Online Chapter 2
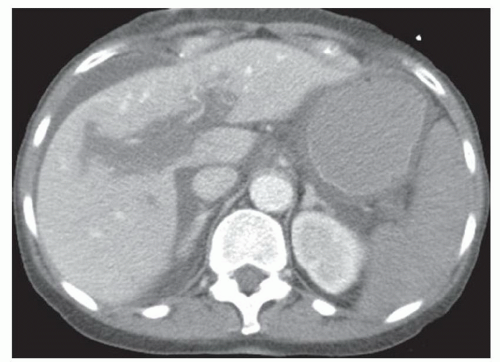
CASE 2.1 CLINICAL HISTORY 34-year-old man with fever and abdominal pain.
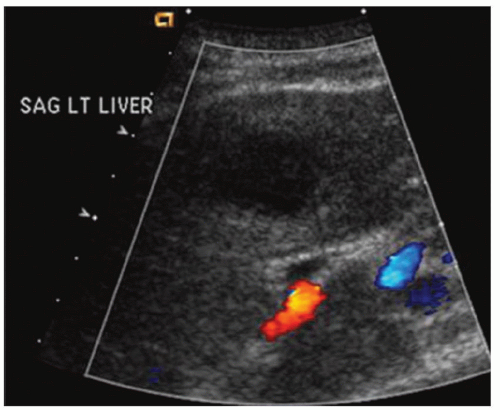
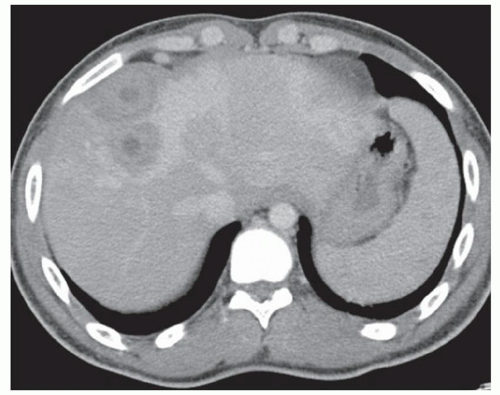
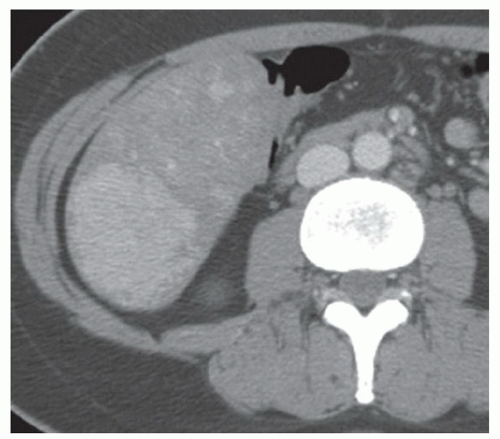
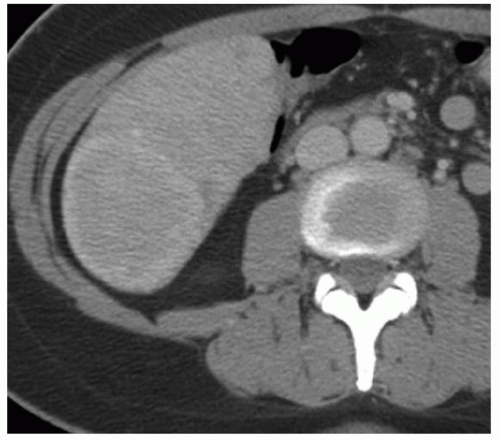
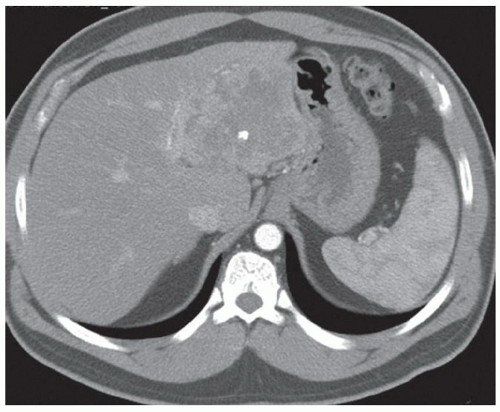
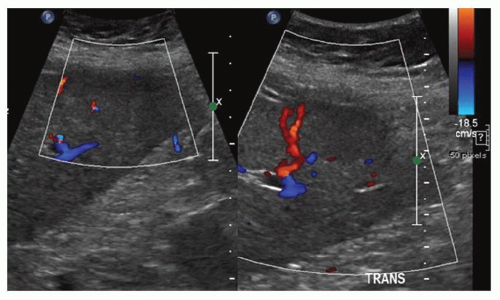
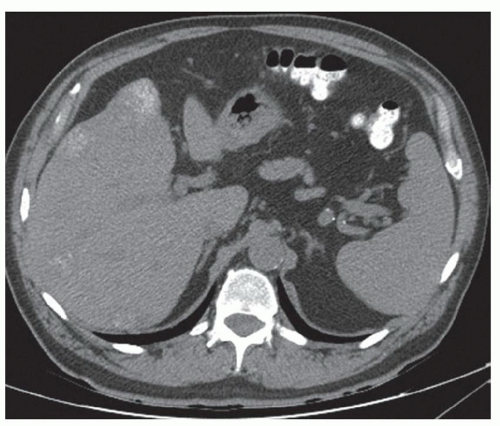
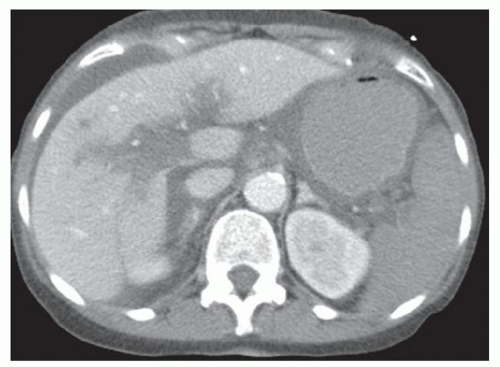
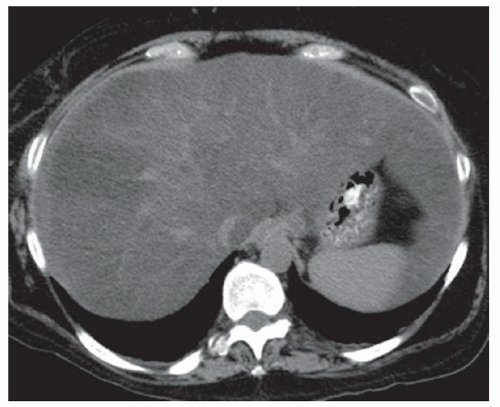
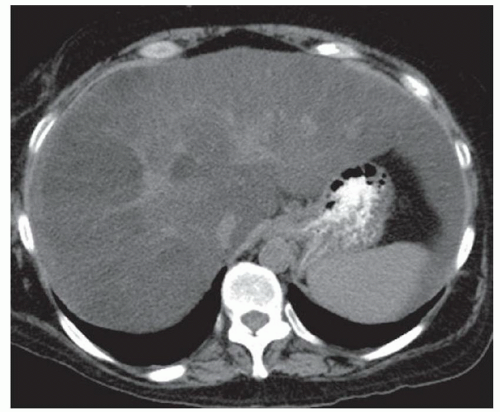
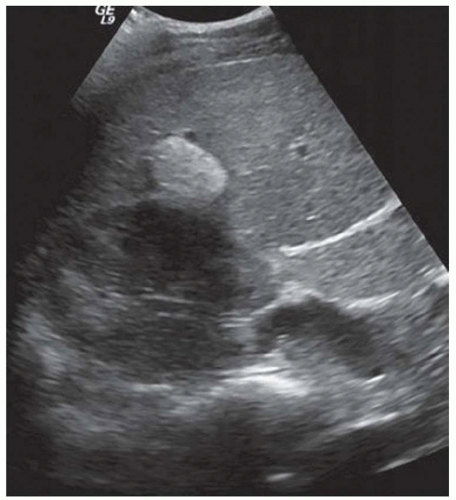
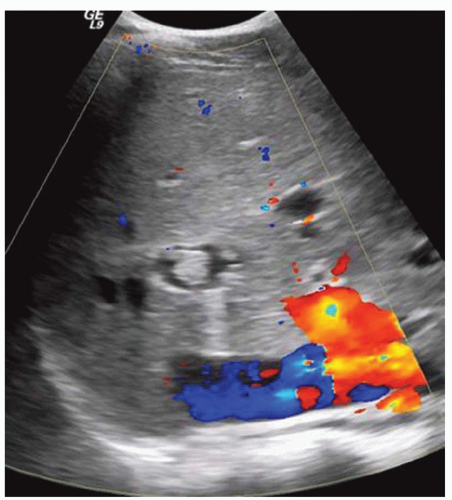
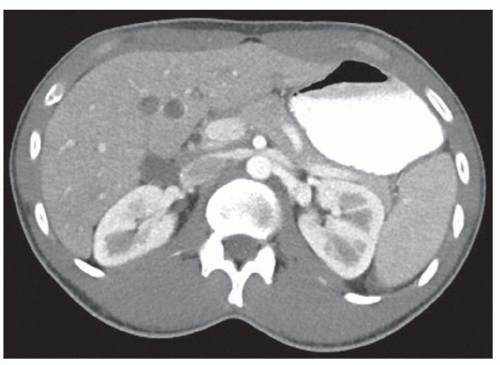
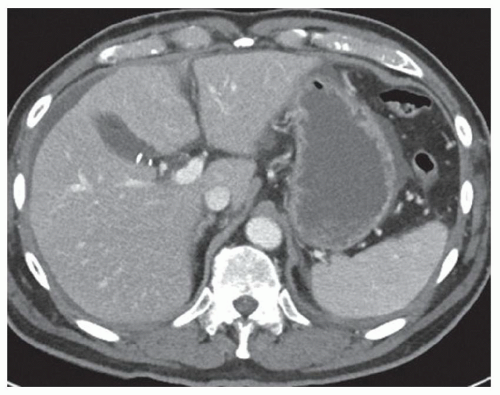
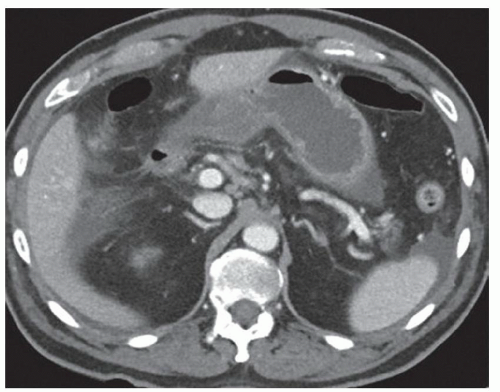
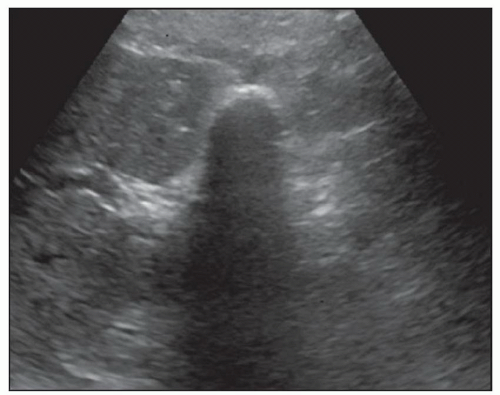
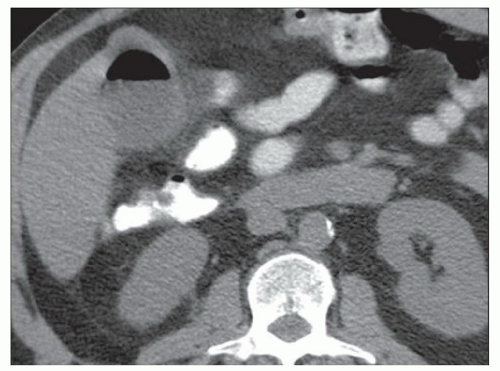
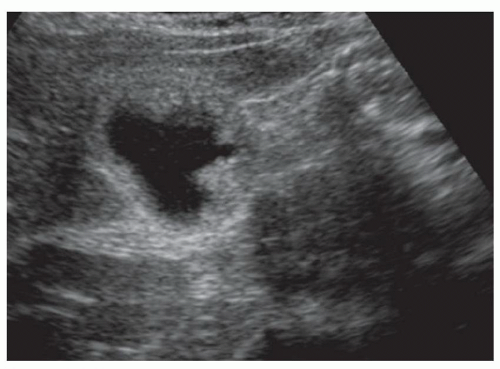
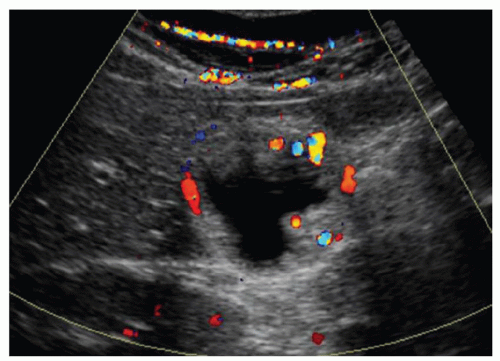
FINDINGS Color Doppler ultrasound (US) (A) demonstrates a 3-cm hypoechoic mass in the left hepatic lobe without definite internal blood flow. Multiple other similar lesions were seen at US. Contrast-enhanced computed tomography (CT) (B) demonstrates multiple low-attenuation hepatic lesions with central cystic spaces and surrounding areas of relative hyperenhancement.
DIFFERENTIAL DIAGNOSIS Abscesses, cysts, cystic metastases.
DIAGNOSIS Abscesses.
DISCUSSION The “double-target” sign seen in the above case refers to the cystic central portion of an abscess surrounded by reactive hyperemia. Abscesses may also appear as a large, multilocular area of low attenuation.
Cystic metastases can occur when hypervascular masses outgrow their blood supply and become necrotic. Treated gastrointestinal stromal tumor (GIST) metastases, for example, can have a cystic appearance. Clinical correlation can help distinguish between liver abscesses and metastases, but percutaneous biopsy is sometimes needed. Cysts appear entirely cystic at CT or MR and do not demonstrate adjacent reactive hyperemia.
Questions for Further Thought
1. What are the most common organisms found in liver abscesses?
View Answer
1. Escherichia coli, Clostridium, and Bacteroides are the most common species found in liver abscesses.
2. By what route(s) do bacteria enter the liver?
View Answer
2. Bacteria enter the liver most frequently via the portal system or the biliary system.
Reporting Requirements
1. Describe the size, number, and location of liver lesions.
2. Include hepatic abscess in the differential diagnosis of a cystic liver mass with surrounding hyperemia.
What the Treating Physician Needs to Know
1. For larger abscesses, image-guided percutaneous drainage catheter placement may be beneficial.
2. Up to 26% of patients may become septic (fever, hypotension, rigors, and hypoxia) immediately following percutaneous drainage catheter placement even in the setting of prophylactic antibiotics.
Answers
1. Escherichia coli, Clostridium, and Bacteroides are the most common species found in liver abscesses.
2. Bacteria enter the liver most frequently via the portal system or the biliary system.
REFERENCES
1. Mortele KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics 2001;21:895-910.
2. Thomas J, Turner SR, Nelson RC, Paulson EK. Postprocedure sepsis in imaging-guided percutaneous hepatic abscess drainage: how often does it occur? Am J Roentgenol 2006;186: 1419-1422.
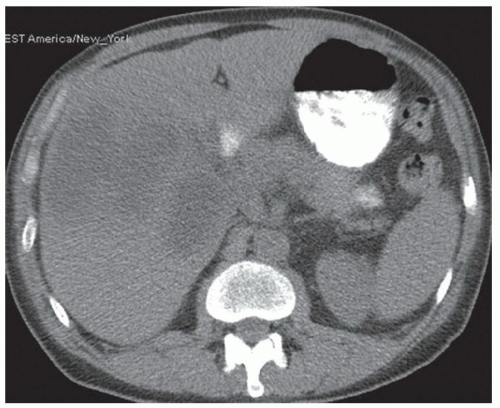
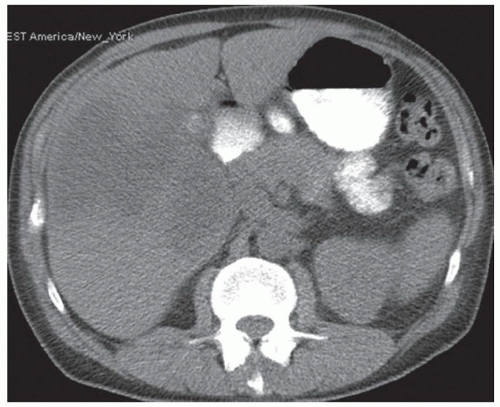
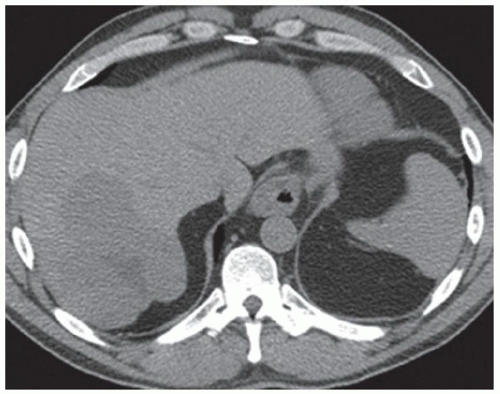
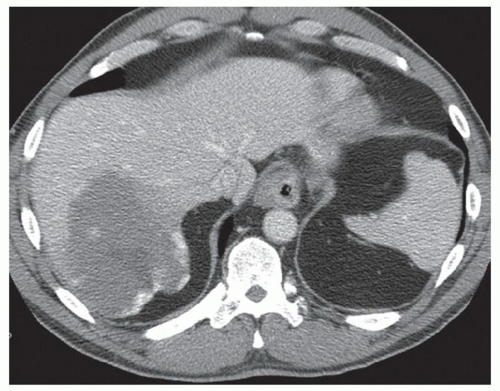
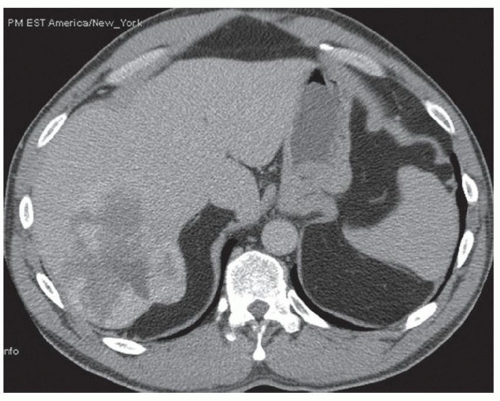
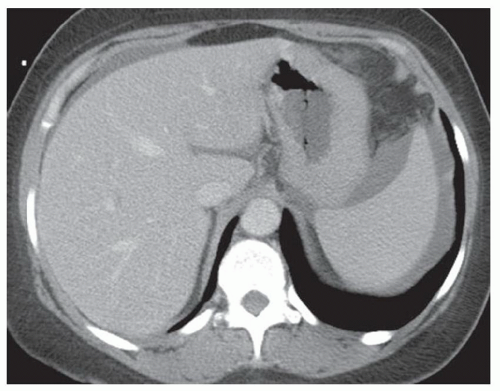
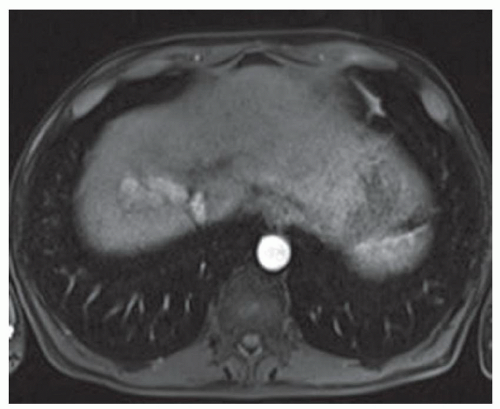
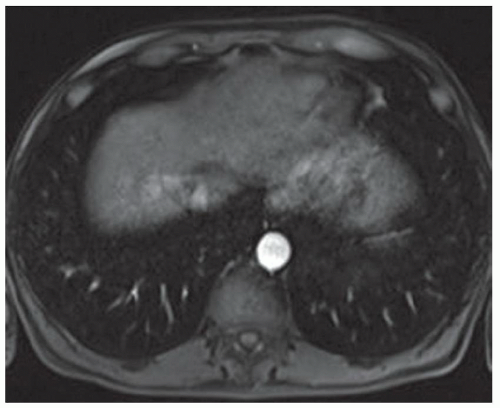
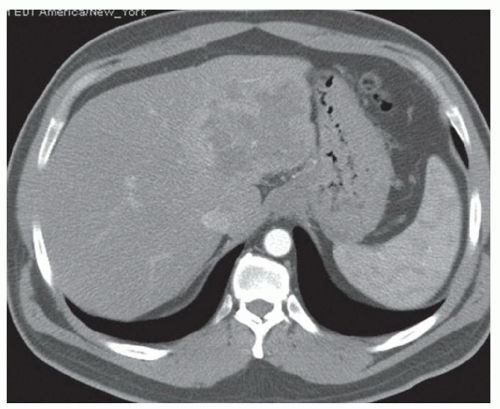
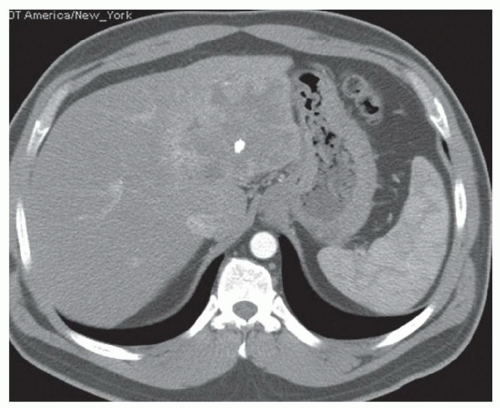
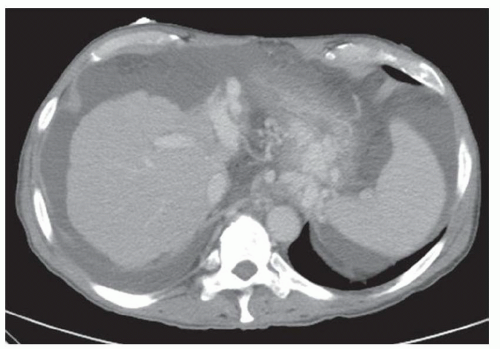
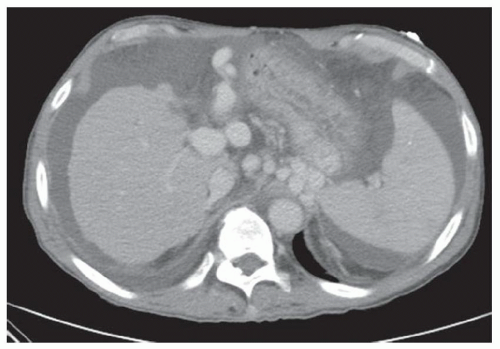
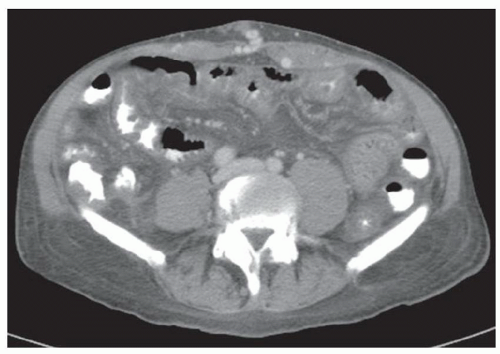
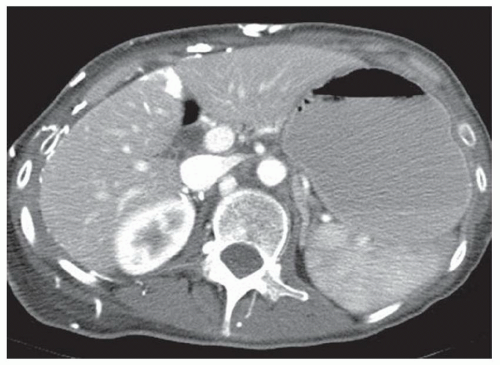
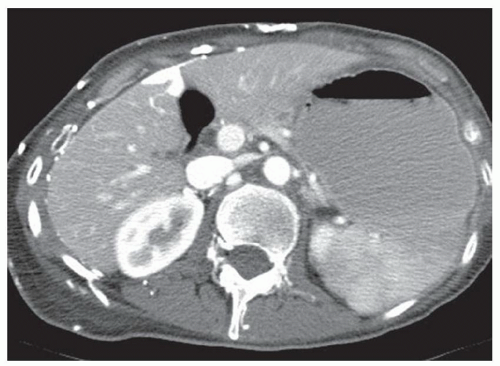
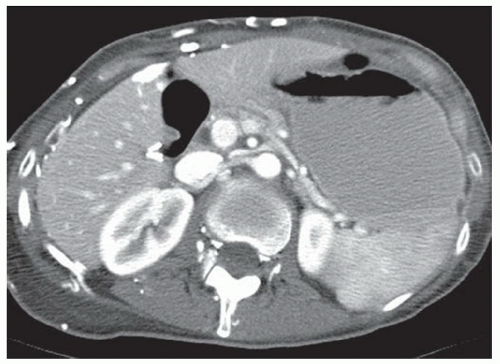
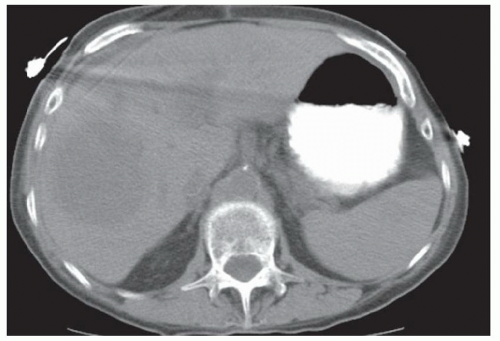
CASE 2.2 CLINICAL HISTORY 54-year-old man with right upper quadrant pain.
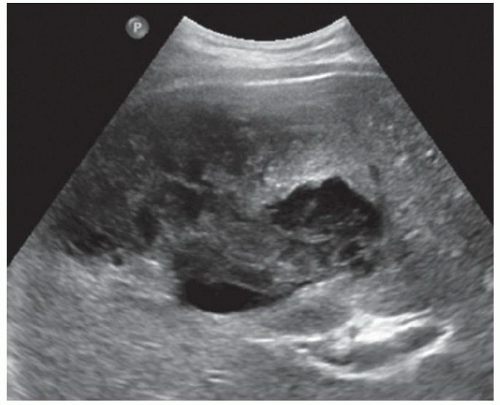
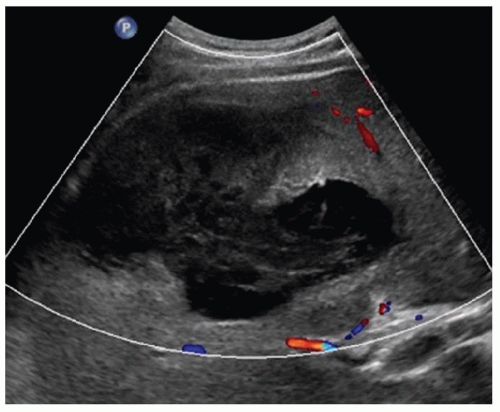
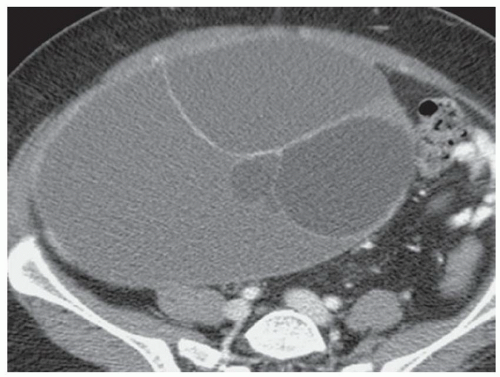
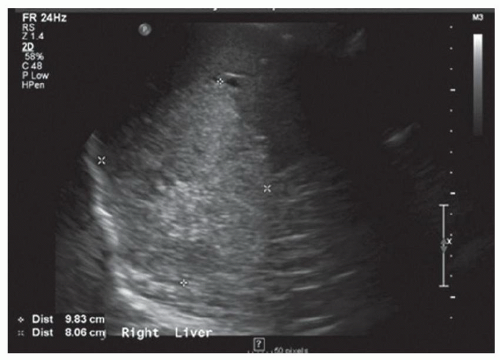
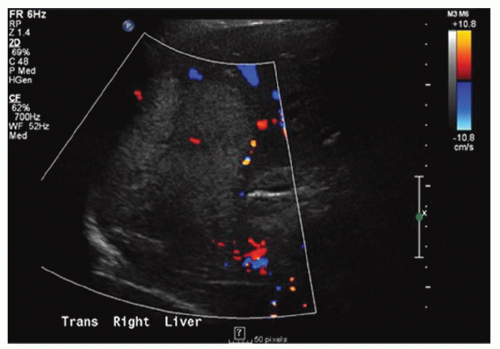
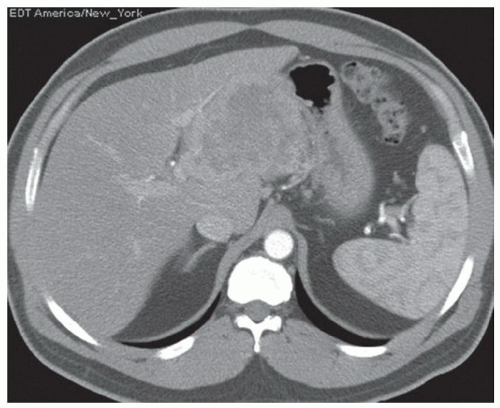
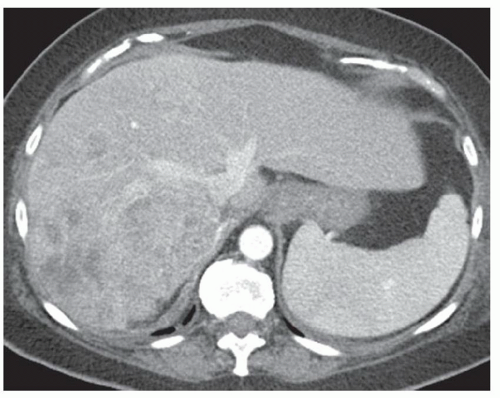
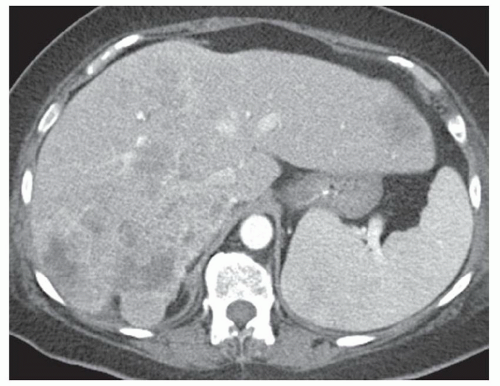
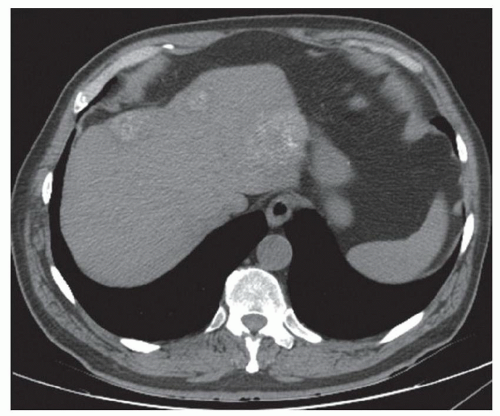
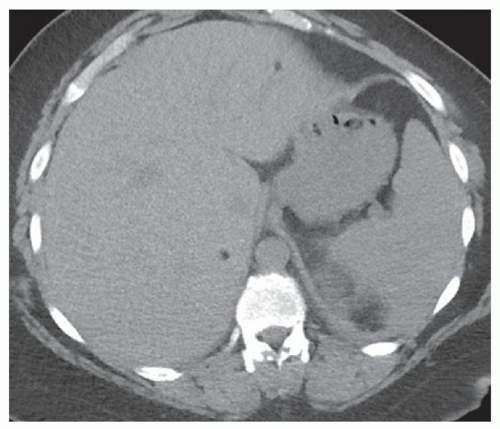
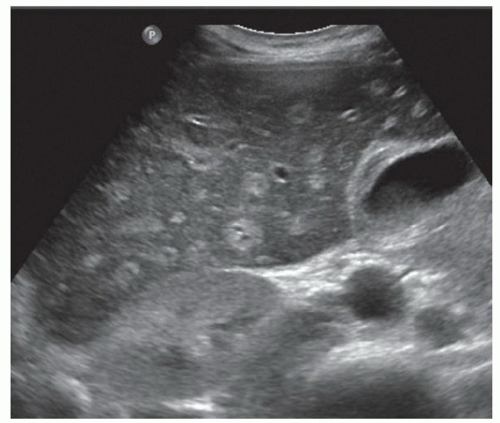
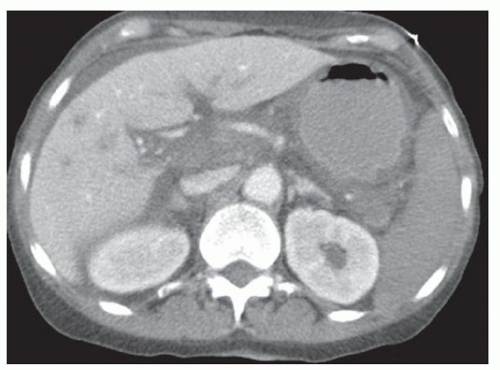
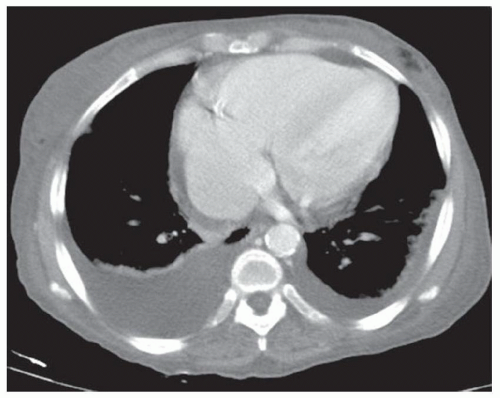
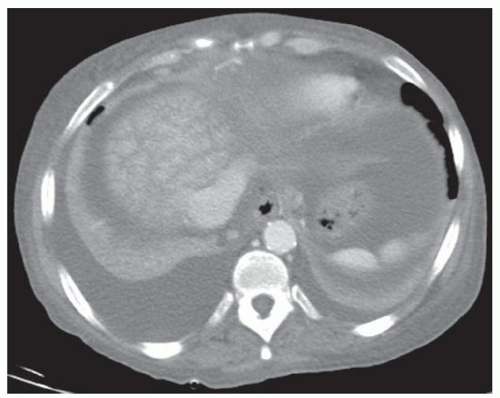
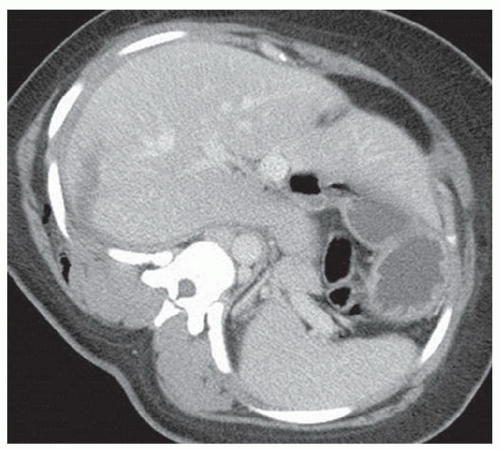
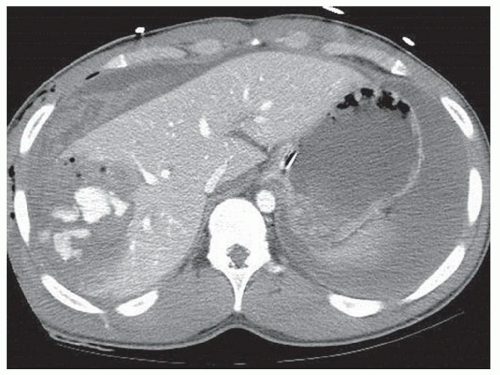
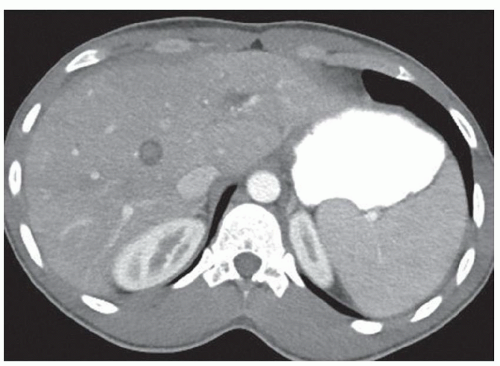
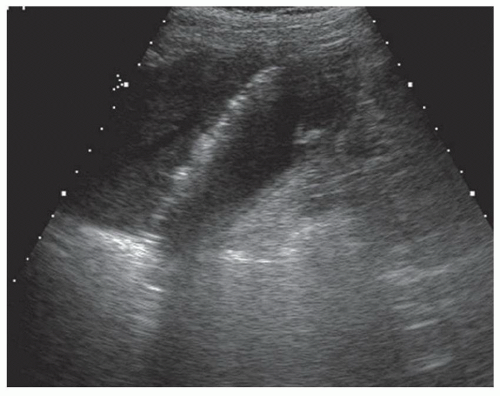
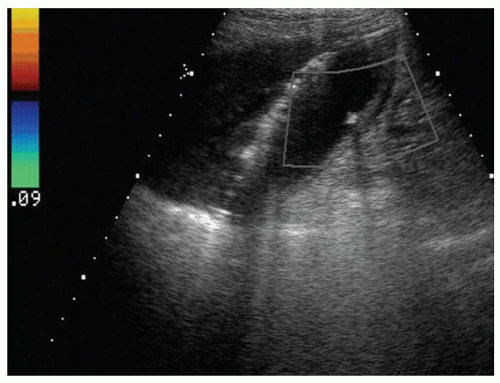
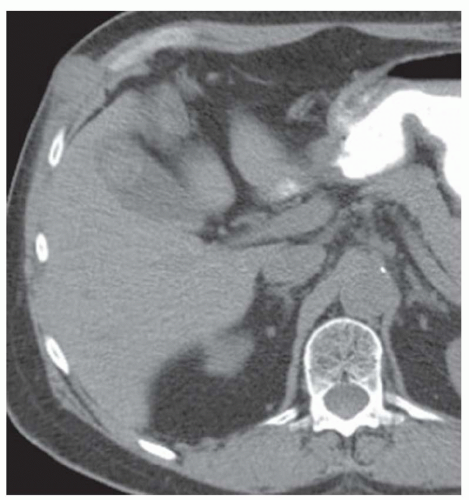
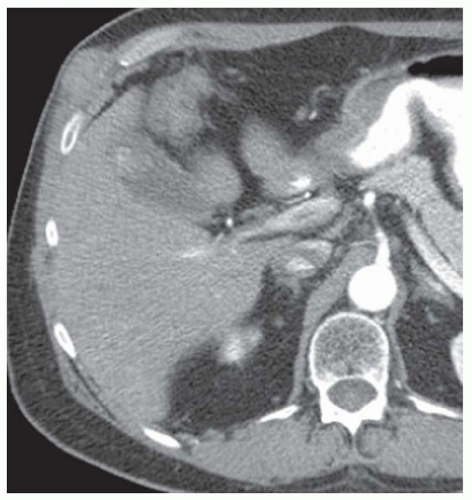
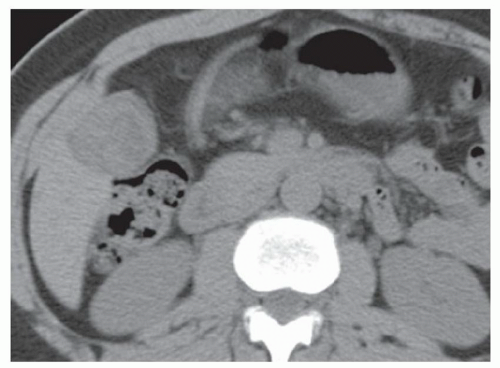
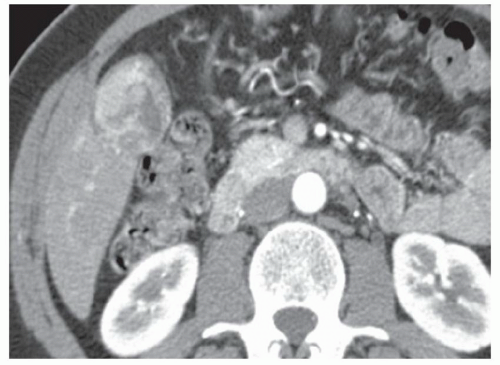
FINDINGS Grayscale US (A) demonstrates a 12-cm bilobed, heterogeneous structure in the right hepatic lobe extending toward the liver dome. No definite internal blood flow is seen in this location at color Doppler imaging (B). Mobile debris was visible at real-time imaging. Noncontrast CT (C, D) demonstrates the lesion to be multilobular and of low attenuation.
DIFFERENTIAL DIAGNOSIS Abscess, biloma, hematoma.
DIAGNOSIS Abscess.
DISCUSSION US demonstrates a large structure with internal debris and no internal blood flow. Based on US alone, the structure could be a large abscess, biloma, or hematoma. Given the low attenuation seen at noncontrast CT, a hematoma is unlikely. Blood products at noncontrast CT of the abdomen usually measure >40 to 50 HU. Attenuation values may be lower if blood mixes with ascitic fluid, in anemic patients, and over time as blood products evolve. Given that the low-attenuation areas in the above liver measure approximately 25 HU, a hematoma is unlikely. Bilomas are usually <20 HU attenuation and appear closer to the attenuation of water than hematomas.
Based on imaging features, abscess is the most likely diagnosis in this case. Clinical history of fever and elevated white blood cell count were confirmatory.
Questions for Further Thought
1. What are the most common causes of liver abscesses?
View Answer
1. Biliary infections or gastrointestinal (GI) infections drained through the portal venous system are the most common causes of liver abscesses. Therefore, in patients with liver abscesses the biliary system should be evaluated for evidence of obstructing stone, stricture, or tumor. The GI tract should also be evaluated for infection such as appendicitis or diverticulitis.
2. The treating physician asks you to drain this abscess. What is your response?
View Answer
2. This abscess would be amenable to percutaneous drainage using CT or US guidance. As patients with liver abscesses are prone to sepsis following manipulation of the abscess, it is important to confirm that the patient has received antibiotics prior to the drainage procedure. Aspirated fluid should be sent for culture.
Reporting Requirements
1. Describe the size and location of the abscess.
2. Report should be called to the ordering physician as, if left untreated, mortality is high in patients with liver abscesses.
What the Treating Physician Needs to Know
1. The above lesion is compatible with an abscess.
2. The above lesion would be amenable to percutaneous drainage.
Answers
1. Biliary infections or gastrointestinal (GI) infections drained through the portal venous system are the most common causes of liver abscesses. Therefore, in patients with liver abscesses the biliary system should be evaluated for evidence of obstructing stone, stricture, or tumor. The GI tract should also be evaluated for infection such as appendicitis or diverticulitis.
2. This abscess would be amenable to percutaneous drainage using CT or US guidance. As patients with liver abscesses are prone to sepsis following manipulation of the abscess, it is important to confirm that the patient has received antibiotics prior to the drainage procedure. Aspirated fluid should be sent for culture.
REFERENCES
1. Mortele KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics 2001;21:895-910.
2. Thomas J, Turner SR, Nelson RC, Paulson EK. Postprocedure sepsis in imaging-guided percutaneous hepatic abscess drainage: how often does it occur? Am J Roentgenol 2006;186:1419-1422.
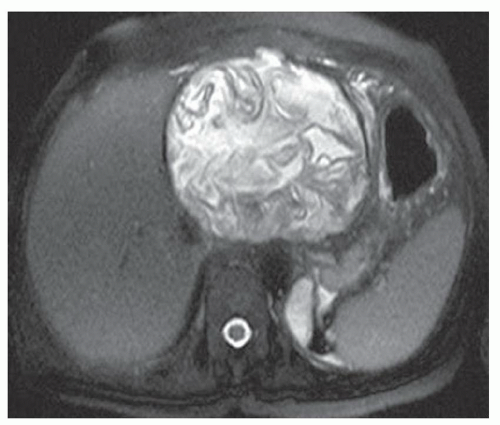
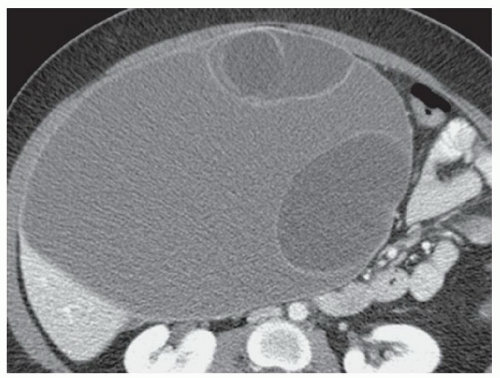
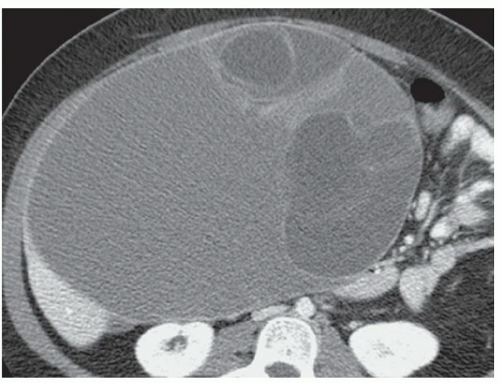
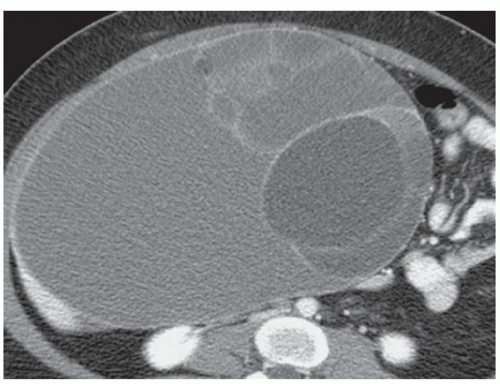
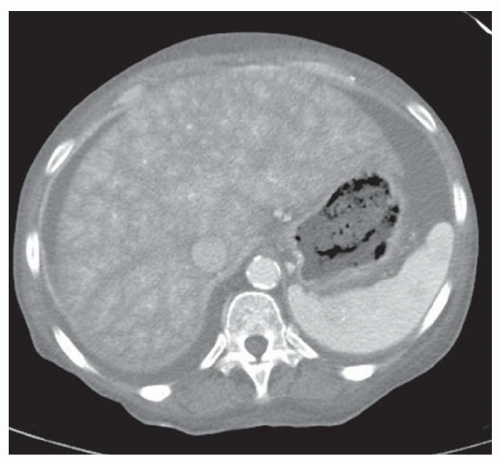
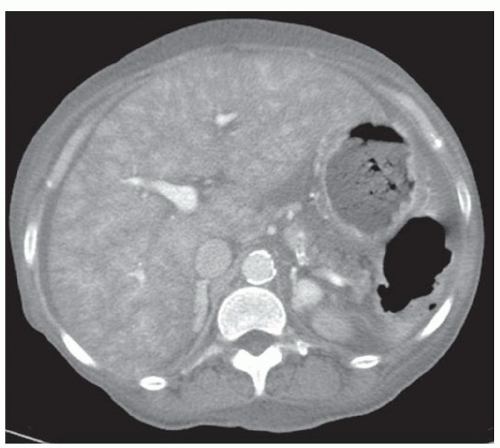
CASE 2.3 CLINICAL HISTORY Three different patients with vague abdominal discomfort.
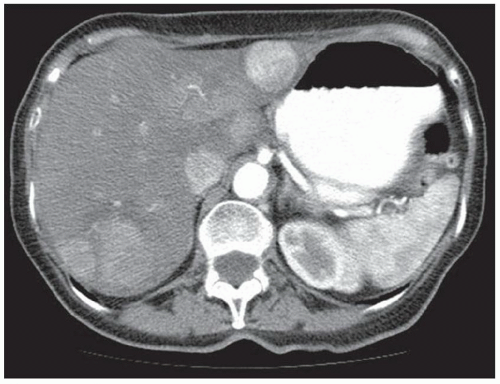
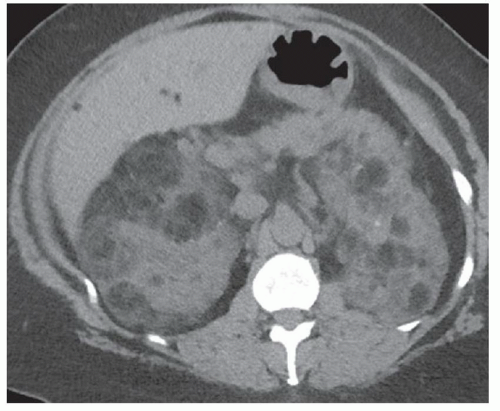
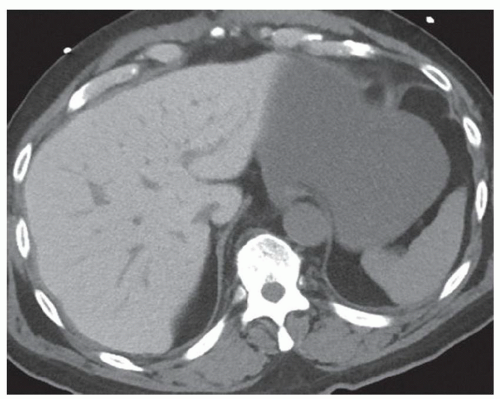
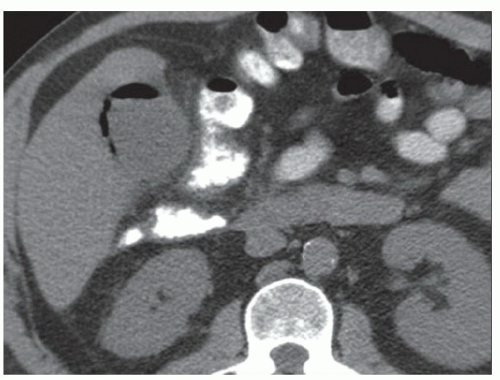
FINDINGS Contrast-enhanced CT (A) demonstrates a 10-cm low-attenuation mass spanning the right and left hepatic lobes. Linear areas of increased attenuation are visible in the nondependent portion of the mass. A small, geographic area of low attenuation is seen along the right aspect of the mass most likely reflecting some adjacent edema.
T2-weighted MR in a different patient (B) demonstrates a 12-cm left hepatic lobe lesion which is T2 bright and contains numerous curvilinear low signal areas. Contrast-enhanced CT in a third patient (C) demonstrates a 15-cm right hepatic lobe mass with several round structures internally.
DIFFERENTIAL DIAGNOSIS Bacterial abscesses, biliary cystadenomas, hydatid cysts.
DIAGNOSIS Hydatid cysts.
DISCUSSION Hydatid disease is endemic in many parts of the world, including the Middle East, the Mediterranean, and South America. At histology, the margin of the cyst is termed the “pericyst” and is composed of compressed liver parenchyma and inflammatory cells. The cyst wall contains an outer acellular layer (ectocyst) and an inner germinal membrane (endocyst).
When all three layers (pericyst, ectocyst, and endocyst) are adherent to one another, the hydatid cyst will appear as a unilocular cystic structure that cannot be distinguished from a simple cyst. When the inner ectocyst and endocyst become detached from the pericyst, they will float within the cyst giving rise to the “water lily sign” (A, B). An additional characteristic pattern of hydatid cyst disease seen at imaging is the daughter cyst appearance where numerous smaller cystic structures are seen within a dominant cyst as in (C) above.
Bacterial abscesses are usually less well defined than the above lesion. Also, bacterial abscesses may be multiloculated and have a lobulated rather than a spherical margin.
Biliary cystadenomas are often well circumscribed, but are usually not as spherical as the above mass.
Question for Further Thought
1. What other organs can be affected by hydatid disease?
View Answer
1. Hydatid cysts may be seen in almost any location in the body, including the lungs, brain, spleen, and kidneys.
Reporting Responsibilities
1. Describe the size and location of the lesion and whether or not there is mass effect on adjacent structures.
2. If a water lily sign or daughter cyst sign is seen, it is highly likely that the lesion is a hydatid cyst.
What the Treating Physician Needs to Know
1. The water lily sign and the daughter cyst sign are highly specific for hydatid disease.
2. Hydatid cysts are usually resected.
3. Many hydatid cysts appear as unilocular cystic structures that cannot be distinguished from simple cysts.
Answer
1. Hydatid cysts may be seen in almost any location in the body, including the lungs, brain, spleen, and kidneys.
REFERENCES
1. Acunas B, Rozanes I, Acunas G, Celik L, Alper A, Gokmen E. Hydatid cyst of the liver: identification of detached cyst lining on CT scans obtained after cyst puncture. Am J Roentgenol 1992;156:751-753.
2. Pedrosa I, Saiz A, Arrazola J, Ferreiros J, Pedrosa CS. Hydatid disease: radiologic and pathologic features and complications. Radiographics 2000;20:795-817.
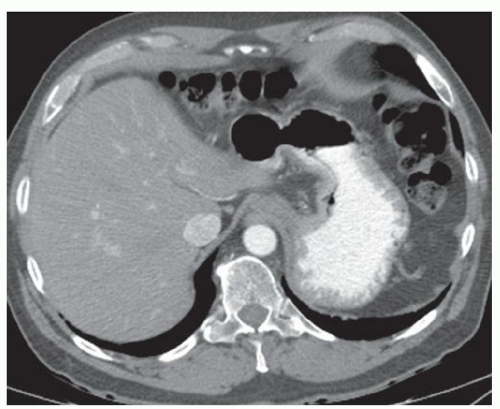
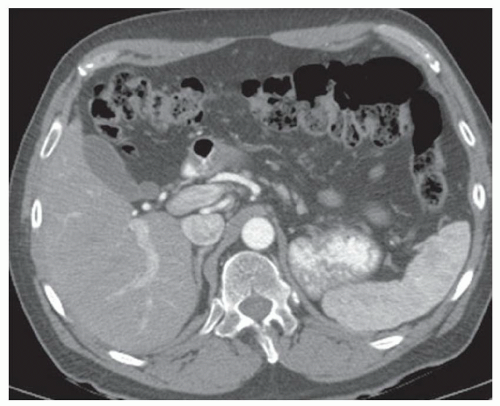
CASE 2.4 CLINICAL HISTORY 38-year-old woman with abdominal distention.
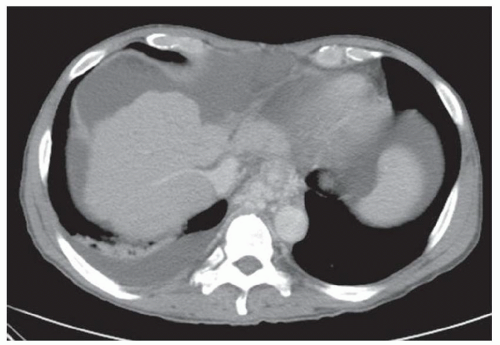
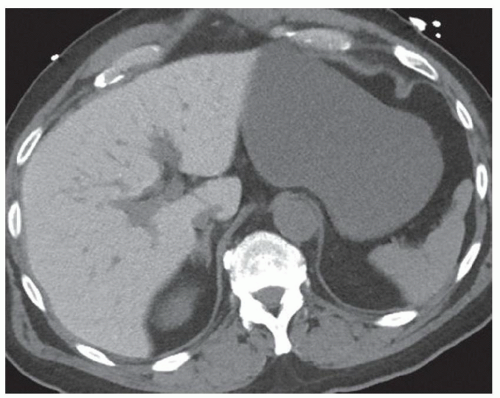
FINDINGS Contrast-enhanced CT (A-D) demonstrates a 20-cm mass involving the left and right hepatic lobes. Multiple enhancing internal septations are seen.
DIFFERENTIAL DIAGNOSIS Abscess, biliary cystadenoma, biliary cystadenocarcinoma.
DIAGNOSIS Biliary cystadenomas are uncommon cystic liver masses. These lesions most commonly occur in middle-aged women who may present with vague abdominal discomfort due to mass effect from the lesion. The etiology of biliary cystadenomas is unknown, but a congenital origin due to aberrant development of a biliary anlage has been proposed.
At CT, biliary cystadenomas appear as multilocular cystic masses that contain internal enhancing septations, mural nodules, and occasionally calcifications. The presence of a solid enhancing component does not necessarily indicate malignancy. Cystadenomas can therefore be difficult to distinguish from cystadenocarcinomas at imaging. These lesions usually demonstrate bright signal at T2-weighted MR. T1 signal intensity varies depending on the protein content of the cyst fluid.
Histologically, biliary cystadenomas usually appear as multilocular cystic masses lined by a cuboidal or columnar epithelium that resembles biliary epithelium. A majority of cystadenomas also contain ovarian stroma.
Biliary cystadenomas can be distinguished from simple cysts based on the presence of internal enhancing septations and enhancing soft tissue. Abscesses are usually less well
defined, do not demonstrate enhancing solid nodules, and may demonstrate adjacent edema. Biliary cystadenomas can be difficult to distinguish from biliary cystadenocarcinomas. Biliary cystadenomas are usually resected due to the risk of malignant transformation and the difficulty in completely excluding cystadenocarcinoma at imaging.
defined, do not demonstrate enhancing solid nodules, and may demonstrate adjacent edema. Biliary cystadenomas can be difficult to distinguish from biliary cystadenocarcinomas. Biliary cystadenomas are usually resected due to the risk of malignant transformation and the difficulty in completely excluding cystadenocarcinoma at imaging.
Questions for Further Thought
1. In what other locations may biliary cystadenomas occur?
View Answer
1. Biliary cystadenomas also may occur in the gallbladder or in the extrahepatic biliary system. The majority of biliary cystadenomas occur in the liver.
2. What imaging features distinguish biliary cystadenoma from biliary cystadenocarcinoma?
View Answer
2. Unfortunately, no imaging features reliably distinguish biliary cystadenoma from biliary cystadenocarcinoma. Both are surgical lesions.
Reporting Responsibilities
1. Report the size and location of the lesion.
2. Report the relationship of the lesion to major vascular structures as this information may impact surgical planning.
What the Treating Physician Needs to Know
1. Biliary cystadenomas are usually surgical lesions and are resected due to the risk of malignant transformation.
2. Biliary cystadenomas cannot be reliably differentiated from biliary cystadenocarcinomas at imaging.
Answers
1. Biliary cystadenomas also may occur in the gallbladder or in the extrahepatic biliary system. The majority of biliary cystadenomas occur in the liver.
2. Unfortunately, no imaging features reliably distinguish biliary cystadenoma from biliary cystadenocarcinoma. Both are surgical lesions.
REFERENCES
1. Mortele KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics 2001;21:895-910.
2. Horton KM, Bluemke DA, Hruban RH, Soyer P, Fishman EK. CT and MR imaging of benign hepatic and biliary tumors. Radiographics 1999;19:431-451.
3. Levy AD, Murakata LA, Abbott RM, Rohrmann Jr CA. From the archives of the AFIP: benign tumors and tumor-like lesions of the gallbladder and extrahepatic bile ducts: radiologicpathologic correlation. Radiographics 2002;22:387-413.
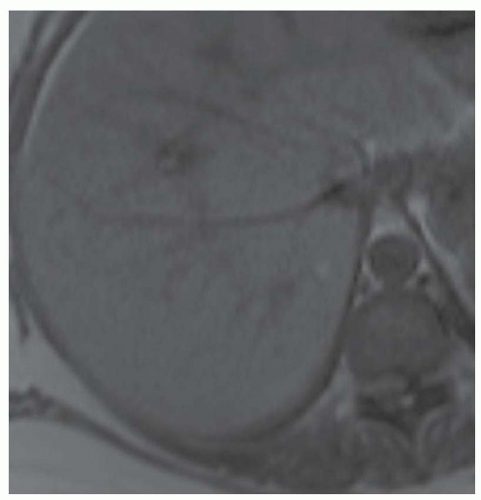
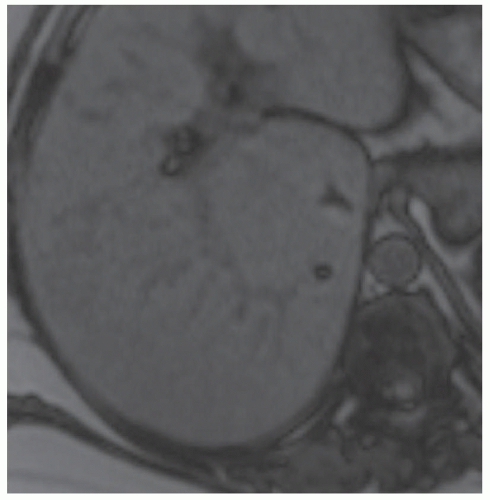
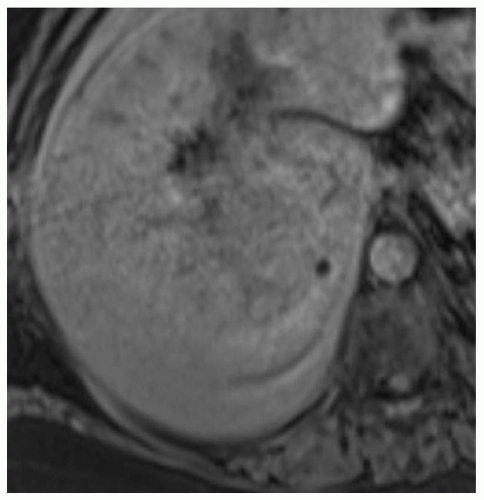
CASE 2.5 CLINICAL HISTORY 39-year-old woman with abdominal discomfort.
FINDINGS Grayscale US (A) demonstrates a 6-cm homogeneous hypoechoic mass in the inferior right hepatic lobe. Blood flow is visible in the mass at color Doppler imaging (B). Patient also underwent CT, and the lesion was low attenuation precontrast (C), hyperenhanced in the arterial phase (D), and remained more attenuating than the surrounding liver at venous phase imaging (E). An additional smaller, 1.5-cm lesion is visible slightly more anteriorly in the arterial phase image (D).
DIFFERENTIAL DIAGNOSIS Focal nodular hyperplasia (FNH), hepatocellular adenoma, hepatocellular cancer.
DIAGNOSIS Hepatocellular adenoma.
DISCUSSION Hepatocellular adenomas are hyperenhancing lesions that most often occur in women taking oral contraceptive pills, in individuals with glycogen storage disease, and in individuals using anabolic steroids.
Histologically, adenomas appear as sheets of relatively normal-appearing hepatocytes with intervening sinusoids and relatively little connective tissue. Blood supply is via peripheral arterial vessels. The paucity of supporting connective tissue in the presence of arterial pressure blood flow are thought to be factors predisposing these lesions to bleeding. Adenomas do not contain bile ducts, a feature that distinguishes them from areas of FNH at histology.
The US appearance of adenomas is nonspecific. Adenomas may appear hypoechoic (as in this case) or hyperechoic due to the presence of internal blood products.
Adenomas may also have a variable appearance at CT. If the adenoma has bled, it will appear heterogeneous at precontrast CT with areas of low and high precontrast attenuation. The adenoma in this case had not bled and appeared homogeneous at noncontrast CT. Adenomas characteristically hyperenhance in the arterial phase of imaging as compared with the neighboring liver parenchyma. Adenomas are also typically visible in the portal venous phase of enhancement though the amount of enhancement relative to the adjacent liver parenchyma is variable.
At MR, signal loss may be visible at out-of-phase imaging due to intracellular fat. At MR, adenomas are typically bright at T2 though not fluid bright-like hemangiomas. T1 signal varies and may be dark or, if blood products are present, bright T1 signal may be visible. As with CT, adenomas hyperenhance in the arterial phase at MR and remain visible in the portal venous phase.
By comparison, FNH appears “stealth” in precontrast images and is often imperceptible or only faintly perceptible. Like adenomas, FNHs hyperenhance in the arterial phase. FNHs are also usually relatively stealth in the portal venous phase. Unlike adenomas, FNHs do not contain fat and therefore do not lose signal at out-of-phase MR.
Adenomas may be difficult to distinguish from hepatocellular cancer as both hyperenhance in the arterial phase and both may contain fat. Hepatocellular cancer usually demonstrates washout at delayed-phase imaging (e.g., 3 minutes post-contrast). Adenomas may also demonstrate relative washout. Clinical history, evaluation of the background liver, and correlation with serum α-fetoprotein (AFP) may help distinguish between hepatocellular adenoma and hepatocellular cancer. For example, hepatocellular cancers most commonly occur in patients with chronic liver disease (e.g., small nodular livers), whereas hepatocellular adenomas often occur in patients with morphologically normal livers. AFP may be elevated in the setting of hepatocellular cancer but is usually not elevated in the setting of hepatocellular adenoma.
Questions for Further Thought
1. What is the usual management of hepatocellular adenomas?
View Answer
1. Depending on patient comorbidities and lesion size, adenomas may be resected due to the risk of bleeding and potential for malignant transformation. Patients with multiple adenomas for which resection of all lesions is not possible may undergo embolization. Alternatively, follow-up imaging following cessation of oral contraceptive pills or anabolic steroids may be recommended to evaluate whether an adenoma regresses.
2. How does the management of adenomas vary from the management of FNH?
View Answer
2. Unlike adenomas for which treatment is often offered, patients with FNH usually require no further treatment. Therefore, management decisions will change depending on whether a lesion is favored to be an adenoma or FNH at imaging.
Reporting Requirements
1. Report the size and location of the lesion.
2. Attempt to narrow differential diagnosis as much as possible.
What the Treating Physician Needs to Know
1. Solitary hepatocellular adenomas may be resected due to the risk of bleeding and malignant transformation. Risk of bleeding correlates with adenoma size. The surgical literature suggests that adenomas <5 cm should be
followed with imaging to assess for interval growth. Adenomas >5 cm should be resected, given the potential for bleeding and malignant transformation. However, some surgeons will also resect adenomas that measure smaller than 5 cm in size.
2. Patients with multiple adenomas may undergo embolization procedures and/or resection.
Answers
1. Depending on patient comorbidities and lesion size, adenomas may be resected due to the risk of bleeding and potential for malignant transformation. Patients with multiple adenomas for which resection of all lesions is not possible may undergo embolization. Alternatively, follow-up imaging following cessation of oral contraceptive pills or anabolic steroids may be recommended to evaluate whether an adenoma regresses.
2. Unlike adenomas for which treatment is often offered, patients with FNH usually require no further treatment. Therefore, management decisions will change depending on whether a lesion is favored to be an adenoma or FNH at imaging.
REFERENCES
1. Faria SC, Iyer RB, Rashid A, Whitman GJ. Hepatic adenoma. Am J Roentgenol 2004;182:1520.
2. Grazioli L, Federle MP, Brancatelli G, Ichikawa T, Olivetti L, Blachar A. Hepatic adenomas: imaging and pathologic findings. Radiographics 2001;21:877-894.
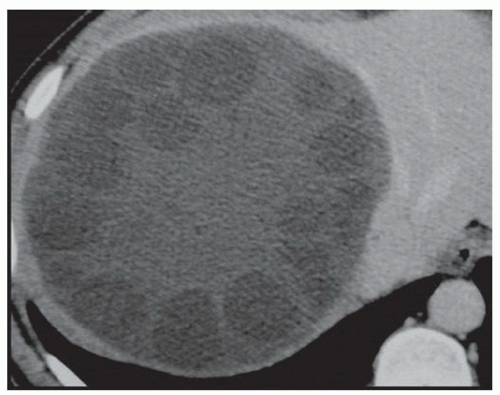
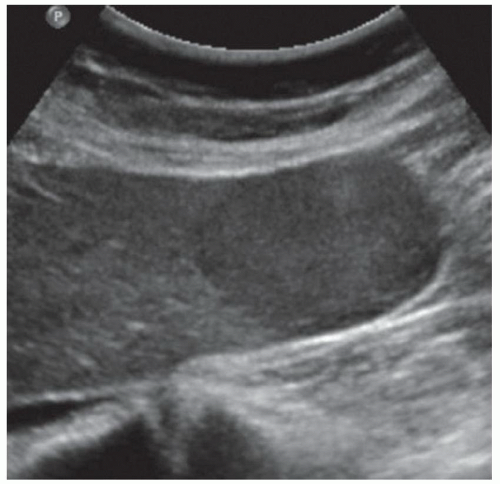
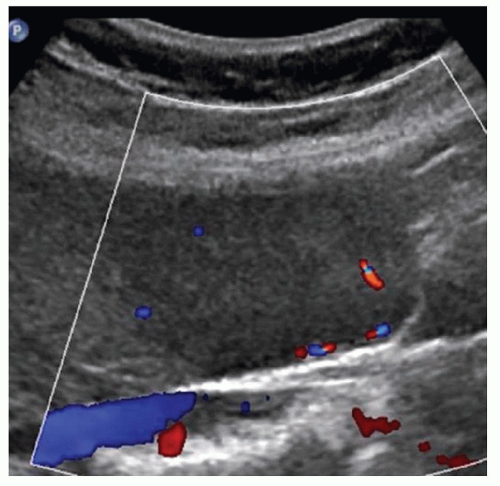
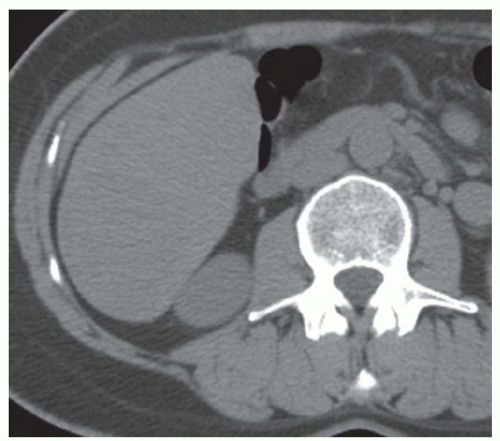
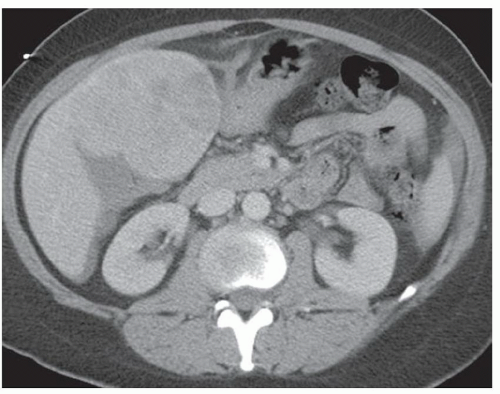
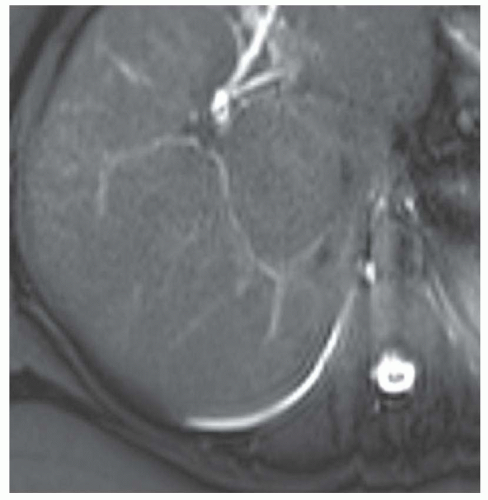
CASE 2.6 CLINICAL HISTORY 57-year-old man undergoing renal US found to have an 8 cm × 9 cm right hepatic lobe mass; CT obtained for further characterization.
FINDINGS Grayscale US (A) demonstrates an 8 cm × 9 cm echogenic mass in the right hepatic lobe. Color Doppler image (B) demonstrates blood flow around the periphery of the mass. A well-circumscribed, low attenuation lobulated mass is identified in the posterior segment right hepatic lobe at noncontrast CT (C). Portal venous phase CT demonstrates discontinuous peripheral nodular enhancement (D) with progressive fill-in at delayed phase imaging (E).
DIFFERENTIAL DIAGNOSIS AT CT Hemangioma.
DIAGNOSIS Hemangioma.
DISCUSSION Hemangiomas are benign tumors and are present in approximately 20% of adults. The peripheral, cloudlike discontinuous nodular enhancement with gradual fill-in seen in the above mass is diagnostic of a hepatic hemangioma, and no differential diagnosis should be given.
At histology, hemangiomas are characterized by large vascular spaces with a single cell layer endothelial lining. Blood supply originates around the periphery of the hemangioma. Blood slowly fills the dilated vascular spaces within a hemangioma from peripheral to central.
The imaging appearance of hemangiomas reflects their histology. Blood enters the hemangioma along the periphery. Discontinuous, cloud-like areas of peripheral enhancement are seen in arterial phase images with gradual fill-in in portal venous and delayed phase images. These peripheral areas of enhancement are often though not always similar in attenuation to the blood pool (in other words aortic enhancement). The central area of low attenuation in the above hemangioma reflects a fibrous scar.
At US, hemangiomas may appear as lobular hyperechoic masses with increased through-transmission. Blood flow within hemangiomas is usually too slow to be detected with color or power Doppler. CT or MR is necessary in many cases to definitively characterize a lesion as a hemangioma.
As hemangiomas are benign tumors, no treatment is required. Occasionally, large hemangiomas may result in abdominal discomfort and/or mass effect on surrounding structures and may be resected.
Questions for Further Thought
1. What is the typical ultrasonographic appearance of hepatic hemangiomas?
View Answer
1. At US, typical hepatic hemangiomas are homogeneous, hyperechoic structures with increased through transmission and well-circumscribed margins. CT or MR is necessary in many cases to definitively characterize a lesion as a hemangioma.
2. What are flash-filling hemangiomas?
View Answer
2. Flash-filling hemangiomas demonstrate immediate homogeneous hyperenhancement at arterial phase imaging. Flashfilling hemangiomas are usually subcentimeter in size.
Reporting Requirements
1. When a lesion demonstrates classic CT or MR features of a hemangioma as above, the diagnosis can be made at cross-sectional imaging and no differential diagnosis is warranted.
2. For large hemangiomas, report whether there is evidence of mass effect on adjacent structures.
What the Treating Physician Needs to Know
1. Hemangiomas are benign tumors and do not require treatment.
2. Imaging follow-up is usually not required for classic hemangiomas.
Answers
1. At US, typical hepatic hemangiomas are homogeneous, hyperechoic structures with increased through transmission and well-circumscribed margins. CT or MR is necessary in many cases to definitively characterize a lesion as a hemangioma.
2. Flash-filling hemangiomas demonstrate immediate homogeneous hyperenhancement at arterial phase imaging. Flashfilling hemangiomas are usually subcentimeter in size.
REFERENCES
1. Vilanova JC, Barcelo J, Smirniotopolous JG, et al. Hemangioma from head to toe: MR imaging with pathologic correlation. Radiographics 2004;24:367-385.
2. Vilgrain V, Boulos L, Vullierme M-P, Denys A, Terris B, Menu Y. Imaging of atypical hemangiomas of the liver with pathologic correlation. Radiographics 2000;20:379-397.
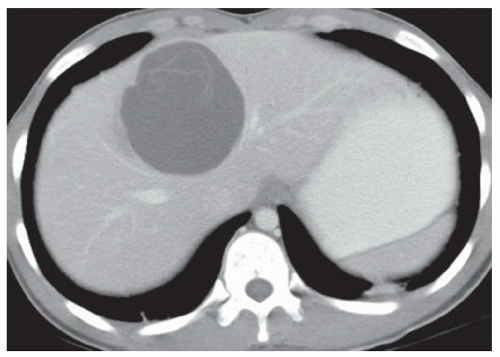
CASE 2.7 CLINICAL HISTORY 48-year-old woman presents to the emergency department with acute onset of right upper quadrant pain.
FINDINGS Portal venous phase contrast-enhanced CT demonstrates normal liver morphology without findings of chronic liver disease (A). A 9-cm heterogeneous, enhancing mass is identified spanning the left and right hepatic lobes (B, C). Additionally, small-to-moderate volume perihepatic and perisplenic fluid measuring 30 to 40 HU is present and tracks inferiorly within the abdomen.
DIFFERENTIAL DIAGNOSIS Ruptured hepatocellular carcinoma (HCC), ruptured hepatic adenoma.
DIAGNOSIS Ruptured HCC.
DISCUSSION HCC and hepatic adenoma are the liver tumors that are most likely to bleed and produce hemoperitoneum. Most, though not all, hepatocellular cancers occur in patients with chronic liver disease. Approximately 5% to 15% of patients with hepatocellular cancer will experience spontaneous tumor bleeding and rupture.
Hepatic adenomas are rare tumors that occur in patients taking oral contraceptive pills and anabolic steroids and in patients with glycogen storage disease. Hepatic adenomas are often electively resected given the risk of spontaneous hemorrhage and a theoretical risk of malignant transformation into HCC. Emergency resection of a bleeding hepatic adenoma is associated with a 5% to 10% mortality rate, whereas the mortality rate associated with elective adenoma resection is <1%. Risk of bleeding correlates with adenoma size. The surgical literature suggests that adenomas <5 cm should be followed with imaging to assess for interval growth. Adenomas >5 cm should be resected given the potential for bleeding and malignant transformation. However, some surgeons will also resect adenomas that measure less than 5 cm in size.
In the above case, a differential diagnosis of ruptured HCC or adenoma was given. The patient continued to bleed after attempted embolization and underwent hepatic resection to try to stop the bleeding. Pathology came back as hepatocellular cancer.
When liver tumors bleed, bleeding may be confined within the tumor resulting in irregular areas of increased attenuation within the tumor at CT. Bleeding may also extend beyond the tumor but be contained by the liver capsule resulting in subcapsular hematoma. The most devastating type of bleeding is intraperitoneal hemorrhage (as in the above case) which can be life threatening.
At MR, precontrast T1 bright material within a liver mass could reflect blood products. Alternatively, fat, glycogen, and copper have also been reported as accounting for T1 bright signal in liver masses. Areas of fat would be expected to demonstrate signal loss at out-of-phase imaging or fat-saturated imaging depending on the quantity of fat present.
A bleeding liver mass in a patient with a small and nodular liver is most likely a hepatocellular cancer. A bleeding liver mass in a patient with an otherwise normal appearing liver, no risk factors for chronic liver disease, and a history of oral contraceptive or anabolic steroid use may be a bleeding adenoma.
A number of theories have been proposed to explain rupture of hepatocellular cancers. It has been proposed that these tumors may bleed due to rupture of a superficial artery or vein supplying or draining the tumor. Alternatively, tumors along the surface of the liver may bleed due to trauma. Additionally, tumor extension into the hepatic veins could increase intravascular pressure in the tumor with resultant hemorrhage.
Question for Further Thought
1. Is rupture of hepatocellular cancer a contraindication to resection?
View Answer
1. No. In the acute setting, resection may be performed to control bleeding if angiographic embolization procedures are unsuccessful. If embolization is successful, previous rupture is not considered a contraindication to elective tumor resection.
Reporting Requirements
1. Notify the ordering physician of the presence of a liver tumor that has bled.
2. Given the normal morphology of the remaining liver, suggest the possibility of hepatocellular cancer or hepatic adenoma.
What the Treating Physician Needs to Know
1. Acutely, angiographic embolization should be performed to try to stop the hemorrhage.
2. Resection of the tumor could be performed emergently if angiographic embolization fails or on an elective basis if angiographic embolization is successful.
Answer
1. No. In the acute setting, resection may be performed to control bleeding if angiographic embolization procedures are unsuccessful. If embolization is successful, previous rupture is not considered a contraindication to elective tumor resection.
REFERENCES
1. Casillas VJ, Amendola MA, Gascue A, Pinnar N, Levi JU, Perez JM. Imaging of nontraumatic hemorrhagic hepatic lesions. Radiographics 2000;20:367-378.
2. Belghiti J, Kianmanesh R. Surgical treatment of hepatocellular carcinoma. HPB 2005;7:42-49.
3. Ribeiro MAF, Chaib E, Saad WA, D’Albuquerque LAC, Cecconello I. Surgical management of spontaneous ruptured hepatocellular adenoma. Clinics (Sao Paulo) 2009;64:775-779.
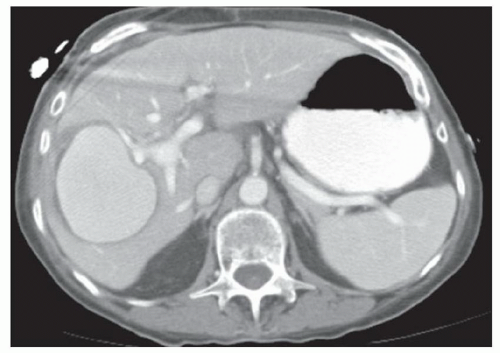
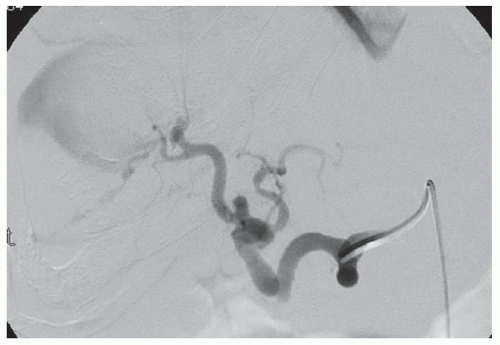
CASE 2.8 CLINICAL HISTORY 53-year-old man with hepatitis C undergoing screening for hepatocellular cancer.
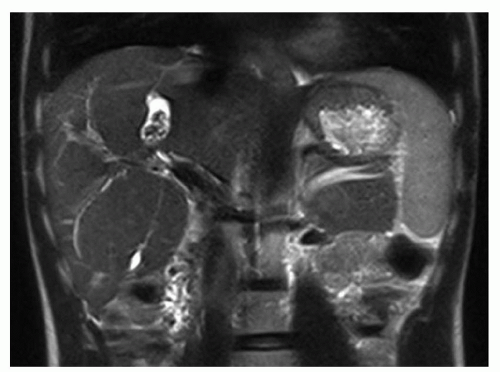
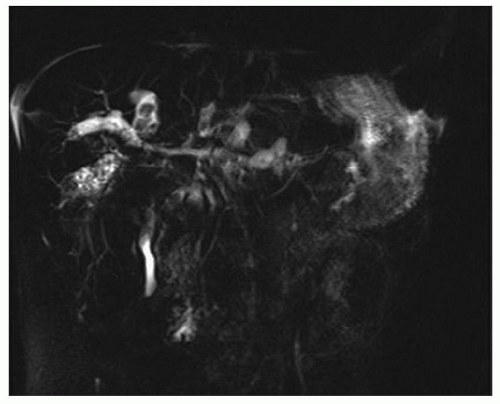
FINDINGS Arterial phase contrast-enhanced CT (A) and MR (C) demonstrate a 2-cm round arterially enhancing structure adjacent to the right hepatic vein. The right hepatic vein is expanded by hyperenhancing material that also extends into the inferior vena cava (IVC) (B, D).
DIFFERENTIAL DIAGNOSIS Hepatocellular carcinoma with bland thrombus, hepatocellular carcinoma with tumor thrombus in the right hepatic vein extending into the IVC.
DIAGNOSIS Hepatocellular carcinoma, tumor thrombus.
DISCUSSION Specific diagnostic criteria for hepatocellular carcinoma (HCC) at CT or MR are arterial hyperenhancement (as above) with washout at delayed-phase imaging with a capsule or pseudocapsule (not shown).
Treatment options for HCC include resection, liver transplant, and chemoembolization. Patients with small tumors and an otherwise normal or near-normal liver may undergo
resection. Patients with unresectable hepatocellular cancers that fall within Milan criteria are candidates for liver transplant. Milan criteria are not more than three tumors ≤3 cm or one tumor ≤5 cm. Based on the 2 cm size of the above tumor, the patient would have been within Milan criteria. However, the presence of tumor thrombus in the IVC or the portal vein currently is considered a contraindication to liver transplant, so the above patient was not a transplant candidate.
resection. Patients with unresectable hepatocellular cancers that fall within Milan criteria are candidates for liver transplant. Milan criteria are not more than three tumors ≤3 cm or one tumor ≤5 cm. Based on the 2 cm size of the above tumor, the patient would have been within Milan criteria. However, the presence of tumor thrombus in the IVC or the portal vein currently is considered a contraindication to liver transplant, so the above patient was not a transplant candidate.
Bland thrombus (usually in the portal vein) can also occur in patients with chronic liver disease and portal hypertension. As bland thrombus is not necessarily a contraindication for liver transplant, it is critically important to accurately distinguish between bland thrombus and tumor thrombus. A key feature that distinguishes tumor thrombus from bland thrombus is enhancement of the tumor thrombus. By comparison, bland thrombus does not enhance. Note in the above case the expansion of the right hepatic vein with hyperenhancing material. Neither the middle hepatic vein nor the left hepatic vein is similarly hyperenhancing.
Question for Further Thought
1. In addition to hepatocellular cancer, what other abdominal tumors may directly extend into major vascular structures.
View Answer
1. Renal cell cancers may extend into the renal vein and IVC. Adrenal cortical carcinomas may extend into the IVC.
Reporting Requirements
1. Report the size and location of the hepatocellular cancer.
2. Report the presence of tumor thrombus extending into the hepatic vein and IVC.
What the Treating Physician Needs to Know
1. This patient has a hepatocellular cancer with tumor thrombus filling the right hepatic vein and extending into the IVC.
Answer
1. Renal cell cancers may extend into the renal vein and IVC. Adrenal cortical carcinomas may extend into the IVC.
REFERENCES
1. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-700.
2. Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification and reporting of hepatocellular cancer. Radiology 2013;266:376-382.
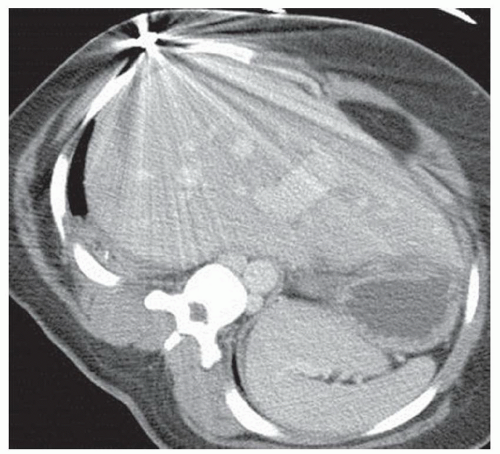
CASE 2.9 CLINICAL HISTORY 39-year-old man with chest pain.
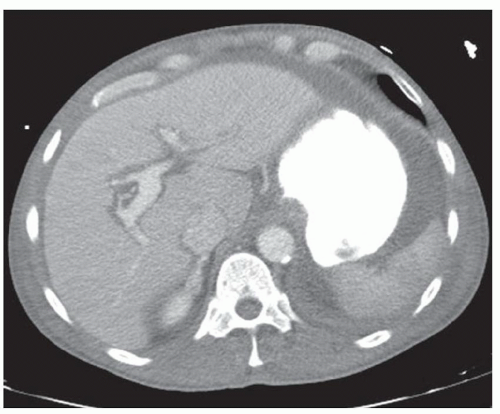
FINDINGS Arterial phase images (A-D) through the upper abdomen obtained as part of a PE protocol chest CT demonstrate an 8-cm mass with a lobular contour in the left hepatic lobe. The mass demonstrates peripheral hyperenhancement and central low attenuation. A central calcification is also present (B, C). No morphologic features of chronic liver disease are seen.
DIFFERENTIAL DIAGNOSIS Fibrolamellar hepatocellular cancer, focal nodular hyperplasia (FNH), intrahepatic cholangiocarcinoma.
DIAGNOSIS Fibrolamellar hepatocellular cancer.
DISCUSSION Fibrolamellar carcinomas are rare tumors occurring in 0.02 per 100,000 people per year. This incidence is 100 times lower than that of hepatocellular cancer. Fibrolamellar cancers most commonly occur in younger patients with a peak incidence at age 25. These tumors usually occur in patients without underlying chronic liver disease. By comparison, hepatocellular cancers most commonly occur in older patients with underlying chronic liver disease. At histology, a diffuse fibrous
stroma with bands of collagen and fibrocytes is present in fibrolamellar carcinomas.
stroma with bands of collagen and fibrocytes is present in fibrolamellar carcinomas.
Imaging features of fibrolamellar HCC include lobulated margins (>80% of cases), central calcification (35% to 68% of cases), areas of low attenuation at noncontrast CT, areas of high attenuation at arterial phase CT, and a central scar (20% to 71% of cases). The central low attenuation in the above images indicates the central scar.
The above mass is not an area of FNH as FNH usually homogeneously enhances in the arterial phase. Also, central calcifications usually are not present in FNH. FNH may have a central scar, but it is usually smaller than the central scars seen with fibrolamellar HCC. At MR, FNH central scars are usually T2 bright, whereas the central scar seen in fibrolamellar carcinomas is usually low signal. Intrahepatic cholangiocarcinomas rarely contain central calcifications. Additionally, intrahepatic cholangiocarcinomas often demonstrate associated biliary ductal dilatation and usually do not hyperenhance in arterial phase images.
Questions for Further Thought
1. Is the prognosis for patients with fibrolamellar carcinoma better or worse than that for patients with traditional hepatocellular cancer?
View Answer
1. Following resection, the prognosis of patients with fibrolamellar carcinoma is better than that of patients with hepatocellular cancer. This better prognosis is attributed in part to the fact that patients with fibrolamellar carcinoma usually do not have underlying chronic liver disease.
2. Are serum AFP levels usually elevated in patients with fibrolamellar hepatocellular cancer?
View Answer
2. No. Serum AFP levels are usually normal in patients with fibrolamellar hepatic cancers.
Reporting Requirements
1. Report the size and location of the mass.
2. Report whether or not there are findings of chronic liver disease.
What the Treating Physician Needs to Know
1. Given the patient’s relatively young age and lack of findings of chronic liver disease, the above mass that demonstrates arterial hyperenhancement, a central scar, and a central calcification is most likely a fibrolamellar carcinoma.
Answers
1. Following resection, the prognosis of patients with fibrolamellar carcinoma is better than that of patients with hepatocellular cancer. This better prognosis is attributed in part to the fact that patients with fibrolamellar carcinoma usually do not have underlying chronic liver disease.
2. No. Serum AFP levels are usually normal in patients with fibrolamellar hepatic cancers.
REFERENCES
1. Smith MT, Blatt ER, Jedlicka P, Strain JD, Fenton LA. Fibrolamellar hepatocellular carcinoma. Radiographics 2008;28: 609-613.
2. Ichikawa T, Federle MP, Grazioli L, Madariaga J, Nalesnik M, Marsh W. Fibrolamellar hepatocellular carcinoma: imaging and pathologic findings in 31 recent cases. Radiology 1999;213: 352-361.
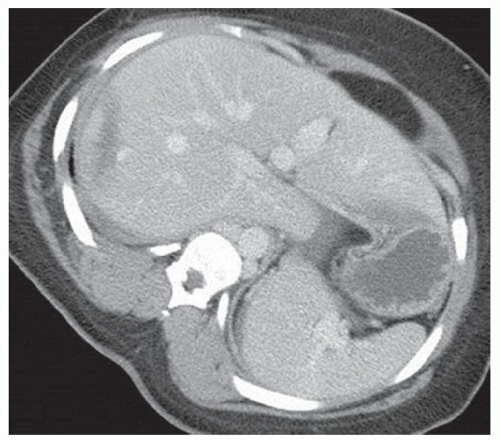
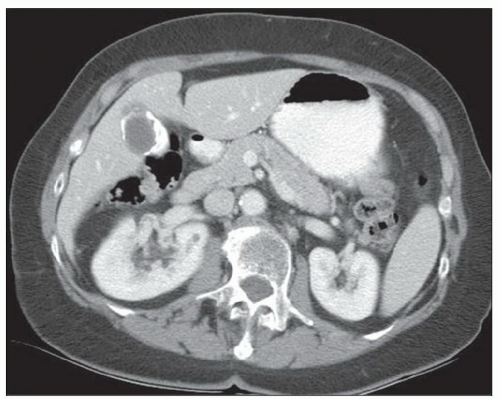
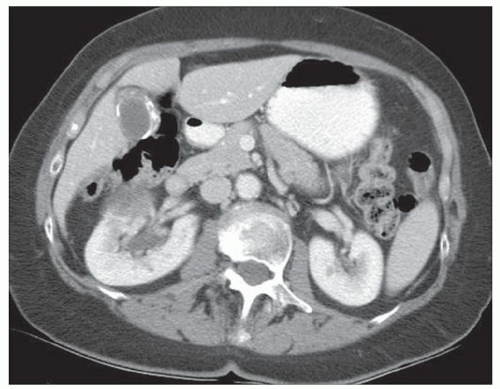
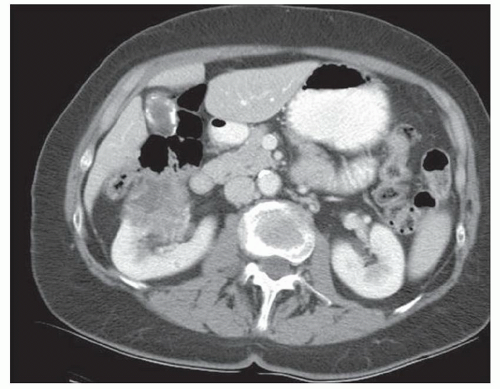
CASE 2.10 CLINICAL HISTORY 63-year-old woman with abdominal pain.
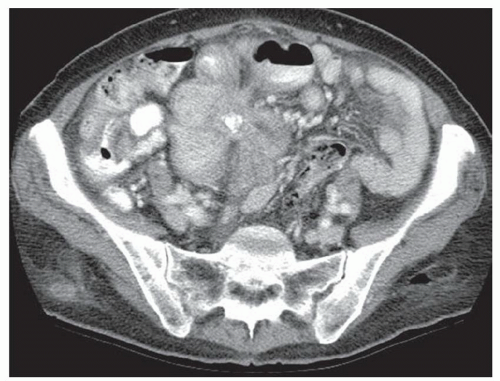
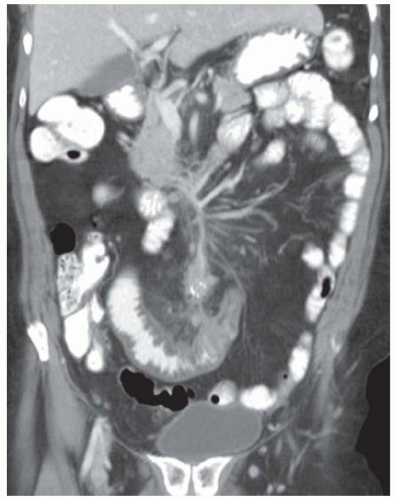
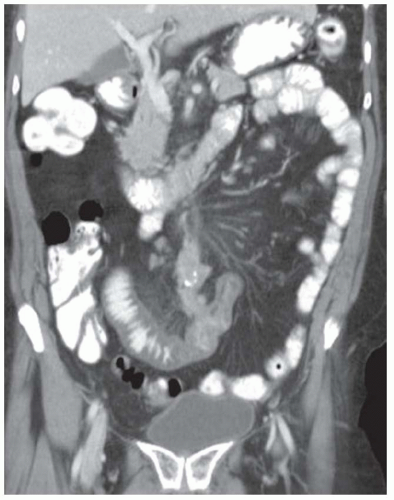
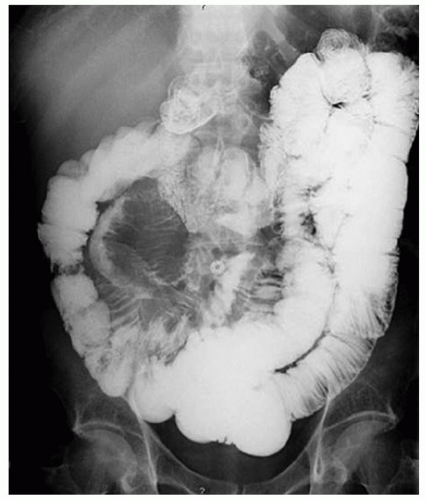
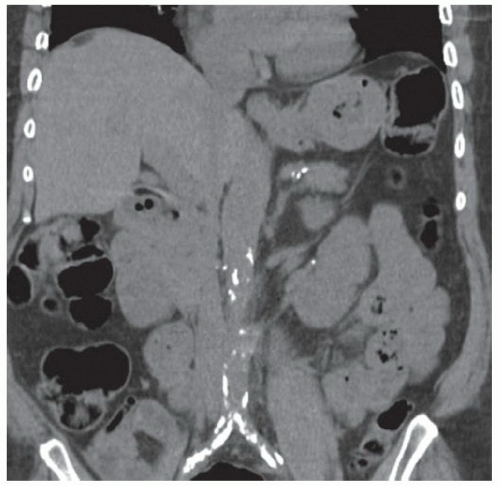
FINDINGS CT image through the liver (A) obtained in the arterial phase of enhancement demonstrates at least five hyperenhancing liver lesions. CT image obtained through the lower abdomen (B) demonstrates a calcified right lower quadrant mesenteric mass with tethering of adjacent small bowel loops that are also thick walled. Coronal reformations from a second patient demonstrate a calcified mesenteric mass extending into distal ileum (C, D). The distal ileum is also thick walled (C, D). Small bowel follow-through (E) demonstrates tethering of small bowel loops in the right lower abdomen.
DIFFERENTIAL DIAGNOSIS Metastatic carcinoid tumor.
DIAGNOSIS Metastatic carcinoid tumor.
DISCUSSION Carcinoid tumors can be classified as foregut (20% to 25%, including lung, thymus, stomach, and proximal duodenum), midgut (40% to 50%, including distal duodenum, small bowel, appendix, and proximal colon), and hindgut (15%, including distal colon and rectum). Carcinoid tumors are generally relatively slow growing. Even with metastatic disease, 5-year survival is 70% to 80%.
At CT, mesenteric masses related to carcinoid tumors are calcified in approximately 70% of cases. These mesenteric masses are metastases, typically from an ileal tumor which is often small, occult at imaging, and may be multifocal. Serotonin and other substances released by these tumors can trigger a desmoplastic reaction in the mesentery. This desmoplastic reaction can compress mesenteric veins resulting in bowel wall thickening as in this case.
Liver metastases are usually hypervascular and therefore best appreciated in the arterial phase of imaging (approximately 30 seconds after contrast administration). Bone metastases from carcinoid tumors are typically sclerotic.
Questions for Further Thought
1. Although most carcinoid tumors are sporadic, they can be associated with syndromes. Name three such syndromes.
View Answer
1. Syndromes associated with carcinoid tumors include multiple endocrine neoplasia (MEN) type 1, MEN type 2, and neurofibromatosis type 1.
2. Name symptoms associated with carcinoid syndrome.
View Answer
2. Symptoms associated with carcinoid syndrome include diarrhea, abdominal cramping, and facial and upper body flushing and usually imply the presence of liver metastases.
Reporting Responsibilities
1. Describe the sites of metastatic disease (most frequently small bowel mesentery and liver for GI carcinoid tumors).
2. Attempt to identify the site of the primary tumor. However, primary GI carcinoid tumors are frequently so small that they are occult at imaging.
What the Treating Physician Needs to Know
1. When carcinoid tumor is suspected, correlation with urine levels of 5-hydroxyindoleacetic acid (a breakdown product of serotonin) can help confirm the diagnosis.
2. 111In-octreotide scintigraphy is frequently used for staging of carcinoid tumors.
Answers
1. Syndromes associated with carcinoid tumors include multiple endocrine neoplasia (MEN) type 1, MEN type 2, and neurofibromatosis type 1.
2. Symptoms associated with carcinoid syndrome include diarrhea, abdominal cramping, and facial and upper body flushing and usually imply the presence of liver metastases.
REFERENCES
1. Scarsbrook AF, Ganeshan A, Statham J, et al. Anatomic and functional imaging of metastatic carcinoid tumors. Radiographics 2007;27:455-477.
2. Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med 1999;340:858-868.
CASE 2.11 CLINICAL HISTORY 48-year-old woman with breast cancer.
FINDINGS Longitudinal and transverse grayscale (A) and color Doppler (B) images demonstrate a 3-cm heterogeneous but primarily hypoechoic mass in the lateral segment left hepatic lobe. Bloodflow is seen within and around the periphery of the mass. Contrast-enhanced CT images (C, D) obtained in the portal venous phase of contrast material enhancement demonstrate numerous illdefined hypoenhancing lesions in both the right and left hepatic lobes.
DIFFERENTIAL DIAGNOSES Abscesses, metastatic disease.
DIAGNOSIS Metastatic disease.
DISCUSSION The liver is a common site of metastatic disease. Adenocarcinomas (e.g., from the colon, pancreas, or lung) usually result in hypoenhancing liver metastases. Portal venous phase imaging is more sensitive than noncontrast CT and arterial phase CT in the detection of hypovascular liver metastases.
Two different strategies employed to acquire images in the portal venous phase of enhancement are (1) scanning at a fixed time interval and (2) bolus tracking software. Initiating image acquisition 70 seconds after the start of administration of intravenous contrast material usually results in adequate venous phase images. However, a number of variables including cardiac output can impact the volume of contrast material that has reached the liver by such a fixed imaging window. With bolus tracking software, a region of interest is placed in the liver, low tube current images are acquired every 2 to 3 seconds, and the scan begins once hepatic
parenchymal enhancement crosses a predetermined threshold (e.g., 50 HU).
parenchymal enhancement crosses a predetermined threshold (e.g., 50 HU).
Abscesses also usually appear hypoenhancing at portal venous phase CT. However, since abscesses often contain fluid they usually appear lower in attenuation than metastases. Abscesses also may be multilocular in appearance. Additionally, patients with liver abscesses usually have a fever and elevated white blood cell count.
Questions for Further Thought
1. List tumors that can result in hypervascular liver metastases.
View Answer
1. Neuroendocrine tumors, melanomas, sarcomas, thyroid cancers, carcinoid tumors, choriocarcinomas, kidney cancers, and some breast cancers are hypervascular tumors that can result in hypervascular liver metastases.
2. List tumors that can result in calcified liver metastases.
View Answer
2. Mucinous adenocarcinomas (e.g., the colon and the ovary) can result in calcified liver metastases.
3. List tumors that can result in cystic liver metastases.
View Answer
3. Rapidly growing tumors with areas of necrosis may appear cystic. Some mucinous tumors may result in cystic liver metastases. Treated GIST metastases may appear cystic.
Reporting Requirements
1. Report the approximate number of metastases.
2. Report which lobes or segments contain metastases.
3. Provide reference measurements of several of the dominant lesions.
What the Treating Physician Needs to Know
1. As locoregional therapy options (e.g., partial hepatic resection, radiofrequency ablation, and selective chemoembolization) become more common, it is important to state the approximate number and distribution of liver metastases. For example, depending on tumor cell type and patient comorbidities, surgeons may resect some liver metastases. A patient with a small number of metastases (e.g., three) may be a candidate for wedge resections or radiofrequency ablations. If all of a patient’s metastases are located in a single segment or a few segments, the patient may undergo segmental resection. By comparison, a patient with 50 liver metastases scattered throughout the right and left hepatic lobes likely would not be a candidate for resection or radiofrequency ablation. Therefore, it is important to describe the number and distribution of liver metastases. Terms such as “numerous,” “several,” and “many” without an actual approximate numerical quantification and distribution description should be avoided.
Answers
1. Neuroendocrine tumors, melanomas, sarcomas, thyroid cancers, carcinoid tumors, choriocarcinomas, kidney cancers, and some breast cancers are hypervascular tumors that can result in hypervascular liver metastases.
2. Mucinous adenocarcinomas (e.g., the colon and the ovary) can result in calcified liver metastases.
3. Rapidly growing tumors with areas of necrosis may appear cystic. Some mucinous tumors may result in cystic liver metastases. Treated GIST metastases may appear cystic.
REFERENCES
1. Soyer P, Poccard M, Boudiaf M, et al. Detection of hypovascular hepatic metastases at triple-phase helical CT: sensitivity of phases and comparison with surgical and histopathologic findings. Radiology 2004;231:413-420.
2. Silverman PM, Roberts S, Tefft MC, et al. Helical CT of the liver: clinical application of an automated computer technique, SmartPrep, for obtaining images with optimal contrast enhancement. Am J Roentgenol 1995;165:73-78.
CASE 2.12 CLINICAL HISTORY 63-year-old woman with a newly diagnosed colon cancer, staging examination.
FINDINGS Noncontrast CT images (A, B) demonstrate numerous masses with central areas of calcification involving the right and left hepatic lobes.
DIFFERENTIAL DIAGNOSIS Echinococcus, metastatic disease, prior granulomatous disease.
DIAGNOSIS Metastatic disease from mucinous colon cancer.
DISCUSSION A variety of benign and malignant causes can result in hepatic calcifications at CT. Prior granulomatous disease is a common cause of hepatic parenchymal calcifications. Calcified granulomas are usually round, subcentimeter, densely calcified structures rather than ill-defined areas of calcification within a larger mass as in the above case.
Other infections such as those caused by Echinococcus can also result in hepatic calcifications. However, calcifications due to Echinococcus infection are usually curvilinear rather than amorphous and ill-defined as in the above case.
Mucin-producing tumors can result in calcified liver metastases and were the etiology of the hepatic calcifications seen in the above case. Mucin-producing tumors may arise from the colon, pancreas, appendix, ovary, and breast.
A variety of benign and malignant primary hepatic tumors occasionally demonstrate areas of calcification, including hemangioma, hepatocellular adenoma, fibrolamellar carcinoma, intrahepatic cholangiocarcinoma, and hepatocellular cancer. The above primary liver tumors often demonstrate typical enhancement patters that can be used to distinguish them from mucinous metastases, which are typically hypoenhancing. For example, hemangiomas typically demonstrate peripheral discontinuous nodular enhancement with gradual fill-in. Intrahepatic cholangiocarcinoma classically demonstrates delayed hyperenhancement. Hepatocellular cancers typically demonstrate arterial hyperenhancement and washout at delayed imaging.
Question for Further Thought
1. What are other potential etiologies of calcified liver metastases?
View Answer
1. Rarely, chondrosarcoma metastasizes to the liver and can produce calcified metastases. Also, chemotherapy can result in calcification of non-mucinous liver metastases.
Reporting Requirements
1. Describe the size, number, and distribution of liver metastases.
2. Report the presence of calcification which is suggestive of a mucinous primary malignancy.
What the Treating Physician Needs to Know
1. The above partially calcified lesions are characteristic of liver metastases from mucin-producing tumors.
Answer
1. Rarely, chondrosarcoma metastasizes to the liver and can produce calcified metastases. Also, chemotherapy can result in calcification of non-mucinous liver metastases.
REFERENCE
1. Stoupis C, Taylor HM, Paley MR, et al. The rocky liver: radiologic-pathologic correlation of calcified hepatic masses. Radiographics 1998;18:675-685.
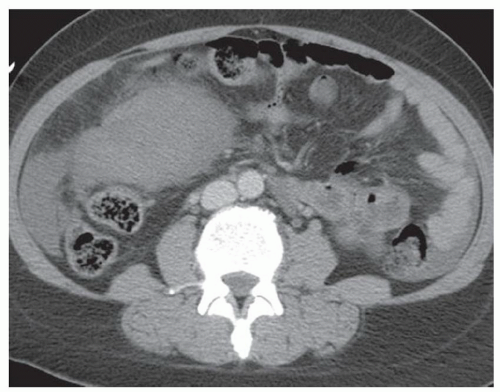
CASE 2.13 CLINICAL HISTORY 36-year-old woman with abdominal pain.
FINDINGS Noncontrast CT (A, B) demonstrates scattered subcentimeter low attenuation lesions in the right and left hepatic lobes. Numerous renal lesions containing large amounts of fat are also present (B). See discussion below for description of (C) to (G).
DIFFERENTIAL DIAGNOSIS FOR LIVER LESIONS angiomyolipomas (AMLs), lipomas, teratomas.
DIAGNOSIS Hepatic AMLs in a patient with tuberous sclerosis.
DISCUSSION AMLs are composed of blood vessels, smooth muscle, and fat. Hepatic AMLs are less common than renal AMLs. Approximately 6% of hepatic AMLs are associated with tuberous sclerosis as compared to 20% of renal AMLs.
The imaging appearances of AMLs vary depending on their fat content with some appearing essentially entirely fat containing and others having more of a soft tissue component. At CT and MR, AMLs demonstrate varying amounts of fat and soft tissue. AMLs containing minimal or no fat may be extremely difficult to distinguish from other solid liver tumors.
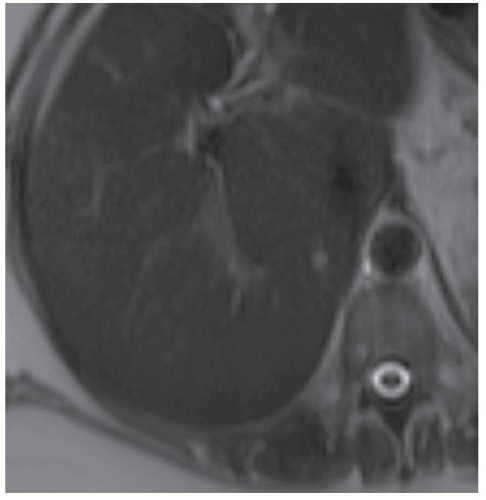
Note how these AMLs appear at MR (C: T2-weighted without fat saturation; D: T2-weighted with fat saturation; E: in-phase; F: out-of phase; G: T1 precontrast with fat saturation). The cerebrospinal fluid (CSF) demonstrates high signal (appears white) in images (C) and (D), indicating that these are T2-weighted images. On the other hand, the CSF demonstrates low signal (appears dark) in images (E) to (G), indicating that these are T1-weighted images.
Note how the 7-mm AML in the posterior segment right hepatic lobe follows the signal intensity of subcutaneous fat in all series appearing bright in (C) and (E) and dark in (D) and (G). Also note the India ink artifact in the out-of-phase image (F) whereby a black line is visible around the lesion. This black line occurs because at the echo time at which the out-of-phase image is acquired, fat and water protons are pointing in opposite directions and cancel each other out. The India ink artifact occurs at all fat-water interfaces (e.g., at the interface between hepatic parenchyma and adjacent fat).
Hepatic lipomas are rare tumors and are composed entirely of fat. These lesions appear hyperechoic at US, are low (<0 HU) attenuation at CT, and follow the signal intensity of subcutaneous fat at MR. Small lipomas may be impossible to distinguish from AMLs if the AML appears entirely fat containing at imaging. Hepatic AMLs and lipomas are both benign tumors, are usually small in size, and usually require no further treatment.
Hepatic teratomas are exceedingly rare tumors. These masses arise from pluripotential cells and often contain areas of calcification, fat, soft tissue, and sometimes fluid.
Questions for Further Thought
1. List other lesions associated with tuberous sclerosis.
View Answer
1. Cortical tubers, subependymal nodules, angiomyolipomas, cardiac rhabdomyomas, ungual fibromas, bone cysts.
2. What is the classic clinical triad that is sometimes seen in patients with tuberous sclerosis?
Reporting Requirements
1. Describe the size and location of the fat-containing lesions.
2. Look for other evidence of tuberous sclerosis such as renal AMLs.
What the Treating Physician Needs to Know
1. Entirely fat-containing lesions are benign (usually AMLs or lipomas) and require no further treatment.
Answer
1. Cortical tubers, subependymal nodules, angiomyolipomas, cardiac rhabdomyomas, ungual fibromas, bone cysts.
2. Epilepsy, mental retardation, adenoma sebaceum.
REFERENCE
1. Umeoka S, Koyama T, Miki Y, et al. Pictorial review of tuberous sclerosis in various organs. Radiographics 2008;28:e32.
CASE 2.14 CLINICAL HISTORY 72-year-old man with left flank pain.
FINDINGS Coronal reformation from a noncontrast CT study demonstrates an ovoid, approximately 2-cm fat attenuation structure along the capsular surface of the liver dome. (The patient also has advanced calcified atherosclerotic disease of the abdominal aorta and branch vessels.)
DIFFERENTIAL DIAGNOSIS Angiomyolipoma (AML), lipoma, pseudolipoma of Glisson capsule.
DIAGNOSIS Pseudolipoma of Glisson capsule.
DISCUSSION The term “pseudolipoma of Glisson capsule” refers to a well-circumscribed fat attenuation structure along the capsular surface of the liver. These pseudolipomas are thought to reflect a detached colonic epiploic appendage that has become lodged between the liver dome and the diaphragm.
Intraparenchymal hepatic lesions that are entirely fat attenuation structures are most commonly lipomas or AMLs. These lesions can be difficult to distinguish at imaging. Both are benign and require no intervention in most cases. AMLs may also contain a visible soft tissue component.
Question for Further Thought
1. Is any intervention required for a pseudolipoma of Glisson capsule?
View Answer
1. No. These pseudolipomas are thought to be asymptomatic, have no known malignant potential, and therefore do not warrant treatment.
Reporting Responsibilities
1. Describe the finding as an incidental finding.
2. Consider mentioning only in the body of the report.
What the Treating Physician Needs to Know
1. Pseudolipoma of Glisson capsule is an incidental finding.
Answer
1. No. These pseudolipomas are thought to be asymptomatic, have no known malignant potential, and therefore do not warrant treatment.
REFERENCES
1. Prasad SR, Wang H, Rosas H, et al. Fat-containing lesions of the liver: radiologic-pathologic correlation. Radiographics 2005;25: 321-331.
2. Sasaki M, Harada K, Nakanuma Y, Watanabe K. Pseudolipoma of Glisson’s capsule. Report of six cases and review of the literature. J Clin Gastroenterol 1994;19:75-78.
CASE 2.15 CLINICAL HISTORY 38-year-old man status post-motor vehicle collision.
FINDINGS Contrast-enhanced CT demonstrates linear low attenuation paralleling both sides of the portal system (A). US demonstrates diffusely increased echogenicity along the portal triads (B).
DIFFERENTIAL DIAGNOSIS Biliary ductal dilatation, periportal edema.
DIAGNOSIS Periportal edema.
Periportal edema can be distinguished from biliary ductal dilatation by noting whether the linear low attenuation seen along the portal system at CT runs along both sides of the portal venous system (indicating periportal edema) or runs along one side of the portal venous system (indicating biliary ductal dilatation). At US, periportal edema appears as increased echogenicity along the portal triads.
Periportal edema is a nonspecific finding and can be seen with hepatitis, congestive heart failure, and postliver transplant rejection. Periportal edema is thought to reflect fluid or dilated lymphatics around the portal triads.
Question for Further Thought
1. What is the treatment for periportal edema?
View Answer
1. No treatment is required for periportal edema. The edema will often improve with improvement of the underlying condition.
Reporting Requirement
1. Accurately differentiate periportal edema from biliary ductal dilatation.
What the Treating Physician Needs to Know
1. Periportal edema is a nonspecific finding that can be seen in a number of settings including third spacing of fluid (e.g., due to liver disease or congestive heart failure), and liver transplant rejection.
Answer
1. No treatment is required for periportal edema. The edema will often improve with improvement of the underlying condition.
REFERENCES
1. Mortele KJ, Segatto E, Ros PR. The infected liver: radiologic-pathologic correlation. Radiographics 2004;24:937-955.
2. Lawson TL, Thorsen KM, Erickson SJ, Perrett RS, Quiroz FA, Foley WD. Periportal halo: a CT sign of liver disease. Abdom Imaging 1993;18:42-46.
CASE 2.16 CLINICAL HISTORY 57-year-old man with abdominal distention.
FINDINGS Multiple intravenous contrast-enhanced CT images of the abdomen and the pelvis demonstrate the liver to have a small and nodular morphology (A-C). Numerous large vessels are seen about the distal esophagus (A) and the gastric fundus (B). A recanalized paraumbilical vein is also present (C). Numerous prominent vascular structures are present in the subcutaneous fat around the umbilicus (D). The colon is also diffusely thick-walled (D).
DIFFERENTIAL DIAGNOSIS Cirrhosis with portal hypertention.
DIAGNOSIS Cirrhosis with portal hypertension.
DISCUSSION Portal hypertension, as the name suggests, indicates increased pressure within the portal venous system. Portal hypertension can result from prehepatic causes (e.g., portal vein thrombus), intraheptic causes (e.g., cirrhosis), and post-hepatic causes (e.g., hepatic vein thrombosis). Imaging findings of portal hypertension include splenomegaly (spleen >13 cm in length), ascites, and portosystemic collaterals.
Portosystemic collaterals become apparent because when portal vein pressures increase, portosystemic collateral vessels dilate as lower resistance pathways via which
portal system blood can return to the inferior vena cava and superior vena cava (SVC). The above patient has a number of portosystemic collaterals including esophageal varices, paraesophageal varices, gastric varices, a recanalized paraumbilical vein, and caput medusa.
portal system blood can return to the inferior vena cava and superior vena cava (SVC). The above patient has a number of portosystemic collaterals including esophageal varices, paraesophageal varices, gastric varices, a recanalized paraumbilical vein, and caput medusa.
Gastroesophageal varices are, in general, the most clinically significant varices as they are the most common varices to result in upper GI bleeding which can be life threatening. Gastroesophageal varices receive blood from the left gastric vein (coronary vein) and the short gastric veins. Gastroesophageal varices empty into the azygos system and SVC.
The falciform ligament normally contains 1 to 3 small paraumbilical veins. In the setting of portal hypertension, paraumbilical veins become dilated and drain into anterior abdominal wall collateral vessels. Dilated anterior abdominal wall collaterals are connected to the superior and inferior epigastric veins and account for the “caput medusae” appearance at physical examination, whereby numerous superficial collateral vessels are seen that resemble the mythological Medusa hair. This patient also has colonic wall thickening as can be seen with colopathy of portal hypertension. Colopathy of portal hypertension is thought to result from increased portal vein pressures, usually involves the right colon, and is believed to be asymptomatic.
Questions for Further Thought
1. What is the difference between esophageal varices and paraesophageal varices?
View Answer
1. Esophageal varices are located in the esophageal wall, are visible at endoscopy, and are prone to bleeding. Paraesophageal varices are located outside of the esophageal wall, are not visible at endoscopy, and are less prone to bleeding. Esophageal varices protrude into the esophageal lumen at imaging.
2. List other portosystemic collateral pathways.
View Answer
2. Other portosystemic collateral pathways include perisplenic varices, omental varices, retroperitoneal-paravertebral varices, mesenteric varices, splenorenal shunts, gastrorenal shunts, and anorectal varices (also known as hemorrhoids).
Reporting Requirements
1. Describe the presence of cirrhosis.
2. Describe the findings of portal hypertension (ascites and varices).
What the Treating Physician Needs to Know
1. Given the patient’s findings of advanced chronic liver disease, screening for hepatocellular cancer with contrast-enhanced CT or MR (precontrast, late arterial phase, portal venous phase, and delayed phase imaging) is likely warranted.
Answers
1. Esophageal varices are located in the esophageal wall, are visible at endoscopy, and are prone to bleeding. Paraesophageal varices are located outside of the esophageal wall, are not visible at endoscopy, and are less prone to bleeding. Esophageal varices protrude into the esophageal lumen at imaging.
2. Other portosystemic collateral pathways include perisplenic varices, omental varices, retroperitoneal-paravertebral varices, mesenteric varices, splenorenal shunts, gastrorenal shunts, and anorectal varices (also known as hemorrhoids).
REFERENCES
1. Georgiades CS. Etymology of selected medical terms used in radiology: the mythological connection. Am J Roentgenol 2002;178:1101-1107.
2. Cho KC, Patel YD, Wachsberg RH, Seeff J. Varices in portal hypertension: evaluation with CT. Radiographics 1995;15:609-622.
CASE 2.17 CLINICAL HISTORY 43-year-old woman with abdominal distention.
FINDINGS Intravenous contrast-enhanced CT images (A-C) demonstrate expansion of the main portal vein, right portal vein, and left portal vein, which contain low attenuation material. Segmental portal vein branches are also filled with low attenuation material. The liver parenchyma is normal in appearance. Also, small-volume ascites is present.
DIFFERENTIAL DIAGNOSIS Bland portal vein thrombus, tumor thrombus.
DIAGNOSIS Bland portal vein thrombus.
DISCUSSION Portal vein thrombosis most commonly occurs in patients with cirrhosis. Other conditions that can lead to portal vein thrombosis include diverticulitis, appendicitis, inflammatory bowel disease, and other systemic hypercoaguable states (e.g., malignancy, factor V Leiden mutation, protein C or S deficiency, oral contraceptives, pregnancy, antiphospholipid syndrome, antithrombin deficiency).
Acute portal vein thrombus usually results in a distended portal vein as in the above case. Patients with acute portal vein occlusion often present with abdominal pain. If untreated, patients with acute portal vein obstruction often develop ascites as in this case and can develop bowel ischemia.
By comparison, chronic portal vein thrombosis may be asymptomatic. At imaging, chronically occluded portal veins are usually small in size. Acute occlusive portal vein thrombosis is usually treated with anticoagulants, whereas chronically occluded veins may not warrant treatment.
Bland thrombus can be distinguished from tumor thrombus by assessing for enhancement within the thrombus. Bland thrombus will not enhance, whereas tumor thrombus enhances. Additionally, tumor thrombus usually directly extends from a visible hepatic parenchymal tumor, most commonly hepatocellular cancer. The lack of thrombus enhancement and the lack of a contiguous hepatic parenchymal mass support the diagnosis of bland thrombus.
Question for Further Thought
1. What tumors are associated with tumor thrombus?
View Answer
1. Hepatocellular cancer may directly invade the portal venous system or hepatic venous system with tumor thrombus. Renal cell cancers and adrenocortical cancers may demonstrate direct extension of tumor thrombus into the renal vein and inferior vena cava, respectively.
Reporting Requirements
1. This acute portal vein thrombus should be called to the ordering physician.
2. Assess for evidence of bowel ischemia such as bowel wall thickening and pneumatosis.
What the Treating Physician Needs to Know
1. Acute portal vein thrombus is present.
2. Whether complications such as ascites and bowel ischemia are visible.
Answer
1. Hepatocellular cancer may directly invade the portal venous system or hepatic venous system with tumor thrombus. Renal cell cancers and adrenocortical cancers may demonstrate direct extension of tumor thrombus into the renal vein and inferior vena cava, respectively.
REFERENCE
1. Ponziani FR, Zocco MA, Campanale C, et al. Portal vein thrombosis: insight into physiopathology, diagnosis and treatment. World J Gastroenterol 2010;16:143-155.
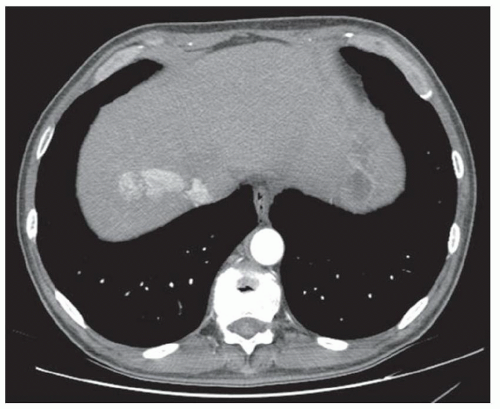
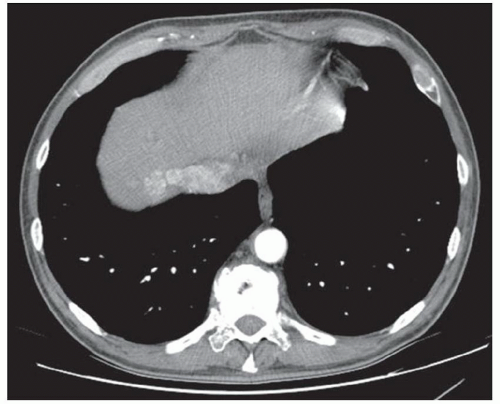
CASE 2.18 CLINICAL HISTORY 58-year-old man with abdominal pain.
FINDINGS Noncontrast CT images (A, B) demonstrate the liver parenchyma to be of high attenuation measuring 110 HU. Chest radiograph (C) demonstrates a pacemaker.
DIFFERENTIAL DIAGNOSIS Drug therapy (gold, amiodarone, and cisplatin), glycogen storage disease, hemosiderosis, Wilson disease
DIAGNOSIS Amiodarone therapy.
DISCUSSION Amiodarone is a medication used to treat arrhythmias. Formulations of amiodarone contain iodine (e.g., 75 mg of iodine in a 200-mg tablet of amiodarone). Amiodarone is excreted primarily via hepatobiliary excretion, and therapy results in dose-dependent iodine deposition in the liver in some patients. That not all patients who take amiodarone develop a hyperdense liver is thought to be due to genetic differences in metabolism of the drug.
Hepatic toxicity due to amiodarone usually manifests as a transient transaminitis that resolves after cessation of amiodarone treatment. Individuals are usually asymptomatic. The incidence of amiodarone-related hepatic toxicity is thought to be low, and clinically significant hepatic toxicity is very uncommon.
Medications including gold, cisplatin, and Thorotrast can result in a hyperdense liver. Gold therapy is occasionally used to treat patients with, for example, rheumatoid arthritis. Cisplatin is a chemotherapy drug used to treat a number of solid organ malignancies. Thorotrast was used as a contrast agent in medical imaging in the 1930s to 1950s. This radioactive α-emitter is taken up by the liver, spleen, lymph nodes, and bone. Thorotrast has a half life of >20 years in the human body with resultant significant radiation exposures to individuals who were administered this agent before it was removed from the market. Individuals administered Thorotrast are at significantly increased risk
for a number of malignancies including hepatic and biliary malignancies.
for a number of malignancies including hepatic and biliary malignancies.
Glycogen storage diseases most commonly result in a low attenuation liver due to fat deposition but can also result in a high attenuation liver due to glycogen deposition. Hemosiderosis refers to diffuse iron deposition in the liver and may be due to a genetic abnormality (termed “hemochromatosis” or “primary hemosiderosis”) or multiple blood transfusions (termed “secondary hemosiderosis”). Wilson disease results in copper deposition in the liver due to a genetic error in copper metabolism.
Questions for Further Thought
2. At what attenuation value should the liver parenchyma be considered hyperattenuating at noncontrast CT?
Reporting Responsibilities
1. Describe increased hepatic parenchymal attenuation.
2. Check for clues to etiology such as cardiomegaly or pacemaker which may indicate an arrhythmia and amiodarone treatment.
What the Treating Physician Needs to Know
1. Increased hepatic attenuation is due to iodine deposition in patients receiving amiodarone therapy.
2. Incidence of amiodarone hepatic toxicity is low, has an unclear relationship to hepatic attenuation at noncontrast CT, and usually manifests as a transient transaminitis.
Answers
1. 50 to 60 HU
2. Roughly 80 HU
REFERENCES
1. Boll DT, Merkle EM. Diffuse liver disease: strategies for hepatic CT and MR imaging. Radiographics 2009;29:1591-1614.
2. Goldman IS, Winkler ML, Raper SE, et al. Increased hepatic density and phospholipidosis due to amiodarone. Am J Roentgenol 1985;144:541-546.
3. Brouse SD, Phillips SM. Amiodarone use in patients with documented allergy to iodine-containing compounds. Pharmacotherapy 2005;25:429-434.
CASE 2.19 CLINICAL HISTORY 47-year-old woman with abnormal liver function tests.
FINDINGS Grayscale US demonstrates the liver to be more echogenic than the right kidney (A). Additionally, internal architecture of the liver is not well-delineated, especially in the posterior liver, at grayscale (B) or color Doppler (C) imaging. Noncontrast CT demonstrates diffusely low attenuation of the liver parenchyma (20 HU) (D, E) with patchy areas of even more low attenuation.
DIFFERENTIAL DIAGNOSIS Hepatic steatosis.
DIAGNOSIS Hepatic steatosis.
DISCUSSION Hepatic steatosis is an abnormality commonly seen at US, CT, and MR. At histology, triglycerides are identified within hepatocytes in patients with fatty
liver. When quantifying the degree of fatty liver, pathologists estimate the percentage of hepatocytes that contain fat droplets.
liver. When quantifying the degree of fatty liver, pathologists estimate the percentage of hepatocytes that contain fat droplets.
A number of conditions are associated with fatty liver including excessive alcohol consumption, obesity, and insulin resistance. Steroids, total parenteral nutrition, and jejunoileal bypass surgery may also result in fatty liver.
At US, fatty liver can be diagnosed if the liver is more echogenic than the kidney and if the US beam has difficulty penetrating the liver such that the internal architecture of the liver is not well-depicted. Often, the internal architecture of the posterior liver is especially poorly seen.
At noncontrast CT, the normal liver parenchyma measures 50 to 60 HU and is more attenuating than the spleen. Fatty liver is suggested at CT if the liver measures <40 HU attenuation or more than 10 HU less than the spleen at noncontrast CT. As the liver and the spleen enhance differently after administration of intravenous contrast material, it is more difficult to accurately diagnose hepatic steatosis at postcontrast imaging. Nonetheless, a liver that is significantly lower in attenuation than the spleen at postcontrast imaging is also most likely fatty infiltrated.
At MR, fatty liver is diagnosed by detecting signal loss at out-of-phase as compared to in-phase imaging. Arterial phase heterogeneous enhancement is suggestive of inflammation (steatohepatitis) in a patient with fatty liver. MR spectroscopy is thought to be the most accurate way to quantify the degree of hepatic steatosis noninvasively.
Fat deposition may be diffuse or focal. Fat sparing may also be focal. Typical areas for focal fat deposition or sparing include along the falciform ligament, porta hepatis, gallbladder fossa, or elsewhere in the subcapsular liver.
Questions for Further Thought
1. Define NAFLD.
View Answer
1. Fatty liver can be divided into two broad categories: (1) alcohol-related fatty liver disease and (2) nonalcohol-related fatty liver disease (NAFLD). NAFLD includes both hepatic steatosis and NASH.
2. Define nonalcoholic steatohepatitis (NASH).
View Answer
2. NASH is a subcategory of NAFLD. NASH describes patients who also demonstrate inflammatory changes and/or fibrosis in addition to hepatic fat deposition.
Reporting Requirements
1. Report that the patient has fatty liver.
2. If the patient has prior imaging, attempt to compare severity of fat deposition to the prior study.
3. Report if there is evidence of inflammation (e.g. heterogeneous arterial phase enhancement).
4. Report if there is evidence of fibrosis (e.g. reticular delayed phase hyperenhancement).
What the Treating Physician Needs to Know
1. The patient has fatty liver.
2. No changes of fibrosis are seen in this patient.
Answers
1. Fatty liver can be divided into two broad categories: (1) alcohol-related fatty liver disease and (2) nonalcohol-related fatty liver disease (NAFLD). NAFLD includes both hepatic steatosis and NASH.
2. NASH is a subcategory of NAFLD. NASH describes patients who also demonstrate inflammatory changes and/or fibrosis in addition to hepatic fat deposition.
REFERENCES
1. Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, Sirlin CB. Fatty liver: imaging patterns and pitfalls. Radiographics 2006;26:1637-1653.
2. Sanval AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 2002;123:1705-1725.
CASE 2.20 CLINICAL HISTORY 54-year-old woman with abnormal liver function tests.
FINDINGS Contrast-enhanced portal venous phase CT (A-D) demonstrates right heart enlargement (A). Note distention of the left hepatic vein (B). The liver parenchyma is extremely heterogeneous with primarily linear and reticular areas of low attenuation (C, D). Note findings of volume overload including bilateral pleural effusions, small volume ascites, and diffusely increased attenuation within the subcutaneous fat reflecting diffuse anasarca.
DIFFERENTIAL DIAGNOSIS Fatty liver, metastatic disease, nutmeg liver.
DIAGNOSIS Nutmeg liver.
DISCUSSION The term “nutmeg liver” comes from the histopathologic changes seen in patients with passive hepatic congestion which are said to appear similar to grated nutmeg. At gross pathology, dilated venules and small hepatic
veins appear dark, whereas the surrounding hepatic parenchyma appears pale.
veins appear dark, whereas the surrounding hepatic parenchyma appears pale.
At CT or MR imaging, findings associated with nutmeg liver include right heart enlargement and distended hepatic veins. Early in the disease process, hepatomegaly may be observed. However, prolonged exposure of hepatocytes to increased venous pressures results in hypoxia, cellular necrosis, and eventually fibrosis as is seen in this patient with resultant heterogeneous enhancement of the liver with reticular areas of low attenuation as in the above case.
Clinically, patients with passive hepatic congestion may present with right upper quadrant pain due to stretching of the liver capsule as a result of increased hepatic vein pressure. Patients may also demonstrate elevated liver function tests.
Metastatic disease more commonly is rounded in appearance unlike the linear reticular areas of low attenuation seen in patients with nutmeg liver. Focal fat deposition may sometimes have a linear or reticular morphology and may in rare instances be difficult to distinguish from nutmeg liver. In cases where focal fat deposition is a differential diagnostic consideration, in- and out-of-phase MR can be used to distinguish nutmeg liver from areas of fat deposition as areas of fat deposition will demonstrate signal loss at out-of-phase MR.
Question for Further Thought
1. List etiologies of passive hepatic congestion.
View Answer
1. Possible etiologies of passive hepatic congestion include right heart failure and pericardial tamponade.
Reporting Requirements
1. Report that this patient has findings of passive hepatic congestion.
2. Report that the patient has findings of diffuse fluid third spacing including pleural fluid, ascites, and anasarca.
What the Treating Physician Needs to Know
1. The patient may have abnormal liver function tests or right upper quadrant pain as a result of passive hepatic congestion.
Answer
1. Possible etiologies of passive hepatic congestion include right heart failure and pericardial tamponade.
REFERENCES
1. Boll DT, Merkle EM. Diffuse liver disease: strategies for hepatic CT and MR imaging. Radiographics 2009;29:1591-1641.
2. Giallourakis CC, Rosenberg PM, Friedman LS. The liver in heart failure. Clin Liver Dis 2002;6:947-967.
CASE 2.21 CLINICAL HISTORY 58-year-old man with abdominal pain.
FINDINGS Contrast-enhanced CT demonstrates an arterial structure in the fissure for the ligamentum venosum (A) and an arterial structure located immediately posterior to the main portal vein (B).
DIFFERENTIAL DIAGNOSIS Normal vascular anatomy, accessory or replaced left hepatic artery (LHA), accessory or replaced right hepatic artery (RHA).
DIAGNOSIS Accessory LHA, replaced RHA (determined based on evaluation of entire study).
DISCUSSION Classically, the left gastric artery (LGA) arises from the proximal celiac trunk. The celiac trunk then bifurcates into the common hepatic artery and the splenic artery. The common hepatic artery gives off the gastroduodenal artery beyond which point the common hepatic artery is termed the proper hepatic artery. The proper hepatic artery then bifurcates into right and left hepatic arteries. This classic arterial anatomy occurs in approximately 50% to 60% of individuals.
Some of the more common hepatic arterial anatomy variants and their respective incidence are as follows:
15% accessory LHA arising from LGA
12% replaced RHA arising from superior mesenteric artery (SMA)
5% replaced LHA arising from LGA
3% accessory RHA from SMA
2% replaced common hepatic artery from SMA
2% replaced common hepatic artery from aorta
To determine whether a vessel is “accessory” or “replaced” it is necessary to interrogate the liver’s vascular supply from the celiac trunk. For example, if the RHA arising from the celiac trunk supplies the anterior segment right hepatic lobe, an RHA branch from the SMA that supplies the posterior segment would be termed an “accessory RHA.” On the other hand, if the entire right hepatic lobe is supplied by an arterial structure arising from the SMA, this vessel would be termed a “replaced RHA.”
Knowledge of variant hepatic arterial anatomy is especially helpful for surgeons prior to hepatic resection or transplant and is helpful for interventional radiologists prior to chemoembolization procedures.
Questions for Further Thought
1. An arterial structure is seen in the fissure for the ligamentum venosum. What could this structure be?
View Answer
1. An arterial structure seen in the fissure for the ligamentum venosum may be an accessory LHA or a replaced LHA from the LGA or an accessory LGA from the LHA.
2. Where are replaced or accessory right hepatic arteries most commonly located in the liver hilum?
View Answer
2. Replaced or accessory right hepatic arteries are usually located immediately posterior to the main portal vein.
Reporting Responsibilities
1. Accurately describe variant hepatic arterial anatomy.
What the Treating Physician Needs to Know
1. If surgery or chemoembolization is planned, the presence of variant hepatic arterial anatomy may alter the surgical or interventional radiology approach to the case.
Answers
1. An arterial structure seen in the fissure for the ligamentum venosum may be an accessory LHA or a replaced LHA from the LGA or an accessory LGA from the LHA.
2. Replaced or accessory right hepatic arteries are usually located immediately posterior to the main portal vein.
REFERENCE
1. Covey AM, Brody LA, Maluccio MA, Getrajdam GI, Brown KT. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology 2002;224:542-547.
CASE 2.22 CLINICAL HISTORY 68-year-old man with lung cancer, restaging examination.
FINDINGS Contrast enhanced CT (A-C) demonstrates an avidly enhancing triangular-shaped area in the periphery of the segment 4 liver. Note also numerous collateral vessels in the subcutaneous tissues.
DIFFERENTIAL DIAGNOSIS Hypervascular metastasis, superior vena cava (SVC) occlusion.
DIAGNOSIS SVC occlusion.
DISCUSSION The triangular shape and characteristic enhancement pattern in the segment 4 liver seen in this case is an “Aunt Minnie” for SVC occlusion. Chest CT should be recommended to evaluate for a thoracic mass resulting in SVC occlusion.
The segment 4 liver hyperenhances in the setting of SVC occlusion because of the shunting of large volumes of blood through this area via a portosystemic collateral pathway that develops between subcutaneous collateral vessels and the left portal vein via the paraumbilical vein. Blood passes through the portal vein, liver, hepatic veins, and inferior vena cava (IVC) and returns to the heart via this alternate route.
Patients with SVC occlusion may present with distended neck veins, headache, head and neck swelling, and dyspnea. At 99mTc-sulfur colloid imaging, patients with SVC occlusion may demonstrate focal accumulation of radiotracer in the segment 4 liver known as the “hot quadrate sign.” The quadrate lobe is the segment 4 liver.
Questions for Further Thought
1. What are the most common etiologies of SVC occlusion?
View Answer
1. Malignancy (e.g., lung cancer) and chronic occlusion due to scarring from multiple central venous access catheters are the most common etiologies of SVC occlusion.
2. Name another collateral pathway in patients with SVC occlusion.
View Answer
2. The azygous/hemiazygous and paravertebral system is another collateral pathway via which blood can return to the heart in patients with SVC occlusion.
Reporting Responsibilities
1. Report that findings most likely reflect SVC occlusion.
2. Recommend chest CT to evaluate for an obstructing mass.
What the Treating Physician Needs to Know
1. The patient most likely has an occluded SVC.
2. A chest CT is needed to evaluate for a neoplasm occluding the SVC.
Answers
1. Malignancy (e.g., lung cancer) and chronic occlusion due to scarring from multiple central venous access catheters are the most common etiologies of SVC occlusion.
2. The azygous/hemiazygous and paravertebral system is another collateral pathway via which blood can return to the heart in patients with SVC occlusion.
REFERENCES
1. Sheth S, Ebert MD, Fishman EK. Superior vena cava obstruction: evaluation with MDCT. Am J Roentgenol 2010;194:W336-W346.
2. Dickson AM. The focal hepatic hot spot sign. Radiology 2005;237:647-648.
CASE 2.23 CLINICAL HISTORY 67-year-old woman with abdominal pain and a history of percutaneous transhepatic cholangiogram three weeks prior complicated by bleeding.
FINDINGS Noncontrast CT (A) demonstrates a 7-cm low attenuation lesion in the right hepatic lobe. Post-contrast portal venous phase CT (B) demonstrates the lesion to be homogeneously enhancing with attenuation as bright at the blood pool (in other words as bright as the aorta). [C described in Discussion.]
DIFFERENTIAL DIAGNOSIS Focal nodular hyperplasia (FNH), hepatic adenoma, hepatocellular cancer, hypervascular metastasis, pseudoaneurysm.
DIAGNOSIS Pseudoaneurysm.
DISCUSSION The homogeneous enhancement equal to the blood pool in the above lesion is diagnostic of a pseudoaneurysm. Although areas of FNH, hepatic adenomas, hepatocellular cancers, and hypervascular metastases all hyperenhance, they do not enhance as homogeneously as the above case. In uncertain cases where the differential includes a pseudoaneurysm, US is a helpful problem-solving tool as the typical ying-yang appearance of to-and-fro flow would be expected with a pseudoaneurysm at US.
Hepatic pseudoaneurysms most commonly occur secondary to trauma. Approximately 2% of biliary procedures are complicated by hepatic arterial injury. Other potential etiologies of hepatic arterial injury include liver biopsy or other blunt or penetrating trauma. Pseudoaneurysms can also form as a complication of liver abscess if the infection erodes through the arterial wall.
Patients with hepatic arterial pseudoaneurysms are at risk for rupture and catastrophic bleeding. The above patient underwent embolization of the pseudoaneurysm. Initial diagnostic image (C) obtained as part of the embolization procedure demonstrates contrast material opacifying the pseudoaneurysm.
Question for Further Thought
1. What is the incidence of hepatic arterial aneurysms in the setting of polyarteritis nodosa?
View Answer
1. Hepatic arterial aneurysms, usually measuring <1 cm in diameter, occur in up to 50% to 60% of patients with polyarteritis nodosa. A diagnosis of polyarteritis nodosa (a necrotizing vasculitis) should be considered in patients with small hepatic artery aneurysms and no history of trauma.
Reporting Requirements
1. Describe the presence of a 7-cm hepatic arterial pseudoaneurysm in the right hepatic lobe.
2. Directly communicate this emergent finding to the treating physician.
What the Treating Physician Needs to Know
1. If left untreated, this pseudoaneurysm is at risk for rupture with subsequent life-threatening hemorrhage.
2. Embolization is a potential treatment option for this pseudoaneurysm.
Answer
1. Hepatic arterial aneurysms, usually measuring <1 cm in diameter, occur in up to 50% to 60% of patients with polyarteritis nodosa. A diagnosis of polyarteritis nodosa (a necrotizing vasculitis) should be considered in patients with small hepatic artery aneurysms and no history of trauma.
REFERENCES
1. Fidelman N, Bloom AI, Kerlan RK Jr, et al. Hepatic arterial injuries after percutaneous biliary interventions in the era of laparoscopic surgery and liver transplantation: experience with 930 patients. Radiology 2008;247:880-886.
2. Lee M-G, Lim HK, Choi C-S, Auh YH. Radiologic findings of abdominal polyarteritis nodosa. Am J Roentgenol 2000;174: 1675-1679.
CASE 2.24 CLINICAL HISTORY 32-year-old woman, history withheld.
FINDINGS Contrast-enhanced CT images demonstrate a high attenuation structure with surrounding streak artifact in the subcutaneous fat of the right upper quadrant (A). A linear area of low attenuation is seen extending through the posterior segment of the right hepatic lobe (B, C).
DIFFERENTIAL DIAGNOSIS Laceration due to gunshot wound, laceration due to knife wound.
DIAGNOSIS Laceration due to gunshot wound.
DISCUSSION Linear structures are usually not naturally occurring in the human body. When a straight linear lesion is identified, traumatic and iatrogenic injuries should be considered. The clue that the above liver laceration is due to a gunshot wound is the presence of a bullet within the subcutaneous fat of the right upper quadrant.
The term “laceration” is used to describe areas of traumatic low attenuation in the liver which have a linear morphology. By comparison, liver hematomas have a more rounded or ill-defined morphology. Hepatic lacerations that extend to the hepatic veins or portal vein may indicate a significant vascular injury. Lacerations that extend into the porta hepatis also may be associated with bile duct injury.
The American Association of Trauma Surgeons has developed a grading system for liver injury that can be used when describing liver hematomas and lacerations.1 The system characterizes traumatic lacerations and hematomas on a scale of 1 to 6 with grade 1 injuries being the least severe and grade 6 injuries being the most severe. In general, parenchymal hematomas are characterized by the percent of liver surface area involved, and lacerations are characterized by parenchymal depth and length.
Hemodynamically stable patients with liver injuries are usually managed with supportive care and do not require surgical intervention or embolization procedures. Unstable patients may undergo surgical packing of the site of liver injury. Complications of liver trauma include delayed
hemorrhage, superinfection of hematoma, hepatic artery pseudoaneurysm, biloma, and bile peritonitis.
hemorrhage, superinfection of hematoma, hepatic artery pseudoaneurysm, biloma, and bile peritonitis.
Questions for Further Thought
1. How long does it take hepatic lacerations to resolve?
2. How long does it take subcapsular hematomas and hemoperitoneum to resolve?
View Answer
2. Hemoperitoneum usually resolves within 1 week. Subcapsular hematomas usually resolve within 6 to 8 weeks.
Reporting Requirements
1. Report the size and location of liver laceration(s).
2. Report whether the laceration extends to major vascular structures or the porta hepatis.
What the Treating Physician Needs to Know
1. The depth and length of the liver laceration.
2. Whether major vascular structures appear involved.
3. If the laceration extends to the porta hepatis as such lesions are more commonly associated with biliary injury.
Answers
1. Hepatic lacerations usually resolve within 3 weeks.
2. Hemoperitoneum usually resolves within 1 week. Subcapsular hematomas usually resolve within 6 to 8 weeks.
REFERENCES
1. Moore EE, Cogbill TH, Jurkovich GJ, et al. Organ injury scaling: spleen and liver. J Trauma 1995;38-323-324.
2. Yoon W, Jeong YY, Kim JK, et al. CT in blunt liver trauma. Radiographics 2005;25:87-104.
CASE 2.25 CLINICAL HISTORY 28-year-old man, history withheld.
FINDINGS Contrast-enhanced CT image demonstrates a moderate to large amount of intermediate attenuation fluid surrounding the liver and the spleen. Higher attenuation material is also noted along the anterior aspect of the liver. Additionally, a large 7-cm area of low attenuation is seen in the right hepatic lobe with central rounded areas of increased attenuation that are as high attenuation as the blood pool (in other words the aorta) but do not conform to the expected shape of a vessel. Air in the subcutaneous tissues is also noted.
DIAGNOSIS Large post-traumatic intraparenchymal hepatic hemorrhage with active contrast material extravasation and large volume hemoperitoneum.
DISCUSSION This patient has a large right hepatic lobe hematoma with active bleeding and large volume hemoperitoneum. The presence of air in the subcutaneous tissues is the clue to the traumatic etiology in this case.
American Association for the Surgery of Trauma (AAST) has devised a grading system for liver trauma,1 which characterizes both lacerations and hematomas. The AAST system defines grades 1 to 6 injuries, with higher grades indicating more severe injuries. Active bleeding indicates at least a grade 3 injury. In general, parenchymal hematomas are characterized by the percent of liver surface area involved, and lacerations are characterized by parenchymal depth and length. Hemodynamically stable patients are usually managed conservatively and do not require treatment. The above patient was hemodynamically unstable and underwent surgical packing of the intraparenchymal hematoma.
Questions for Further Thought
1. What findings indicate that a hematoma has become superinfected?
View Answer
1. Development of air within the hematoma and clinical findings of fever and elevated white blood cell count could indicate superinfection of the hematoma.
2. How can active extravasation be distinguished from a pseudoaneurysm at CT?
View Answer
2. Both areas of active contrast material extravasation and pseudoaneurysms will appear as areas of increased attenuation that are as bright as the blood pool but do not conform to the expected shape of a vessel. In general, pseudoaneurysms appear more rounded and well-circumscribed when compared with areas of active extravasation. In cases in which it is difficult to distinguish between these two possibilities, acquiring delayed images (e.g., at 90 seconds) would be expected to show increased contrast material accumulation at a site of active extravasation and relative washout in the setting of a pseudoaneurysm.
Reporting Requirements
1. Contact the ordering physician immediately regarding this severe injury.
2. Describe the size and location of the hematoma.
3. Describe the presence of contrast material extravasation, which indicates active bleeding.
4. Describe the large amount of hemoperitoneum.
What the Treating Physician Needs to Know
1. The above patient has a large intrahepatic hematoma with active bleeding and large volume hemoperitoneum.
Answers
1. Development of air within the hematoma and clinical findings of fever and elevated white blood cell count could indicate superinfection of the hematoma.
2. Both areas of active contrast material extravasation and pseudoaneurysms will appear as areas of increased attenuation that are as bright as the blood pool but do not conform to the expected shape of a vessel. In general, pseudoaneurysms appear more rounded and well-circumscribed when compared with areas of active extravasation. In cases in which it is difficult to distinguish between these two possibilities, acquiring delayed images (e.g., at 90 seconds) would be expected to show increased contrast material accumulation at a site of active extravasation and relative washout in the setting of a pseudoaneurysm.
REFERENCES
1. Moore EE, Cogbill TH, Jurkovich GJ, et al. Organ injury scaling: spleen and liver. J Trauma 1995;38-323-324.
2. Yoon W, Jeong YY, Kim JK, et al. CT in blunt liver trauma. Radiographics 2005;25:87-104.
CASE 2.26 CLINICAL HISTORY 21-year-old man with abdominal pain.
FINDINGS Grayscale US (A) demonstrates a 2.7-cm intrahepatic echogenic, shadowing structure in keeping with a stone. Color Doppler US (B) in a different area of the liver demonstrates an additional echogenic shadowing structure surrounded by anechoic material. Note also intrahepatic biliary ductal dilatation (B). Contrast-enhanced CT (C, D) demonstrates high attenuation structures surrounded by low attenuation fluid. T2-weighted coronal MR image (E) demonstrates that the echogenic, high attenuation structures seen in (A) to (D) are stones within a dilated intrahepatic biliary system. Slab MRCP image (F) demonstrates moderate intrahepatic biliary ductal dilatation. In summary, this patient has numerous stones within a dilated intrahepatic biliary system.
DIFFERENTIAL DIAGNOSIS Recurrent pyogenic cholangitis (RPC).
DIAGNOSIS RPC.
DISCUSSION Patients with RPC have intrahepatic biliary strictures, which lead to irregular intrahepatic biliary ductal dilatation, bile stasis, and eventual pigment stone formation. Strictures of the extrahepatic biliary system are uncommon. Pigment stones are hypointense (black) at T2-weighted imaging and may have intrinsic T1 bright signal. The left hepatic lobe is affected more often than the right, but bilobar involvement is also common.
If bile ducts are acutely infected or inflamed, wall hyperemia may be seen at arterial phase MR imaging. Intrahepatic abscesses may occur in up to 20% of patients. Over time, portal vein thrombosis and eventual parenchymal atrophy may develop.
Patients often experience repeated episodes of cholangitis due to superimposed infection and may present with fever, right upper quadrant pain, and jaundice. The infectious organism in the acute setting is usually bacteria. Clonorchis sinensis and Ascaris lumbricoides infestation have also been found in patients with RPC, but a direct link between the two conditions has not been established definitively.
RPC has also been described by a number of other names such as oriental cholangiohepatitis and hepatolithiasis. Although previously thought to be more common in the Far East, the prevalence of RPC is thought to be increasing in western countries.
Questions for Further Thought
1. True or false: Patients with RPC are at increased risk for cholangiocarcinoma?
2. What is the management of RPC?
View Answer
2. In the acute setting, management includes antibiotics, endoscopic or radiologic biliary drainage, and supportive care. Long-term management goals include removing ductal stones (e.g., via ERCP or surgically) and dilating strictured areas when possible. Depending on disease burden, surgical biliary exploration with stone clearance, choledochojejunostomy, or hepatic lobar resection may be required.
Reporting Responsibilities
1. Evaluate for acute complications such as intrahepatic abscess formation.
2. Describe the distribution of ductal dilatation and stricturing as well as the distribution of stones as this information will help guide long-term management (see answer 2 below).
What the Treating Physician Needs to Know
1. Patients with RPC are at increased risk for cholangiocarcinoma. Routine follow-up imaging should be considered for cholangiocarcinoma screening.
Answers
1. True. Patients with RPC are at increased risk for cholangiocarcinoma.
2. In the acute setting, management includes antibiotics, endoscopic or radiologic biliary drainage, and supportive care. Long-term management goals include removing ductal stones (e.g., via ERCP or surgically) and dilating strictured areas when possible. Depending on disease burden, surgical biliary exploration with stone clearance, choledochojejunostomy, or hepatic lobar resection may be required.
REFERENCES
1. Park M-S, Yu J-S, Kim KW, et al. Recurrent pyogenic cholangitis: comparison between MR cholangiography and direct cholangiography. Radiology 2002;220:677-682.
2. Catalano OA, Sahani DV, Forcione DG. Biliary infections: spectrum of imaging findings and management. Radiology 2009;29:2059-2080.
CASE 2.27 CLINICAL HISTORY 65-year-old man with worsening abdominal pain 2 days following laparoscopic cholecystectomy.
FINDINGS Contrast-enhanced CT demonstrates surgical clips in the gallbladder fossa along with fluid (A). Fluid is also present around the liver, stomach, and spleen (A, B). Hepatobiliary iminodiacetic acid (HIDA) scan demonstrates normal hepatic uptake of the radiopharmaceutical approximately 2 minutes post injection (C). In the 30-minute image (D), radiopharmaceutical is visible accumulating in the gallbladder fossa and also tracking around the liver including extending down the right paracolic gutter. In the 60-minute image (E), increased accumulation is seen along the right paracolic gutter.
DIFFERENTIAL DIAGNOSIS (BASED ON CT) Ascites, bile duct leak.
DIAGNOSIS Bile duct leak.
DISCUSSION Bile duct leaks occur in <1% of patients following laparoscopic cholecystectomy. The perihepatic fluid visible in the above CT images is nonspecific but is suspicious for a bile duct leak in an otherwise healthy patient following cholecystectomy. Ascites, for example due to liver disease, could have a similar appearance in the appropriate clinical setting. A small amount of fluid is not unexpected in the gallbladder fossa following cholecystectomy, but fluid should not be visible surrounding the liver and spleen in an otherwise healthy patient.
The above HIDA scan images are diagnostic of a bile duct leak as radiopharmaceutical is seen accumulating in the gallbladder fossa and tracking along the right paracolic gutter. Normally the radiopharmaceutical is excreted by the liver into the common bile duct and duodenum and then advances through the rest of the small and large intestines.
Bile duct leaks may be due to leakage of the cystic duct stump, injury to the hepatic duct, or to disruption of accessory hepatic ducts that drain from the liver parenchyma directly into the gallbladder. Such accessory ducts occur in 5% to 6% of individuals at autopsy and if identified during surgery are usually clipped.
Other complications of cholecystectomy include bile duct occlusion and gallstone spillage. Bile duct occlusion is usually due to accidental clipping of the main hepatic duct, right duct, or left duct during surgery. At imaging, such bile duct injuries typically appear “dry” manifesting as ductal dilatation without significant associated perihepatic fluid.
Question for Further Thought
1. What is the usual management of a bile leak?
View Answer
1. Bile leaks are often managed with CT- or US-guided placement of a drainage catheter into the fluid. A stent may be placed across the common bile duct via ERCP. Bile leaks usually stop with this type of minimally invasive approach.
Reporting Requirements
1. Based on the CT, report the presence of perihepatic fluid that is suspicious for a bile leak.
2. Suggest a HIDA scan or MR study with a hepatobiliary contrast agent and delayed imaging for further evaluation.
3. Evaluate for rim-enhancing fluid collection that could indicate abscess.
What the Treating Physician Needs to Know
1. This patient has generalized free fluid in the upper abdomen that is suspicious for a bile leak following cholecystectomy.
Answer
1. Bile leaks are often managed with CT- or US-guided placement of a drainage catheter into the fluid. A stent may be placed across the common bile duct via ERCP. Bile leaks usually stop with this type of minimally invasive approach.
REFERENCES
1. Duca S, Bala O, Al-Hajjar N, Iancu C, Puia IC, Munteanu D, Graur F. Laparoscopic cholecystectomy: incidence and complications. A retrospective analysis of 9542 consecutive laparoscopic operations. HPB 2003;5:152-158.
2. Ahmad F, Saunders RN, Lloyd GM, Lloyd DM, Robertson GSM. An algorithm for the management of bile leak following laparoscopic cholecystectomy. Ann R Coll Surg Engl 2007;89:5-56.
CASE 2.28 CLINICAL HISTORY 62-year-old woman with right upper quadrant pain.
FINDINGS CT images (A-C) demonstrate circumferential high attenuation material within the gallbladder wall. [D described in Discussion.]
DIFFERENTIAL DIAGNOSIS Cholelithiasis, porcelain gallbladder.
DIAGNOSIS Porcelain gallbladder.
DISCUSSION Determining whether calcification is located in the gallbladder wall or gallbladder lumen is a key to distinguishing between cholelithiasis and wall calcification (“porcelain gallbladder”). Gallbladder wall calcification is thought to result from chronic inflammation.
Historically, gallbladder wall calcification was thought to be highly associated with increased rates of gallbladder cancer. However, a relatively newer study1 of nearly 26,000 gallbladder specimens obtained over a 37-year period found a more nuanced relationship between gallbladder wall calcification and gallbladder cancer. Specifically, a 7% incidence of gallbladder cancer was found in patients with scattered wall calcification. By comparison, no gallbladder
cancers were identified in patients with a diffusely calcified gallbladder wall.
cancers were identified in patients with a diffusely calcified gallbladder wall.
Question for Further Thought
1. What is the wall echo shadow sign?
View Answer
1. The “wall echo shadow sign” is an US finding that can help distinguish a gallbladder full of stones from a gallbladder with a calcified wall. The wall echo shadow sign is visible in a gallbladder full of stones (not shown). Specifically, the echogenic wall is visible. Just deep to the wall is anechoic bile followed by echogenic gallstones and then shadowing behind the gallstones. By comparison, a calcified gallbladder wall will manifest as an echogenic shadowing wall at US as seen in image D.
Reporting Requirement
1. Describe the presence of diffuse gallbladder wall calcification.
What the Treating Physician Needs to Know
1. Historically, diffuse wall calcification was thought to be highly associated with gallbladder cancer. More recent literature suggests that scattered gallbladder wall calcification is associated with a slightly higher risk of gallbladder cancer, whereas diffuse gallbladder wall calcification is not.
Answer
1. The “wall echo shadow sign” is an US finding that can help distinguish a gallbladder full of stones from a gallbladder with a calcified wall. The wall echo shadow sign is visible in a gallbladder full of stones (not shown). Specifically, the echogenic wall is visible. Just deep to the wall is anechoic bile followed by echogenic gallstones and then shadowing behind the gallstones. By comparison, a calcified gallbladder wall will manifest as an echogenic shadowing wall at US as seen in image D.
REFERENCE
1. Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery 2001;129:699-703.
CASE 2.29 CLINICAL HISTORY 78-year-old diabetic patient with right upper quadrant pain.
FINDINGS Grayscale (A) and color Doppler (B) US images demonstrate increased echogenicity along the nondependent gallbladder wall. Note also ill-defined shadowing with internal echoes extending away from this echogenic area. Compare this shadowing to the well-defined anechoic shadowing extending posterior to the small echogenic gallstone seen in the dependent portion of the gallbladder (B). Noncontrast CT images demonstrate air in the gallbladder wall (C) and in the gallbladder lumen (D).
DIFFERENTIAL DIAGNOSIS Emphysematous cholecystitis, gallstone ileus, prior sphincterotomy.
DIAGNOSIS Emphysematous cholecystitis.
DISCUSSION Emphysematous cholecystitis is usually considered a surgical emergency and is characterized by infiltration of the gallbladder wall by gas-forming organisms such as Escherichia coli and Clostridium. Patients with diabetes mellitus are at increased risk for emphysematous cholecystitis.
Emphysematous cholecystitis is often due to narrowing or stenosis of the cystic artery. Rates of acalculous cholecystitis and perforation are more common in patients with
emphysematous when compared with nonemphysematous cholecystitis.
emphysematous when compared with nonemphysematous cholecystitis.
Air can also occur in the gallbladder lumen in patients who have undergone a prior sphincterotomy of the sphincter of Oddi and in patients with an enterobiliary fistula. An enterobiliary fistula may occur when a gallstone perforates into small bowel, sometimes with associated gallstone ileus.
Questions for Further Thought
1. Define clean shadowing.
View Answer
1. Clean shadowing is defined as well-defined, anechoic shadowing as seen behind the gallstone in the above case.
2. Define dirty shadowing.
View Answer
2. Dirty shadowing is characterized by ill-defined margins and areas of internal echoes within the shadowed area as seen behind the air in the gallbladder wall in the above case.
Reporting Requirements
1. Describe the presence of emphysematous cholecystitis.
2. Report this abnormality directly to the ordering clinician as it is considered a surgical emergency.
What the Treating Physician Needs to Know
1. This patient has emphysematous cholecystitis.
2. Emphysematous cholecystitis is a surgical emergency.
Answers
1. Clean shadowing is defined as well-defined, anechoic shadowing as seen behind the gallstone in the above case.
2. Dirty shadowing is characterized by ill-defined margins and areas of internal echoes within the shadowed area as seen behind the air in the gallbladder wall in the above case.
REFERENCES
1. Rosenthal SJ, Cox GG, Wetzel LH, Batnitzky S. Pitfalls and differential diagnosis in biliary sonography. Radiographics 1990;10:285-311.
2. Grayson DE, Abbott RM, Levy AD, Sherman PM. Emphysematous infections of the abdomen and pelvis: a pictorial review. Radiographics 2002;22:543-561.
CASE 2.30 CLINICAL HISTORY 64-year-old man with a history of melanoma, restaging examination.
FINDINGS Noncontrast CT (A) demonstrates a 1.5-cm structure in the gallbladder fundus and a 2-cm mass involving the musculature and soft tissue of the right anterolateral abdominal wall. Intravenous contrast-enhanced CT (B) demonstrates enhancement of the gallbladder mass and of the abdominal wall mass.
DIFFERENTIAL DIAGNOSIS Metastatic gallbladder cancer, other metastatic disease.
DIAGNOSIS Metastatic melanoma.
DISCUSSION The differential diagnosis of an enhancing gallbladder mass includes gallbladder cancer, polyp, and metastatic disease. The identification of an additional soft tissue mass in the abdominal wall narrows the differential diagnosis to gallbladder cancer with a metastasis or metastatic disease from a different primary tumor.
Melanoma accounts for approximately 50% of metastases to the gallbladder. Other tumors including renal cell cancer and hepatocellular cancer may also metastasize to the gallbladder. The gallbladder lesion in the above case and the abdominal wall lesion were both melanoma metastases.
Question for Further Thought
1. What is the most common site of hematogenous metastatic disease in patients with gallbladder cancer?
View Answer
1. The liver is the most common site of hematogenous metastatic disease in patients with gallbladder cancer. Hematogenous metastases may also occur in the lungs and the bones.
Reporting Requirement
1. Report the presence of gallbladder and anterior abdominal wall metastases.
What the Treating Physician Needs to Know
1. This patient has gallbladder and abdominal wall metastases.
Answer
1. The liver is the most common site of hematogenous metastatic disease in patients with gallbladder cancer. Hematogenous metastases may also occur in the lungs and the bones.
REFERENCE
1. Kim M-J, Kim KW, Kim H-C, et al. Unusual malignant tumors of the gallbladder. Am J Roentgenol 2006;187:473-480.
2. Levy AD, Murakata LA, Rohrmann CA Jr. Gallbladder carcinoma: radiologic-pathologic correlation. Radiographics 2001;21: 295-314.
CASE 2.31 CLINICAL HISTORY 55-year-old woman with right upper quadrant pain.
FINDINGS Grayscale US (A) demonstrates marked gallbladder wall thickening. Color Doppler imaging (B) demonstrates marked blood flow within the gallbladder wall. Noncontrast CT (C) also demonstrates abnormal gallbladder wall thickening. Contrast-enhanced CT (D) demonstrates marked gallbladder wall thickening which is avidly enhancing.
DIFFERENTIAL DIAGNOSIS Acute cholecystitis, gallbladder carcinoma.
DIAGNOSIS Gallbladder carcinoma.
DISCUSSION The abnormally thickened and avidly enhancing gallbladder wall in this case is diagnostic of gallbladder cancer. Other potential morphologies of gallbladder cancer are a large mass that obliterates the gallbladder and invades the liver or a polypoid mass in the gallbladder lumen.
Acutely inflamed gallbladders also appear thick-walled, but the wall is usually hypoattenuating and edematous in the setting of acute cholecystitis rather than avidly enhancing. Furthermore, pericholecystic inflammatory changes are often visible in patients with acute cholecystitis, and no adjacent inflammatory changes were visible in this case.
Gallbladder cancer survival is poor as many patients have advanced disease including hepatic invasion at time of diagnosis. Chronic inflammation due to gallstones is thought to increase the risk of gallbladder cancer. Scattered areas of gallbladder wall calcification are also associated with slightly higher rates of gallbladder cancer.
Questions for Further Thought
1. What is the usual treatment of gallbladder cancer?
View Answer
1. Cholecystectomy may be performed in patients whose tumors do not invade the perimuscular connective tissue. Cholecystectomy with en-bloc resection of the adjacent liver is the treatment of choice for tumor that reaches the perimuscular connective tissue.
2. What are the most common sites of metastatic disease for gallbladder cancer?
View Answer
2. Gallbladder cancer most commonly directly invades the liver or metastasizes to the liver via hematogenous spread. Other less commonly involved sites of disease include lung, bone, pancreas, kidneys, adrenal glands, and brain.
Reporting Requirements
1. Describe the presence of abnormal gallbladder wall thickening which is suspicious for gallbladder cancer.
2. Evaluate for evidence of direct hepatic extension.
3. Evaluate for evidence of porta hepatis or peripancreatic lymphadenopathy.
What the Treating Physician Needs to Know
1. This patient most likely has gallbladder cancer.
Answers
1. Cholecystectomy may be performed in patients whose tumors do not invade the perimuscular connective tissue. Cholecystectomy with en-bloc resection of the adjacent liver is the treatment of choice for tumor that reaches the perimuscular connective tissue.
2. Gallbladder cancer most commonly directly invades the liver or metastasizes to the liver via hematogenous spread. Other less commonly involved sites of disease include lung, bone, pancreas, kidneys, adrenal glands, and brain.
REFERENCE
1. Levy AD, Murakata LA, Rohrmann CA. Gallbladder carcinoma: radiologic-pathologic correlation. Radiographics 2001;21: 295-314.
CASE 2.32 CLINICAL HISTORY 53-year-old woman, history withheld.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree