients is controversial, owing to a lack of evidence to support its long-term use. In the UK practice differs from one renal unit to another.
- Water-soluble vitamins should be supplemented – Water-soluble vitamin supplementation may be necessary because of factors such as dialysis losses (Wright & Jones 2010), dietary restrictions, observed low plasma vitamins and poor oral intake in the population. Additionally, water-soluble vitamins are linked with homocysteine levels and anaemia management.
- Vitamin D supplements are generally recommended – Vitamin D deficiency is widespread in the general population. In patients with CKD, vitamin D3 should be corrected as necessary in the standard manner. With CKD there is impaired renal synthesis to the ‘active’ form, 1,25-dihydroxycholecalciferol, and a vitamin D analogue such as calcitriol, alfacalcidol or paracalcitrol is usually required. Dosing is according to serum levels of calcium, phosphate, PTH and other bone markers, which must be regularly monitored.
- Vitamin A supplements should be avoided – An excessive intake of vitamin A is associated with elevated serum calcium levels. General multivitamins and supplements containing high quantities of vitamin A, such as cod liver oil, should be avoided.
Acidosis and nutrition
In CKD, acidosis occurs because the kidneys are less able to synthesise ammonia and excrete hydrogen ions. Correction of acidosis may lead to improvement in nutritional status and bone health. Treatment with sodium bicarbonate may also help to delay the progression of CKD and does not appear to have an adverse effect on blood pressure (de Brito-Ashurst et al. 2009). National recommendations suggest maintaining bicarbonate levels above 22 mmol/L (Wright & Jones 2010).
Haemodialysis
Standard haemodialysis (HD) takes place three times each week. Due to its intermittent nature there is a need to limit accumulation of waste products between sessions, especially fluid and potassium. The British Dietetic Association (BDA) Renal Nutrition Group recommends the European Best Practice Guidelines on Nutrition to provide guidance for nutrients other than protein requirements (BDA Renal Nutrition Group 2011) (Table 9.4).
Protein and energy
Patients undergoing HD are at high risk of malnutrition. Causes include multiple comorbidities and older age, socioeconomic issues, depression, chronic inflammation and poor intake due to time spent travelling and waiting for treatment, or due to multiple dietary restrictions. An adequate energy intake in combination with sufficient protein is essential to achieve an optimal metabolic balance (BDA Renal Nutrition Group 2011).
Patients with a normal or higher BMI may still have inadequate protein and micronutrient intakes, especially if they are inactive, because of the difficulty in selecting a sufficiently high-quality, nutrient-rich diet. It is important to encourage patients to be as active as possible in their daily life.
Table 9.4 Summary of the European and British guidelines and recommendations.
Data from Fouque et al, 2007; BDA Renal Nutrition Group, 2011.
| Nutrient | Guideline and recommendation |
| Protein | 1.1 g/kg oedema-free body weight/day where energy intake is adequate (BDA recommendation) |
| Energy | 30–40 kcal/kg IBW/day in a clinically stable chronic haemodialysis patient, adjusted for age, gender and the best estimate of physical activity level |
| Potassium | 50–70 mmol (1950–2730 mg) or 1 mmol/kg IBW/ day in patients with a pre-dialysis serum potassium > 6 mmol/L |
| Phosphate | 800–1000 mg phosphate/day |
| Sodium | No more than 80–100 mmol (2000–2300 mg) sodium or 5–6 g (75 mg/kg BW) of sodium chloride per day |
| Fluid | Daily fluid intake of 500–1000 mL in addition to volume equal to daily urine output. Individual fluid allowances need to be adapted for patients living in warmer climates, during periods of hot weather, working in hot environments and as a result of clinical conditions such as high fever. Inter-dialytic weight gain (IDWG) should not exceed 4–4.5% of dry body weight. |
IBW, ideal body weight.
Fluid and salt
A restricted fluid intake is required to limit accumulation of fluid between HD sessions to a maximum of 4–4.5% dry body weight (usually 2–3 kg). Control of fluid will improve blood pressure control and tolerance of HD, reduce tissue oedema and reduce the risk of left ventricular hypertrophy and breathlessness. All fluid-restricted patients require a salt restriction to minimise thirst and fluid retention.
Potassium and phosphate
Many HD patients need to limit potassium intake to control potassium accumulation between dialysis sessions. Fruit and vegetable intakes should be optimised within the restriction to maintain micronutrient and fibre intake. Phosphate is removed by HD, but patients will usually need to limit phosphate intake and take phosphate binders for adequate phosphate control. It is important to maintain adequate protein intake. Patients with low serum phosphate should have their binder dosage reduced and diet reviewed for overall nutritional adequacy.
Peritoneal dialysis
The BDA Renal Nutrition Group recommends the 2000 KDOQI National Kidney Foundation clinical practice guidelines for nutrition in chronic renal failure to guide practice in areas other than protein and energy requirements (BDA Renal Nutrition Group 2011).
Protein and energy
Most PD fluids are glucose-based, and approximately 30–70% of the glucose will be absorbed, depending on factors such as the dialysis prescription and individual peritoneal membrane characteristics. This will provide an energy load of about 250–500 kcal for most patients. The glucose absorption will also directly affect diabetes control. Exposure of the peritoneal membrane to higher-strength glucose solutions should be minimised because of the increased risk of premature technique failure.
During PD there are incidental losses of protein of approximately 5–15 g/day depending on individual membrane characteristics and dialysis regimen. The British Dietetic Association recommends a minimum protein intake of 1.0–1.2 g/kg oedema-free body weight per day. There is an emphasis on the need for an adequate total energy intake (from diet and dialysate) of 30–35 kcal/kg oedema-free body weight per day (BDA Renal Nutrition Group 2011). Despite the additional calorie source, malnutrition is widespread due to the general factors related to CKD and its treatment and additional PD-specific causes. These include peritonitis and other infections and abdominal discomfort due to the presence of dialysis fluid. For some patients the peritoneal absorption of glucose can be a useful extra source of energy. For others it represents ‘empty calories’ and causes excess weight gain and a relative deficit of protein and micronutrients. Regular dietary assessment and advice on achieving a high-quality, nutrient-rich diet are essential. It is important to encourage patients to be as active as possible in their daily life and to advise on suitable forms of exercise.
Fluid and salt
Control of fluid intake will help to reduce reliance on high-strength glucose solutions which remove more water but increase glucose absorption. Recommended fluid allowance is approximately 800 mL in addition to a volume equal to the 24-hour urine output (Ash et al. 2006). This will vary according to individual peritoneal membrane characteristics and dialysis regimen, as well as climate and clinical condition. Patients following a fluid restriction will require advice on salt restriction to reduce thirst and fluid retention.
Potassium and phosphate
Most PD regimens remove potassium efficiently on a daily basis. Patients with low potassium intakes are at risk from hypokalaemia due to the continuous removal of potassium. Patients may require education on increasing potassium in their diet. Many PD patients will need to limit phosphate intake, and will require phosphate binders for adequate phosphate control. However, hypophosphataemia is a risk in patients with poor intakes, especially if dietary protein is lacking.
Fibre
Prevention of constipation is important for functioning PD. Patients should be encouraged to have adequate fibre in their diet with plenty of fruit and vegetables within any potassium restriction if this applies. Soluble fibre supplements may be beneficial in preventing constipation without the side effects of stimulant laxatives (Sutton et al. 2007).
Transplant
A successful kidney transplant gives a patient increased dietary freedom. Diet plays an important role in preventing and managing obesity, diabetes, cardiovascular disease, hypertension, dyslipidaemia and bone disease, which can all occur in the transplant recipient. Despite a renal transplant, most patients will still have a degree of CKD, which can make them particularly complex to manage. There is little research regarding diet and outcomes in this population, and many dietary recommendations are therefore similar to those for healthy individuals.
Dyslipidamia is present in approximately 60% of renal transplant recipients and is strongly associated with cardiovascular disease (GMCT 2008). Immunosuppressive regimes, together with pre-existing dyslipidaemia and lifestyle factors, can contribute to abnormal lipid profiles.
Hypertension is a risk factor for cardiovascular disease and can adversely affect graft survival. Patients should be encouraged to continue with a low-salt diet after a transplant. Additionally, alcohol consumption should be based on recommendations for the general population, because of its influence on cardiovascular disease.
Patients should be made aware of the risk of weight gain after a renal transplant. This is multifactorial and associated with increased dietary flexibility and improved wellbeing. Steroid use is also known to stimulate appetite. The majority of the weight gained tends to occur in the first year and can be as high at 35% (GMCT 2008). Obesity is associated with poor graft survival and contributes to hypertension, diabetes and dyslipidaemia. New-onset diabetes after transplantation (NODAT) is associated with graft failure and cardiovascular disease and has been reported to affect 20% of patients at one year post transplant. Immunosuppressive regimens, in addition to traditional risk factors such as age, ethnicity and family history, will influence NODAT risk.
During the initial few weeks following a kidney transplant, patients should be encouraged to take a higher-protein diet, particularly if graft function is delayed and the patient continues to need dialysis. Additionally, a higher protein intake promotes wound healing. A protein intake of 1.3 g/kg in the first four weeks is suggested. Subsequently, stable patients should be encouraged to have a moderate protein intake in line with general healthy eating guidelines (GMCT 2008).
The management of bone disease is complex in kidney disease, and prior to transplantation there are likely to be several bone health abnormalities.The risk of fracture is higher in this group than in the general population. Factors contributing to poorer bone metabolism include poor calcium absorption related to steroid use, hypophosphatemia, particularly in the first few months post transplant, and abnormal vitamin D metabolism (GMCT 2008). Patients should be encouraged take a high-phosphate diet, as early as possible after a renal transplant to help correct hypophosphataemia. Some patients will require oral phosphate supplementation if levels cannot be normalised with a high-phosphate diet and if they are symptomatic. However, if supplemented late in the post-transplant phase, hyperparathyroidism can be worsened (GMCT 2008). See the section on phosphate, above, for practical suggestions.
Calcium intake should follow guidelines for the general population. Physical activity, particularly weight-bearing activity, is important for bone health and should be encouraged. Vitamin D supplements (or analogue) have been shown to have a favourable effect on bone mineral density.
Establishing a fluid goal as early as possible is important. Many patients have been so used to restricting fluid intake that they can find drinking sufficient fluid difficult.
There is thought to be an increased risk of food-borne illnesses such as listeriosis due to immunosuppressant medications, particularly in the early stages after a transplant when doses are higher. Patients are advised on their vulnerability regarding food-borne illnesses and on standard food safety and hygiene, including storage and preparation of raw and cooked food.
When should a kidney transplant patient see a dietitian?
The recently published Evidenced Based Practice Guidelines for the Nutritional Management of Adult Kidney Transplant Recipients (GMCT 2008) suggest that patients should be seen routinely by a dietitian as soon as practicable after a transplant and followed up monthly for the first three months. An annual review is recommended thereafter. Interim referral and review may be required for various reasons including management of comorbidities and symptoms as they occur.
Summary for diet in renal transplant recipients
- Eat a diet based on the eatwell plate which is high in vegetables, fruit and whole grains (Figure 9.1).
- Have a diet low in saturated fat. Use fats which are based on unsaturated fats such as olive oil, margarines and sunflower oil.
- Continue to follow a low-salt diet.
- Aim for a moderate protein intake. However, aim to include oily fish regularly.
- Choose low-fat dairy foods.
- Use a vitamin D supplement as appropriate for the individual.
- Drink alcohol in moderation.
- Aim for regular physical activity.
- Ensure adequate fluid intake.
- Follow good food hygiene practices.
Malnutrition, nutritional assessment and intervention
Malnutrition is widespread in patients with CKD, especially in its more advanced stages, and can have a significant impact on morbidity and mortality (Fouqueet al. 2007). Malnutrition is also referred to more specifically as protein-energy wasting (PEW). It can develop quickly and, once established, is difficult to treat.
Causes of malnutrition in CKD
There are many possible causes of PEW in CKD. Factors include increased requirements, increased losses and multiple barriers to intake. These vary from a non-specific loss of appetite, chronic infections and dialysis protein losses to the economic, social and psychological implications of a chronic illness (Table 9.5).
Table 9.5 Causes of malnutrition in CKD.
| Cause of malnutrition | Inadequate intake | Increased requirements |
| Examples of contributing factors | Socioeconomic, including social isolation, financial difficulties, inability to work Psychological, such as depression, anxiety and stress Uraemic symptoms such as anorexia, acid reflux, nausea, vomiting, taste changes, food aversions especially with under-dialysis or delayed dialysis Pharmaceutical, including polypharmacy and drug-related side effects Inappropriate, unbalanced or inadequately monitored ‘therapeutic’ diets Treatment-related, including time and effort attending the HD unit or doing PD and early satiety with PD fluid in situ Recurrent/prolonged hospital admissions Anaemia, inter-current illnesses, physical infirmity or disability Comorbidities such as cancer, HIV, gastrointestinal disease | Inflammation Infection, including dialysis-specific infections such as HD line sepsis and PD peritonitis Increased protein catabolism associated with factors such as energy deficit, low activity levels, increased infections, acidosis, insulin resistance and hyperparathyroidism Drug side effects Increased losses, including proteinuria and protein losses on HD and PD Comorbidities |
Nutritional assessment
The diagnosis of malnutrition in renal patients can be particularly challenging, as many indices of nutritional status may be directly altered by renal failure or other disease processes. There is currently no single method of nutritional assessment that is considered ideal, but using a combination of approaches is recommended (Wright & Jones 2010).
Anthropometric assessment
Anthropometry includes the measurement of height, weight, circumference of various body parts and skin fold thickness at different sites. The procedures are generally simple, inexpensive, safe and non-invasive. However, certain aspects of kidney disease, such as fluid retention, can affect their use and interpretation.
Fluid retention may mask loss of muscle or fat mass. The best estimation of oedema-free weight should be used for nutritional assessment and calculations such as BMI (weight/height2). In dialysis patients the current ‘dry’ or ‘target’ weight should be obtained from the renal unit records.
There is a lack of knowledge generally amongst patients regarding malnutrition, and improving awareness of weight changes can help. However, this is not always easy. Those with fluid retention may report that they feel fat or overweight – sometimes to the extent that they will intentionally limit food intake and exacerbate PEW. Conversely, a resolution of oedema may be reported as ‘weight loss’. It is useful to obtain a weight history to quantify and explain changes in body weight over time. It is important to clarify weight terminology such as dry weight, target weight and ideal weight. Patients can be advised to self-monitor and report other indicators such as upper-body wasting, anorexia and reduced intake.
Waist and hip circumference measurements can be used to assess for central obesity and cardiovascular disease risk unless the abdomen is distended, for example by the presence of peritoneal fluid, ascites or polycystic organs.
Measurements of triceps skin fold and mid-upper arm circumference can be used to assess and monitor local changes in muscle and fat in individual patients over time. These measurements can be affected by fluid status and should ideally be taken post-haemodialysis or when the subject is at a dry weight. Handgrip dynamometry measures grip strength, and in renal patients it has been found to correlate well with lean body mass and have prognostic value (Wang et al. 2005).
The right side is preferred for upper-arm anthropometry and handgrip measurements, as this is consistent with standard reference data. In practice, it will need to be the side without vascular access, dressings or other obstacles. Comparison of measurements with reference tables can be difficult and needs to take into account any differences in methodology, age and other selection criteria of the reference population. In practice an individual’s measurements will be taken and compared over time to monitor progress.
Biochemical assessment
Serum albumin concentration is a less reliable indicator of nutritional status. Low albumin levels may be related to fluid overload, inflammation, sepsis or other factors and require medical investigation. Conversely, normal albumin levels do not preclude a diagnosis of malnutrition. C-reactive protein (CRP) increases with the acute-phase response and can help in interpreting albumin levels (Wright & Jones 2010).
Subjective global assessment
Subjective Global Assessment (SGA) is widely recommended in renal patients (Wright & Jones 2010). Requiring minimal resources and modest training for the doctor, nurse or dietitian, the SGA combines a short clinical history with a physical examination. This results in an overall impression of nutritional status without the need for precise body composition analysis. This subjective classification of the patient by nutritional status has been shown to correlate well with future morbidity and mortality (Table 9.6). The Patient-Generated Subjective Global Assessment (PG-SGA) has been developed to include a numerical score as well as an overall subjective rating. It is also validated for use with renal patients.
Nutritional screening
Nutritional screening is undertaken to detect those individuals within a group who are at risk of malnutrition and to determine if a more detailed assessment or other intervention is needed. Because of the increased risk of malnutrition with CKD it should be integral to the role of all health professionals caring for these patients (Wright & Jones 2010). Generic methods, such as the Malnutrition Universal Screening Tool (MUST), are in widespread use. However, these may not be fully applicable to patients with CKD, and work to develop renal-specific malnutrition screening tools is ongoing (Wright & Jones 2010).
Table 9.6 Subjective Global Assessment (SGA) tool outline.
Jeejeebhoy, K. (2012). Subjective Global Assessment: A highly reliable nutritional assessment tool. Available at: http://subjectiveglobalassessment.com/.
| History Weight/weight change – using best estimation of dry weight Dietary intake – change/no change Gastrointestinal symptoms (anorexia, nausea, vomiting, diarrhoea) – frequency/duration Functional capacity Disease state/comorbidities as related to nutritional needs Physical examination
|
Overall SGA rating: A, very mild to well-nourished; B, mild to moderately malnourished; C, severely malnourished.
Renal Association recommended frequency of screening for under-nutrition in CKD (Wright & Jones 2010):
- Weekly for inpatients.
- 2–3-monthly for outpatients with eGFR < 20 but not on dialysis.
- Within one month of commencement of dialysis then 6–8 weeks later.
- 4–6-monthly for stable haemodialysis patients.
- 4–6-monthly for stable peritoneal dialysis patients.
- Screening may need to occur more frequently if risk of under-nutrition is increased, for example by inter-current illness.
Dietary treatment of malnutrition
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







