Online Chapter 1
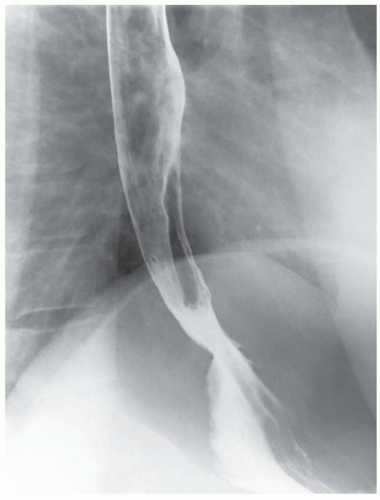
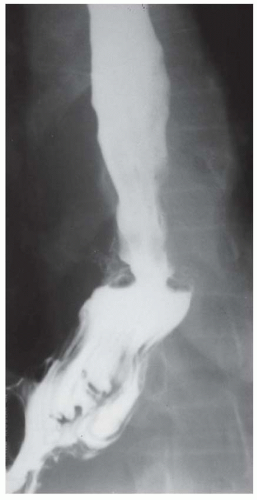
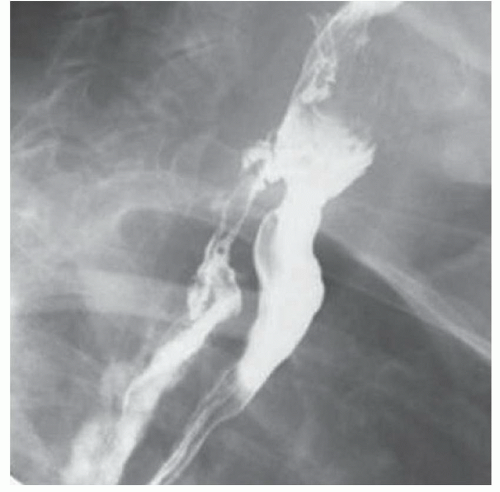
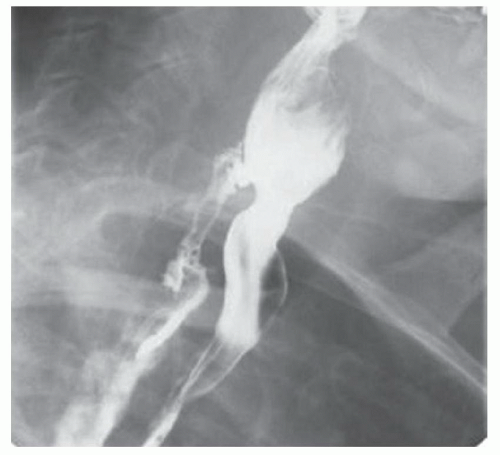
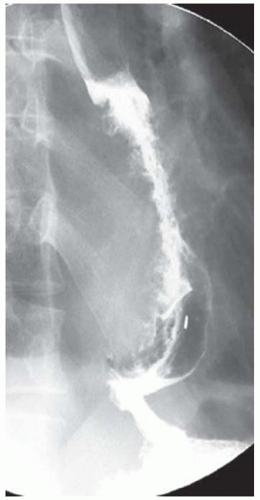
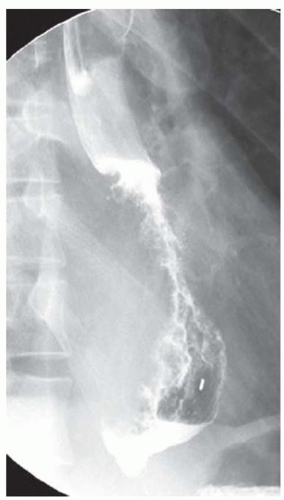
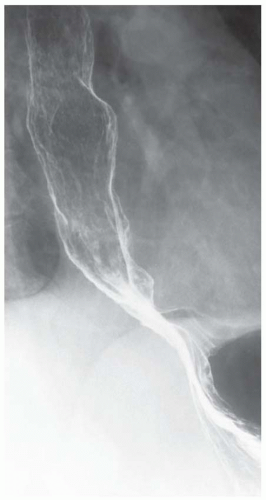
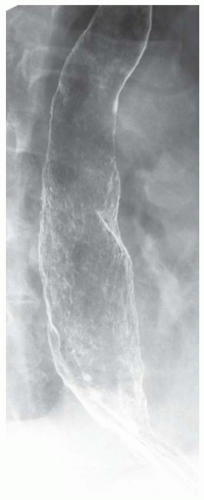
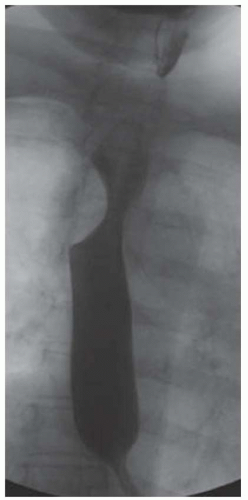
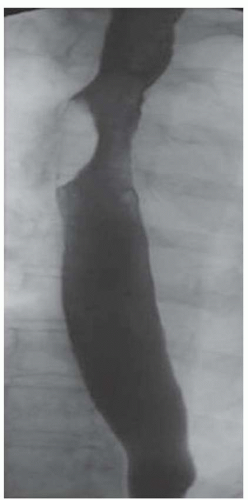
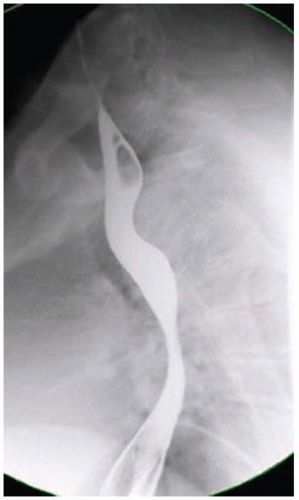
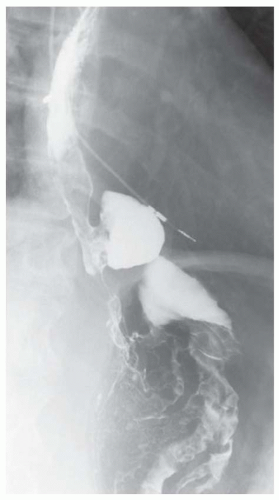
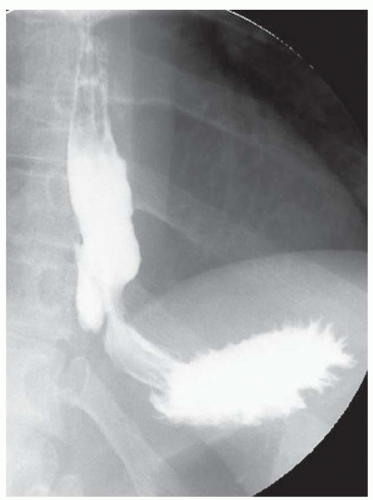
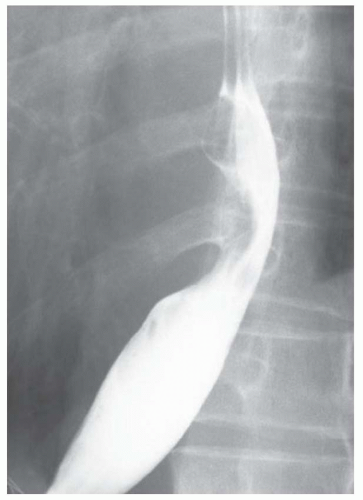
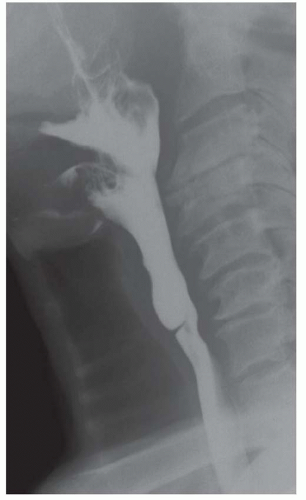
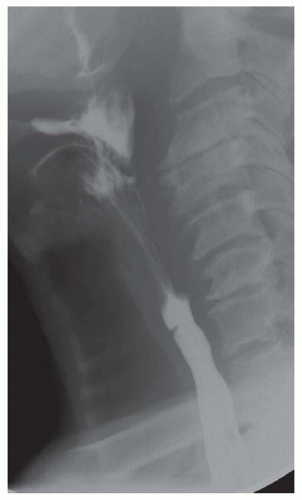
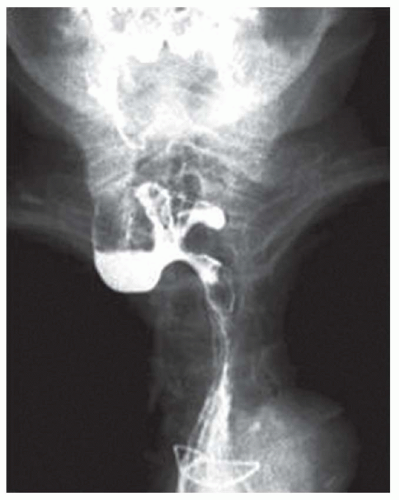
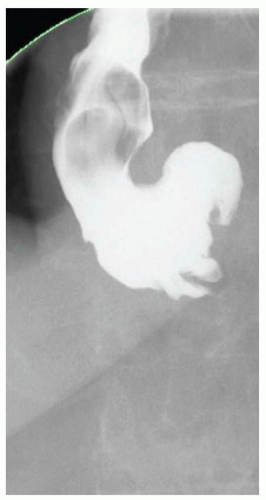
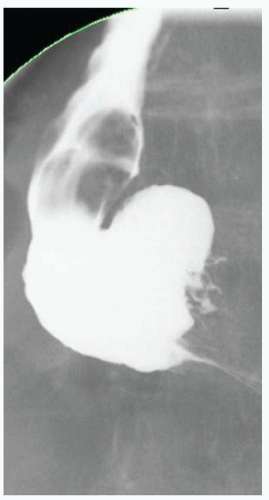
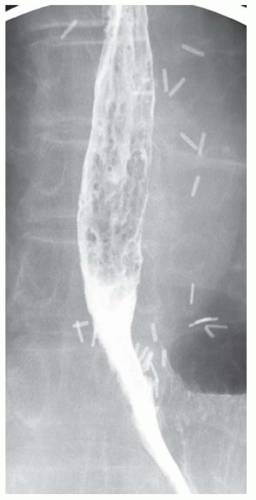
CASE 1.1 CLINICAL HISTORY 46-year-old man with dysphagia.
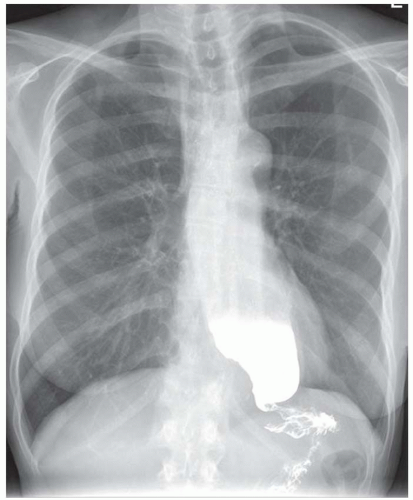
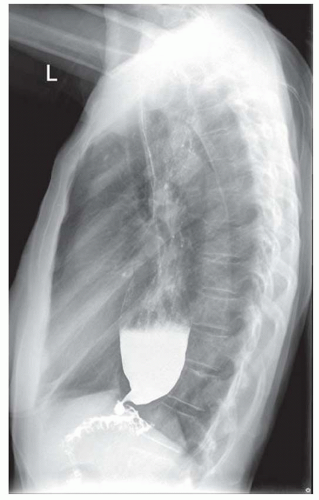
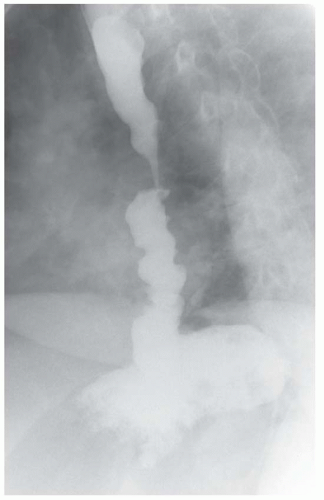
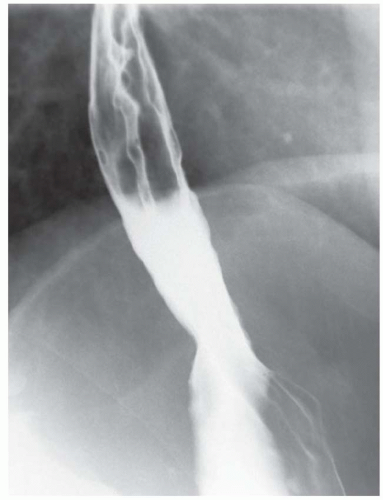
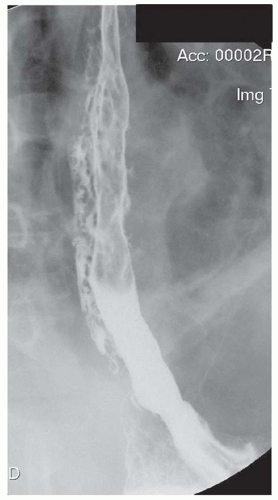
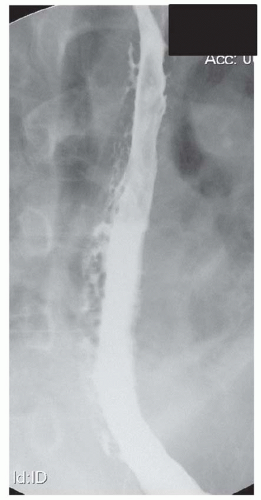
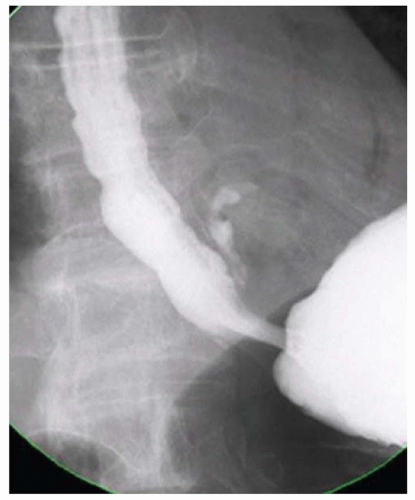
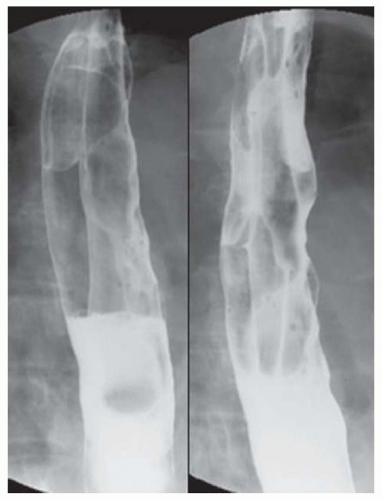
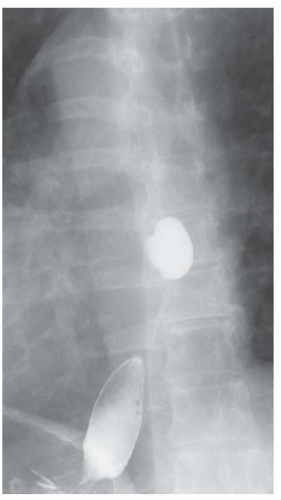
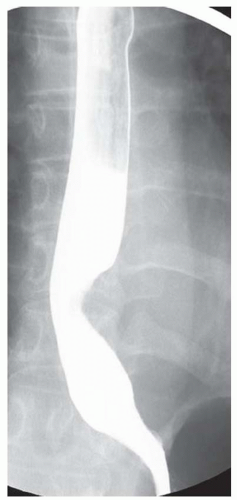
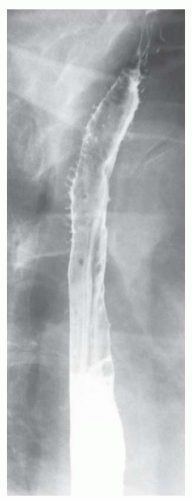
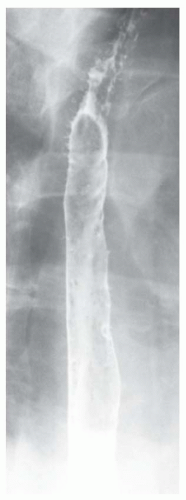
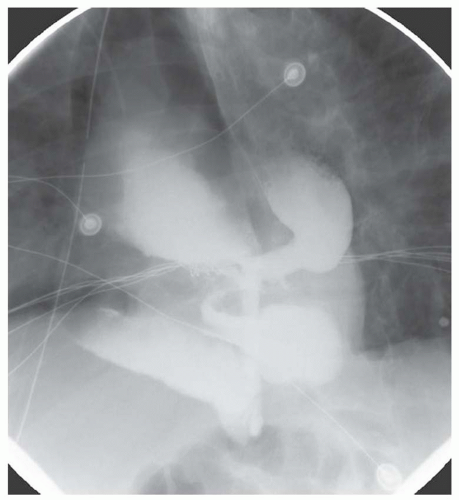
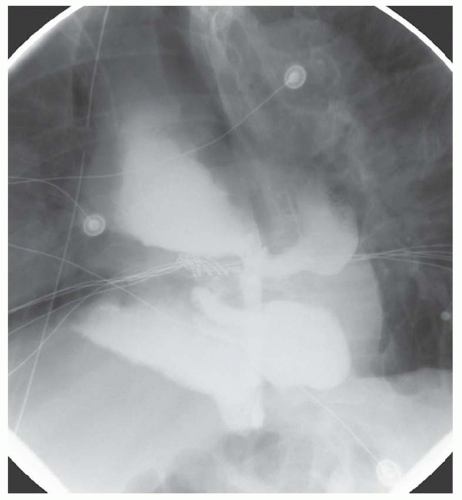
FINDINGS Frontal and lateral radiographs (A, B) obtained at the conclusion of a barium swallow demonstrate a dilated, patulous esophagus. Distally, the esophagus is narrowed. At real-time imaging, ingested contrast material appeared to slide down the walls of the esophagus without any real assistance from primary or secondary peristaltic waves. Ingested contrast material pooled in the distal esophagus and eventually slowly emptied into the stomach. At the conclusion of the study, a moderate amount of contrast material remained in the esophagus as seen in these radiographs.
DIFFERENTIAL DIAGNOSIS Achalasia, distal esophageal cancer.
DIAGNOSIS Achalasia.
DISCUSSION Achalasia is a primary esophageal motility disorder characterized by the failure of the lower esophageal sphincter to relax. At barium swallow, the characteristic “bird-beak” or narrowed appearance of the distal esophagus reflects this failure of the distal esophageal sphincter to relax. At initial presentation, all patients in whom achalasia is suspected should undergo upper endos-copy to exclude a distal esophageal mass causing a pseudoachalasia appearance.
Early in the disease process, achalasia may be misdiagnosed as an isolated distal esophageal stricture. As the disease progresses, increasingly impaired peristalsis is noted due to progressively impaired function of neural networks in the smooth muscle of the distal esophagus. Large-volume food debris may be present in the esophagus at the time of barium swallow even if the patient has not consumed any food by mouth for many hours prior to the study.
A variety of treatment options are available for patients with achalasia (please see Question 2). At some centers, timed barium swallow is performed as a way to evaluate esophageal clearance pre- and post-treatment. Patients ingest 100 to 200 mL of contrast material (volume ingested should be recorded and repeated in follow-up studies), and upright left posterior oblique images are obtained 1, 2, and 5 minutes post contrast ingestion. The percentage of contrast material cleared at 5 minutes is estimated by comparing the computed area of contrast material (width × height of esophageal contrast column) in the 1- and 5-minute films.
Questions for Further Thought
1. What is the etiology of achalasia?
View Answer
1. The etiology of achalasia is unknown. Theories include a genetic, autoimmune, or infectious trigger that incites an inflammatory process that damages the neural networks in esophageal smooth muscle.
2. What are current treatment options for achalasia patients?
View Answer
2. At the present time there is no way to reverse the nerve damage that produces achalasia. Current treatments are aimed at relieving symptoms. Myotomy (via open or laparoscopic approach) or endoscopic balloon dilatation may be performed to dilate a distal esophageal stricture. Botulinum toxin injection via endoscopy can relax the lower esophageal sphincter and provide short-term symptom palliation. Medical management including calcium channel blockers or long-acting nitrates can provide short-term symptomatic relief because of their muscle relaxation properties. The last line treatment option for patients with a megaesophagus who do not respond to other treatments is esophagectomy.
Reporting Requirements
1. Suggest the diagnosis of achalasia.
2. Recommend esophagogastroduodenoscopy (EGD) at initial presentation to rule out an obstructing mass resulting in pseudoachalasia.
What the Treating Physician Needs to Know
1. At initial presentation, achalasia can be difficult to distinguish from a distal esophageal malignant stricture.
2. At initial presentation, all patients with suspected achalasia based on barium swallow should undergo upper endoscopy to exclude a distal esophageal mass resulting in pseudoachalasia.
Answers
1. The etiology of achalasia is unknown. Theories include a genetic, autoimmune, or infectious trigger that incites an inflammatory process that damages the neural networks in esophageal smooth muscle.
2. At the present time there is no way to reverse the nerve damage that produces achalasia. Current treatments are aimed at relieving symptoms. Myotomy (via open or laparoscopic approach) or endoscopic balloon dilatation may be performed to dilate a distal esophageal stricture. Botulinum toxin injection via endoscopy can relax the lower esophageal sphincter and provide short-term symptom palliation. Medical management including calcium channel blockers or long-acting nitrates can provide short-term symptomatic relief because of their muscle relaxation properties. The last line treatment option for patients with a megaesophagus who do not respond to other treatments is esophagectomy.
REFERENCES
1. de Oliveira JMA, Birgisson S, Doinoff C, et al. Timed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasia. Am J Roentgenol 1997;169: 473-479.
2. Vaezi MF, Richter JE. Diagnosis and management of achalasia. Am J Gastroenterol 1999;94:3406-3412.
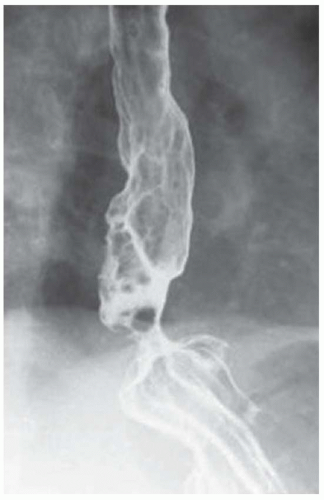
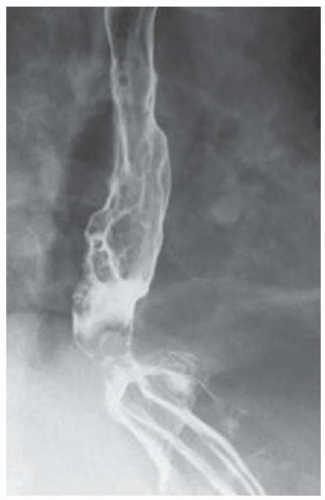
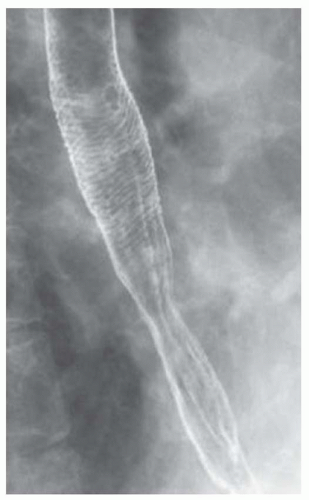
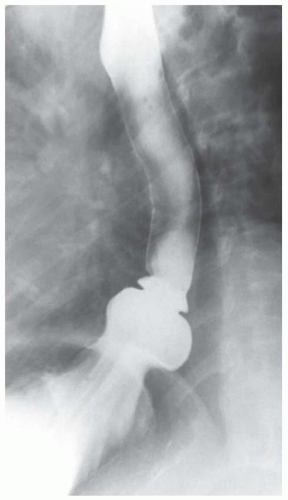
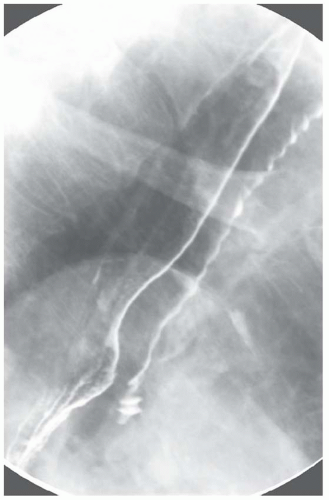
CASE 1.2 CLINICAL HISTORY 75-year-old woman with dysphagia.
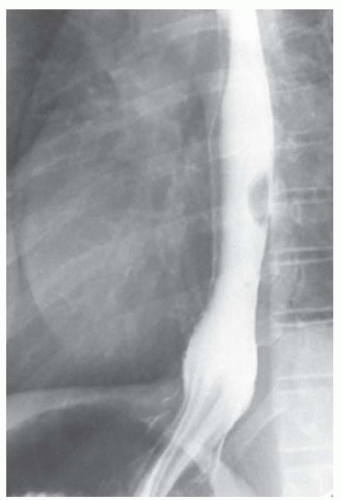
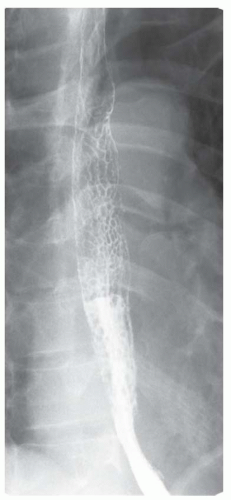
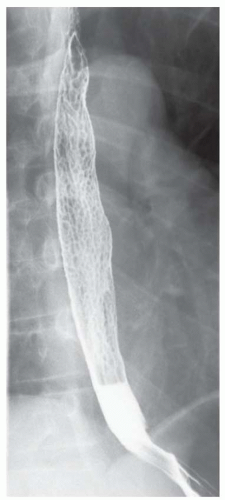
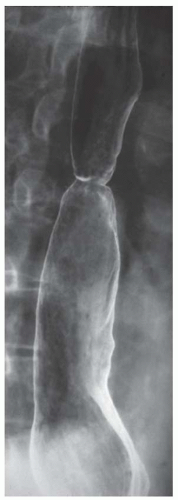
FINDINGS Barium swallow demonstrates intermittent, non-peristaltic esophageal contractions.
DIFFERENTIAL DIAGNOSIS Diffuse esophageal spasm (“corkscrew esophagus”), nutcracker esophagus, presbyesophagus.
DIAGNOSIS Diffuse esophageal spasm.
DISCUSSION Diffuse esophageal spasm refers to simultaneous, nonperistaltic contractions of multiple portions of the esophagus resulting in a corkscrew appearance at barium swallow as demonstrated above. Chest pain or dysphagia may be the primary symptom in patients with diffuse esophageal spasm.
By comparison, barium swallow is often normal in patients with nutcracker esophagus. Nutcracker esophagus is a motility disorder diagnosed at manometry. Isolated high-pressure esophageal contractions greater than 180 mmHg are diagnostic of nutcracker esophagus. Such high-pressure recordings are generally recorded in a portion of the esophagus rather than the entire esophagus as with diffuse esophageal spasm. Chest pain or dysphagia also may be the primary symptom in patients with nutcracker esophagus.
Presbyesophagus refers to abnormal esophageal motility, which is more frequently seen in older patients. “Presby-” is a prefix that comes from the Greek word for old age and is present in a number of medical terms, including presbyopia (age-related difficulty focusing the eye on near objects) and presbycusis (age-related hearing loss). Age-related changes in esophageal motility include a weakened or absent primary peristaltic wave, often with tertiary contractions. These age-related changes may be asymptomatic or result in dysphagia.
Questions for Further Thought
1. Define primary, secondary, and tertiary peristalsis.
View Answer
1. The primary peristaltic wave is triggered by swallowing and propels the food or liquid bolus down the esophagus. The secondary peristaltic wave is triggered by stretch receptors and propels the remaining bolus not cleared by the primary peristaltic wave. Tertiary contractions are uncoordinated, nonpropulsive contractions seen with increasing frequency in elderly patients.
2. Define primary motility disorder and secondary motility disorder.
View Answer
2. Primary motility disorders are defined as isolated esophageal abnormalities of primary, secondary, and/or tertiary peristalsis (e.g., achalasia). Secondary motility disorders are those that are related to other illness (e.g., diabetes or scleroderma). A motility disorder related to an obstructing or near-obstructing esophageal malignancy is usually referred to as a secondary motility disorder.
Reporting Requirement
1. Report the presence of intermittent diffuse esophageal spasm.
What the Treating Physician Needs to Know
1. Report whether episodes of diffuse esophageal spasm correlated with patient symptoms of chest pain or dysphagia during the study.
Answers
1. The primary peristaltic wave is triggered by swallowing and propels the food or liquid bolus down the esophagus. The secondary peristaltic wave is triggered by stretch receptors and propels the remaining bolus not cleared by the primary peristaltic wave. Tertiary contractions are uncoordinated, nonpropulsive contractions seen with increasing frequency in elderly patients.
2. Primary motility disorders are defined as isolated esophageal abnormalities of primary, secondary, and/or tertiary peristalsis (e.g., achalasia). Secondary motility disorders are those that are related to other illness (e.g., diabetes or scleroderma). A motility disorder related to an obstructing or near-obstructing esophageal malignancy is usually referred to as a secondary motility disorder.
REFERENCES
1. Prabhakar A, Levine MS, Rubesin S, Laufer I, Katzka D. Relationship between diffuse esophageal spasm and lower esophageal sphincter dysfunction on barium studies and manometry in 14 patients. Am J Roentgenol 2004;183:409-413.
2. Tack J, Vantrappen G. The aging oesophagus. Gut 1997;41: 422-424.
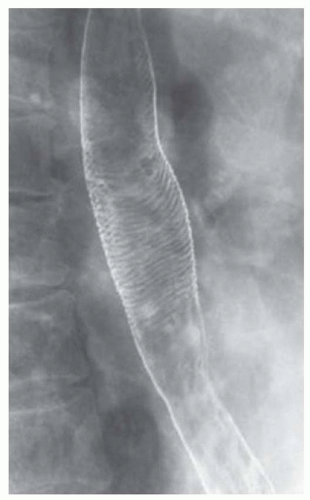
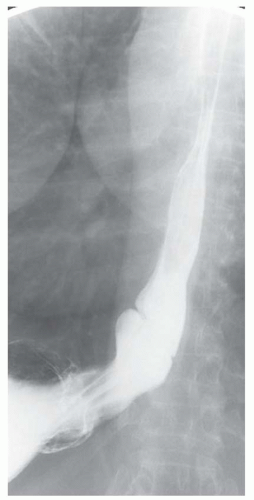
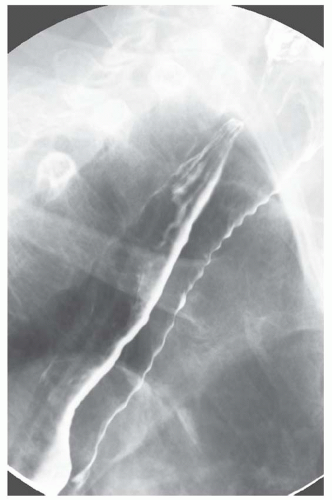
CASE 1.3 CLINICAL HISTORY 55-year-old man with dysphagia.
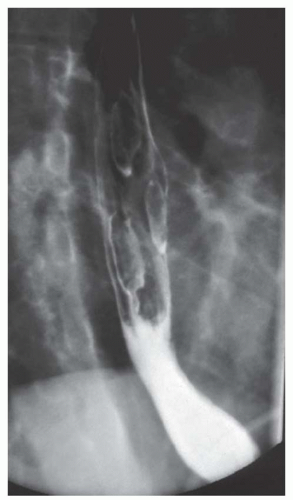
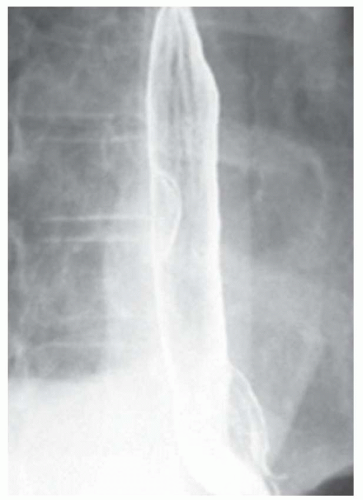
FINDINGS Air-contrast barium swallow (A, B) demonstrates linear, tubular filling defects running parallel to the longitudinal axis of the esophagus. These structures were intermittently visible during the barium swallow.
DIFFERENTIAL DIAGNOSIS Normal longitudinal folds, varices, varicoid esophageal cancer.
DIAGNOSIS Varices.
DISCUSSION Esophageal varices are dilated submucosal veins within the wall of the esophagus. Varices most commonly occur in patients with portal hypertension and are prone to bleeding. Bleeding from esophageal varices is the cause of death in approximately one-third of patients with chronic liver disease and portal hypertension.
Esophageal varices are graded based on their appearance at endoscopy. Grade 1 varices are small, straight varices. Grade 2 varices are large, tortuous varices that occupy less than one-third of the esophageal lumen. Grade 3 varices are large, tortuous varices that occupy more than one-third of the lumen.
Fluoroscopy is a relatively insensitive test for the demonstration of varices. Meticulous attention to technique is necessary to demonstrate varices as they may be intermittently visible during barium studies as in the above case. For example, full-column barium may efface varices rendering them invisible. Varices also may change caliber depending on intrathoracic pressure. Placing the patient in a prone, right anterior oblique position can aid in the demonstration of varices by (1) increasing variceal distention due to gravity effects and (2) improving mucosal coating by slowing the transit of barium. Valsalva maneuver may increase the conspicuity of varices. Single small sips of barium may improve detection of varices as repeated peristalsis may efface varices.
Varicoid esophageal cancer is usually more irregular in appearance and a more fixed abnormality when compared with esophageal varices. However, when in doubt contrast-enhanced computed tomography (CT) or magnetic resonance (MR) imaging is a noninvasive way to rule in or rule out most varices.
The collapsed esophagus often demonstrates longitudinal folds. However, these folds generally run the entire length
of the esophagus and can be distinguished from varices that are usually isolated to the upper third or lower third of the esophagus (see below).
of the esophagus and can be distinguished from varices that are usually isolated to the upper third or lower third of the esophagus (see below).
Questions for Further Thought
1. Define uphill varix.
View Answer
1. Uphill varices occur in patients with portal hypertension and are usually confined to the lower third of the esophagus. These collateral pathways allow blood to bypass the liver and return to the heart via enlarged esophageal collateral vessels and the superior vena cava.
2. Define downhill varix.
View Answer
2. Downhill varices occur in patients with superior vena cava obstruction and are usually located in the upper third or middle third of the esophagus. These dilated esophageal collateral vessels allow blood to return to the heart via the portal venous system and the inferior vena cava.
Reporting Requirement
1. Report the presence of probable esophageal varices along the distal esophagus.
What the Treating Physician Needs to Know
1. Endoscopy is the gold standard test for diagnosing and grading varices.
2. CT and MR are more sensitive than barium swallow for the identification of varices.
Answers
1. Uphill varices occur in patients with portal hypertension and are usually confined to the lower third of the esophagus. These collateral pathways allow blood to bypass the liver and return to the heart via enlarged esophageal collateral vessels and the superior vena cava.
2. Downhill varices occur in patients with superior vena cava obstruction and are usually located in the upper third or middle third of the esophagus. These dilated esophageal collateral vessels allow blood to return to the heart via the portal venous system and the inferior vena cava.
REFERENCES
1. Levine MS, Rubesin SE. Diseases of the esophagus: diagnosis with esophagography. Radiology 2005;237:414-427.
2. de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis 2001;5:645-663.
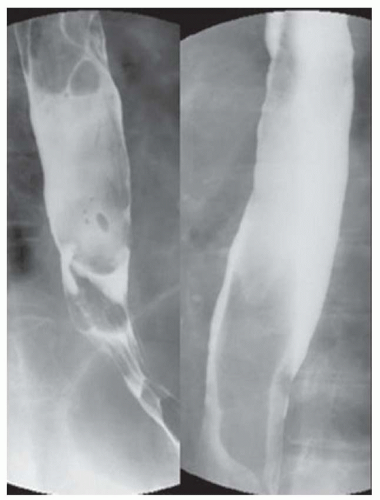
CASE 1.4 CLINICAL HISTORY 67-year-old man with dysphagia.
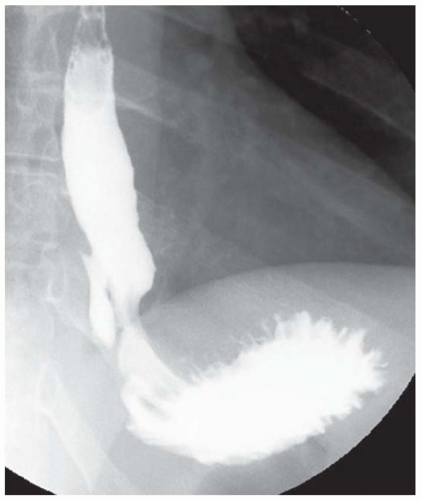
FINDINGS Image from a barium swallow demonstrates an approximately 5 cm in length area of circumferential narrowing of the distal esophagus extending to the level of the esophagogastric junction and also involving the proximal stomach. The esophageal mucosa is smooth.
DIFFERENTIAL DIAGNOSIS Esophageal carcinoma, submucosal mass, extrinsic compression.
DIAGNOSIS Submucosal mass (lymphoma based on EUS-guided biopsy).
DISCUSSION The smooth appearance of the mucosa in this case makes esophageal carcinoma unlikely. The differential diagnosis in this case is therefore narrowed to a submucosal mass or an area of extrinsic compression. In the present case, the patient underwent EUS with tissue sampling, and a diagnosis of esophageal lymphoma (a submucosal process) was confirmed. CT imaging to evaluate for an extraesophageal mass resulting in extrinsic compression would also have been an appropriate next step.
Primary esophageal lymphoma is very rare. The esophagus is the least commonly involved portion of the GI tract in patients with extranodal GI tract lymphoma. The majority of lymphomas involving the GI tract are non-Hodgkin lymphomas.
Esophageal lymphoid tissue is located in both the mucosal layer of the esophagus and the submucosal layer. Primary lymphomatous involvement of the esophagus therefore may manifest as an area of ulceration, a polypoid mass, or as an area of submucosal nodularity or thickening as in the above case.
Question for Further Thought
1. What location in the GI tract is most commonly involved by extranodal lymphoma?
Reporting Requirements
1. Describe the location and extent of the abnormality.
2. Suggest EUS with possible tissue sampling or CT for further evaluation.
What the Treating Physician Needs to Know
1. This patient has an abnormal, mass-like area of distal esophageal narrowing which is suspicious for malignancy.
2. EUS with tissue sampling should be considered to establish the diagnosis.
3. Alternatively, CT could be performed to evaluate for a mediastinal mass resulting in extrinsic compression.
Answer
1. The stomach is the most common location for extranodal primary GI lymphoma.
REFERENCE
1. Levine MS, Rubesin SE, Pantongrag-Brown L, Buck JL, Herlinger H. Non-Hodgkin’s lymphoma of the gastrointestinal tract: radiographic findings. Am J Roentgenol 1997;168:165-172.
CASE 1.5 CLINICAL HISTORY 65-year-old woman, history withheld.
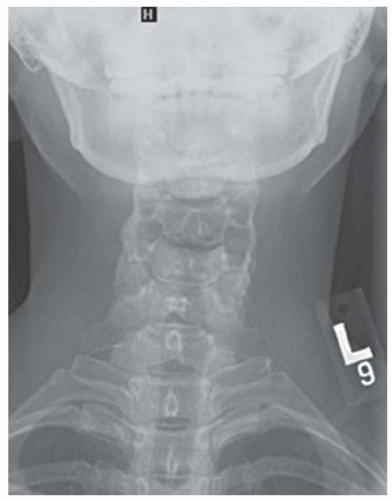
FINDINGS Images (A, B) from a swallow study demonstrate fixed narrowing of the cervical esophagus just above the thoracic inlet. Just above this level of narrowing, contrast material is seen extending posteriorly from the esophagus as well as within the esophageal lumen.
DIAGNOSIS Esophageal perforation.
DISCUSSION This patient sustained an esophageal perforation during attempted endoscopic stricture dilation. Clues to the diagnosis of an esophageal perforation include (1) continued accumulation of contrast material at the site of perforation and (2) absence of peristalsis of the leaked contrast material.
If esophageal perforation is suspected, water-soluble contrast material should initially be administered as water-soluble contrast material will be resorbed if it leaks into the mediastinum. By comparison, leaked barium will not be resorbed and can result in a mediastinitis.
The water-soluble study was positive for leak in this patient. In situations where the initial swallows with water-soluble contrast material are negative and a large leak is excluded, the patient should then ingest barium. Barium is more conspicuous at fluoroscopy and may better demonstrate small leaks. Additionally, as barium is less foul-tasting, patients may be able to swallow larger gulps of barium thereby resulting in increased esophageal distention and better demonstration of a leak. The study should immediately be terminated as soon as any leaked barium is confidently identified.
Question for Further Thought
1. What contrast agent should be used in patients with suspected aspiration or a tracheoesophageal fistula?
View Answer
1. Small sips of barium should be used in patients with suspected aspiration or a tracheoesophageal fistula. Water-soluble contrast material should be avoided as aspiration of water-soluble contrast material can result in pneumonitis. If a patient is observed to aspirate barium, he/she should be instructed to cough up as much as possible as aspiration of large amounts of barium can also result in a severe pneumonitis, especially in debilitated elderly patients.
Reporting Requirements
1. Describe the presence of a leak arising from the proximal cervical esophagus.
2. Directly communicate these findings to the treating physician.
What the Treating Physician Needs to Know
1. The location of the leak.
2. The subjective size of the leak.
3. If the patient has a drain in place, report whether the drain is adequately draining the leaked contrast material.
Answer
1. Small sips of barium should be used in patients with suspected aspiration or a tracheoesophageal fistula. Water-soluble contrast material should be avoided as aspiration of water-soluble contrast material can result in pneumonitis. If a patient is observed to aspirate barium, he/she should be instructed to cough up as much as possible as aspiration of large amounts of barium can also result in a severe pneumonitis, especially in debilitated elderly patients.
REFERENCE
1. Franquet T, Gimenez A, Roson N, Torrubia S, Sabate JM, Perez C. Aspiration diseases: findings, pitfalls, and differential diagnosis. Radiographics 2000;20:873-685.
CASE 1.6 CLINICAL HISTORY 66-year-old woman with dysphagia.
FINDINGS Air-contrast barium swallow demonstrates a large, approximately 7-cm segmental area of luminal narrowing and mucosal irregularity involving the distal third of the esophagus (A, B).
DIFFERENTIAL DIAGNOSIS Neoplasm, severe inflammation caused by gastroesophageal reflux disease (GERD).
DIAGNOSIS Neoplasm (adenocarcinoma).
DISCUSSION An estimated 17,990 new cases of esophageal cancer were diagnosed in the United States in 2012, and 15,210 individuals died from the disease. In the United States, adenocarcinoma is the most common esophageal cancer subtype, the distal esophagus is the most common location, and GERD is the most common risk factor. Worldwide, squamous cell carcinoma is the most common subtype, the upper esophagus is the most common location, and alcohol and tobacco use are the major risk factors.
GERD results in chronic esophageal irritation and inflammation which can progress to esophageal metaplasia (also known as Barrett esophagus) and eventually to esophageal cancer. Patients with esophageal cancer often present with dysphagia (difficulty swallowing) and odynophagia (painful swallowing). Raspy cough may also occur if the tumor involves the recurrent laryngeal nerve.
Tumors are generally large by the time they become symptomatic which accounts for the poor prognosis of patients with esophageal cancer. Additionally, the esophagus lacks a serosal layer which normally serves as a barrier to extension of disease elsewhere in the gastrointestinal (GI) tract. Chemoradiation followed by esophagogastrectomy is the usual treatment for distal esophageal cancers. Palliative treatment includes the placement of metallic stents to palliate obstructing lesions.
The hallmark of esophageal malignancy at barium swallow is mucosal irregularity. Mucosal irregularity distinguishes mucosal lesions from submucosal and extrinsic lesions. Compare the mucosal irregularity seen in this case with the predominantly smooth appearance of the mucosa seen with the submucosal masses in other cases in this volume. Esophageal cancers can also appear as plaque-like or polypoid masses.
Question for Further Thought
1. What methods are used to stage esophageal cancer?
View Answer
1. Endoscopic ultrasound (EUS) is used to determine the local extent of disease and sometimes nodal disease. CT and positron emission tomography (PET)/CT are also used to evaluate for nodal disease and to evaluate for metastases (e.g., liver and lung).
Reporting Requirements
1. Describe the location of the abnormality.
2. Recommend EGD and tissue sampling to determine the diagnosis.
What the Treating Physician Needs to Know
1. This patient has a large esophageal mass that is highly suspicious for malignancy.
2. Further evaluation is required with tissue sampling.
3. Whether the mass is obstructing based on the transit of liquid barium and the 12.5-mm compressed barium tablet.
Answer
1. Endoscopic ultrasound (EUS) is used to determine the local extent of disease and sometimes nodal disease. CT and positron emission tomography (PET)/CT are also used to evaluate for nodal disease and to evaluate for metastases (e.g., liver and lung).
REFERENCES
1. www.cancer.gov. Accessed March 5, 2013.
2. Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825-831.
3. Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet 2009;373: 850-861.
CASE 1.7 CLINICAL HISTORY 53-year-old woman with dysphagia.
FINDINGS Air-contrast barium swallow (A, B) demonstrates an approximately 4-cm segmental plaque-like area of wall thickening and mucosal irregularity involving the distal esophagus.
DIFFERENTIAL DIAGNOSIS Neoplasm, severe inflammation caused by GERD.
DIAGNOSIS Neoplasm (adenocarcinoma).
DISCUSSION The mucosal irregularity seen in this case indicates that this is a mucosal process. This abnormality is compatible with esophageal cancer, and EGD is needed for tissue confirmation. Distal esophageal cancers are most commonly adenocarcinomas. GERD is a major risk factor.
Staging of esophageal cancer often involves endoscopy, CT or PET/CT studies to evaluate local disease extent, nodal stations, and for the presence of distant metastatic disease. Since the esophagus lacks a serosal surface, there is not a significant barrier to prevent esophageal malignancies from directly extending into adjacent structures including the tracheobronchial tree and lung.
The esophagus contains an extensive lymphatic system with bidirectional flow and is unusual in its lymphatic drainage as the esophagus is drained by nodal stations located both above and below the diaphragm. The lymphatics of the upper third of the esophagus, in general, drain craniad with involvement of nodal stations including internal jugular and supraclavicular stations. The lymphatics of the distal third of the esophagus, in general, drain below the diaphragm to involve gastrohepatic ligament and celiac lymph nodes. However, because of bidirectional flow in the extensive esophageal lymphatic plexus distal cancers may result in supraclavicular lymph node disease and vice versa.
Location of hematogeneous metastases in decreasing order of frequency are as follows: liver, lungs, bones, adrenal glands, kidneys, and brain.
Questions for Further Thought
1. Describe the different morphologies of esophageal cancer seen at barium swallow.
View Answer
1. Esophageal cancers may appear as areas of mucosal irregularity, plaque-like lesions, or polypoid lesions.
2. What modality is recommended to determine the depth of tumor invasion?
View Answer
2. Endoscopic ultrasound (EUS) is used to determine the depth of tumor invasion and is often used to evaluate for disease in regional lymph nodes.
Reporting Requirements
1. Describe the location of the abnormality.
2. Recommend EGD and tissue sampling to determine the diagnosis.
What the Treating Physician Needs to Know
1. This patient has a large esophageal mass that is highly suspicious for malignancy.
2. Further evaluation is required with tissue sampling.
Answers
1. Esophageal cancers may appear as areas of mucosal irregularity, plaque-like lesions, or polypoid lesions.
2. Endoscopic ultrasound (EUS) is used to determine the depth of tumor invasion and is often used to evaluate for disease in regional lymph nodes.
REFERENCES
1. www.cancer.gov. Accessed March 5, 2013.
2. Levine MS, Rubesin SE. Diseases of the esophagus: diagnosis with esophagography. Radiology 2005;237:414-427.
3. Sharma A, Fidias P, Hayman LA, Loomis SL, Taber KH, Aquino SL. Patterns of lymphadenopathy in thoracic malignancies. Radiographics 2004;24:419-434.
CASE 1.8 CLINICAL HISTORY 68-year-old man with history of GERD.
FINDINGS Air-contrast barium swallow (A, B) demonstrates an abnormal appearance of the distal esophageal mucosa with rounded and linear crevices filled with contrast material.
DIFFERENTIAL DIAGNOSIS Barrett esophagus, esophageal adenocarcinoma.
DIAGNOSIS Barrett esophagus.
DISCUSSION At fluoroscopy, a reticular mucosal pattern with barium-filling thin grooves or crevices is a highly specific but insensitive sign of Barrett esophagus. Barrett esophagus is defined as metaplasia with columnar epithelium replacing the usual esophageal squamous epithelium. The squamocolumnar junction is displaced proximally in patients with Barrett esophagus. Diagnosis is made based on mucosal biopsies at EGD.
Barrett esophagus is considered to be a premalignant condition. This abnormality is found in approximately 10% to 15% of adults with GERD who undergo endoscopy. Barrett esophagus is found in the majority of patients with esophageal and gastroesophageal cancers in the United States.
Most major GI societies recommend endoscopic surveillance of patients with Barrett esophagus at 1- to 3-year intervals to include detailed inspection of the esophageal mucosa and systematic biopsies. Treatment of Barrett esophagus includes medical management of GERD and antireflux surgery (e.g., hiatal hernia reduction and Nissen fundoplication) for patients who do not respond to medical management.
Barrett esophagus is named after Norman Barrett (1903 to 1979), a thoracic surgeon who described the condition in 1950.
Questions for Further Thought
1. What percentage of patients with reflux esophagitis develop Barrett esophagus?
View Answer
1. Approximately 10% of patients with reflux esophagitis will develop Barrett esophagus.
2. What percentage of patients with Barrett esophagus develop esophageal cancer?
View Answer
2. Roughly 0.5% of patients with Barrett esophagus per year develop esophageal adenocarcinoma.
Reporting Requirements
1. Describe the location and approximate length of disease.
2. Recommend that EGD and possibly tissue sampling be performed.
What the Treating Physician Needs to Know
1. EGD with tissue sampling is needed to confirm the diagnosis of Barrett esophagus.
Answers
1. Approximately 10% of patients with reflux esophagitis will develop Barrett esophagus.
2. Roughly 0.5% of patients with Barrett esophagus per year develop esophageal adenocarcinoma.
REFERENCES
1. Levine MS, Rubesin SE. Diseases of the esophagus: diagnosis with esophagography. Radiology 2005;237:414-427.
2. Sharma P. Barrett’s esophagus. N Engl J Med 2009;361: 2548-2556.
CASE 1.9 CLINICAL HISTORY 63-year-old woman with dysphagia.
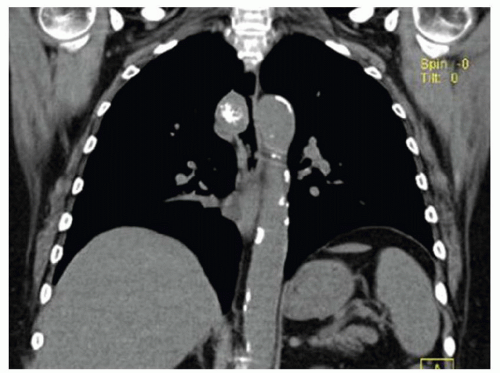
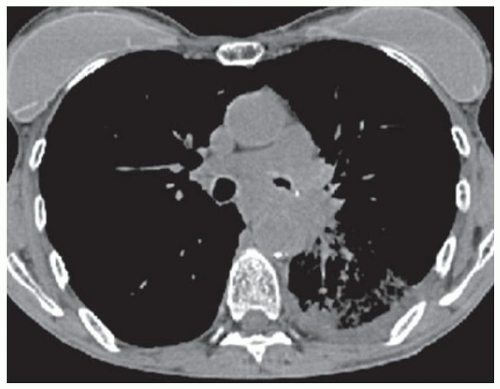
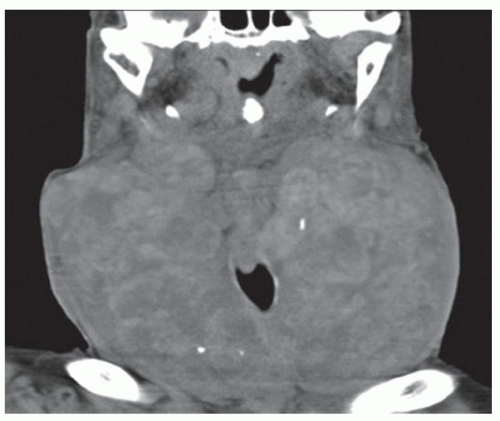
FINDINGS Fluoroscopic images from a single-contrast barium swallow (A, B) demonstrate mass effect on the proximal esophagus resulting in greater than 50% luminal narrowing. The area of mass effect has smooth margins. Coronal reformation from a noncontrast CT examination (C) demonstrates that the area of mass effect is from a calcified mediastinal lymph node.
DIFFERENTIAL DIAGNOSIS OF A SMOOTH, INTRAMURAL,\\ OR EXTRINSIC ESOPHAGEAL MASS Mediastinal lymph node, -omas (lipoma, leiomyoma, neuroma, fibroma, and neurofibroma), enteric duplication cyst, vascular impression.
DIAGNOSIS Calcified mediastinal lymph node.
DISCUSSION The smooth appearance of the mucosa in the above case is characteristic of a submucosal mass or extrinsic process. The appearance of such masses is that of a “marble under a rug.” When an intramural or extrinsic mass is suspected, CT is helpful to distinguish between lymph nodes (as in this case), other solid masses, vascular structures, or fatty masses such as lipomas.
Additionally, EUS rather than basic EGD is usually performed when tissue sampling of a submucosal or extrinsic process is necessary. With EUS the endoscopist can rule out any large vascular structures in the needle trajectory prior to sampling. Additionally, submucosal or extrinsic processes with an overlying smooth mucosa may not be detectable with basic EGD which provides direct visualization of the mucosal layer only.
Question for Further Thought
1. Define dysphagia, dysphasia, and odynophagia.
View Answer
1. Dysphagia = difficulty swallowing; dysphasia = difficulty using or understanding language due to a brain injury; odynophagia = painful swallowing.
Reporting Requirements
1. Describe the area of narrowing and estimate the degree of luminal narrowing.
2. Suggest that the mass may be submucosal or extrinsic and suggest CT as the next step for diagnosis.
3. Report whether patient’s symptoms are reproduced when a 12.5-mm compressed barium tablet lodges at or traverses the area of narrowing.
What the Treating Physician Needs to Know
1. A submucosal or extrinsic mass is suspected.
2. Suggested next steps for diagnosis (e.g., CT for an extrinsic mass, EUS for a submucosal or extrinsic mass, and EGD for a mucosal mass).
Answer
1. Dysphagia = difficulty swallowing; dysphasia = difficulty using or understanding language due to a brain injury; odynophagia = painful swallowing.
REFERENCE
1. Levine MS, Rubesin SE. Diseases of the esophagus: diagnosis with esophagography. Radiology 2005;237:414-427.
CASE 1.10 CLINICAL HISTORY 57-year-old man with no known past medical history presents with chest pain.
FINDINGS Air-contrast barium swallow demonstrates extensive mucosal irregularity of the mid- and distal esophagus with extensive ulcerations (A, B).
DIFFERENTIAL DIAGNOSIS Candida esophagitis, reflux esophagitis, Crohn esophagitis.
DIAGNOSIS Candida esophagitis.
DISCUSSION Candida albicans is the most common etiology of infectious esophagitis. The earliest manifestation of Candida esophagitis is esophageal dysmotility. The esophageal mucosa may appear normal at barium swallow early in the disease. As esophageal candidiasis worsens in severity, mucosal plaques may be visible followed by erosions and ulcerations. The above image is from a patient with very advanced disease. Crohn esophagitis could have a similar appearance, and EGD with biopsy was required to confirm the diagnosis.
Patients who develop candidiasis usually have a predisposing condition including immunosuppression (e.g., due to human immunodeficiency virus [HIV], steroids, chemotherapy, or transplant patients) or patients who have delayed esophageal emptying (e.g., due to esophageal strictures, scleroderma, or achalasia). Treatment of esophageal candidiasis is with fluconazole or itraconazole.
Question for Further Thought
1. What would be the next step to establish the diagnosis?
View Answer
1. Endoscopy with possible tissue sampling would be the next step to establish the diagnosis.
Reporting Requirements
1. Describe the location of the mucosal abnormality.
2. Suggest EGD for further evaluation and possible tissue sampling.
What the Treating Physician Needs to Know
1. The imaging findings, though markedly abnormal, are nonspecific and EGD is needed for definitive diagnosis.
Answer
1. Endoscopy with possible tissue sampling would be the next step to establish the diagnosis.
REFERENCES
1. Roberts L, Gibbons R, Gibbons G, et al. Adult esophageal candidiasis: a radiographic spectrum. Radiographics 1987;7: 289-307.
2. Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis 2004;38:161-189.
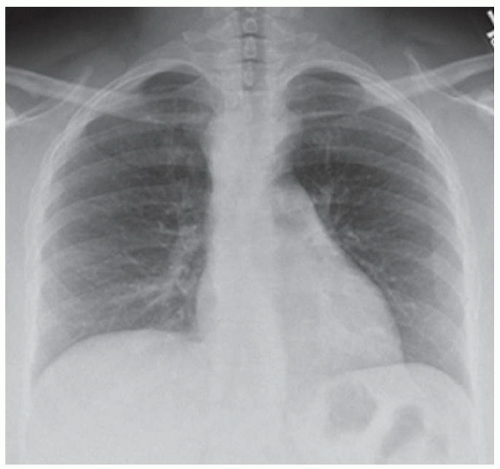
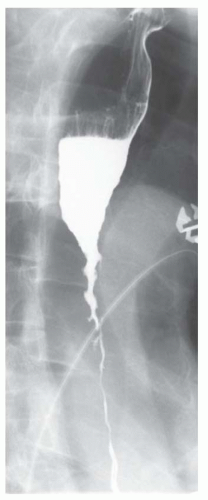
CASE 1.11 CLINICAL HISTORY 72-year-old man with chest pain following vomiting.
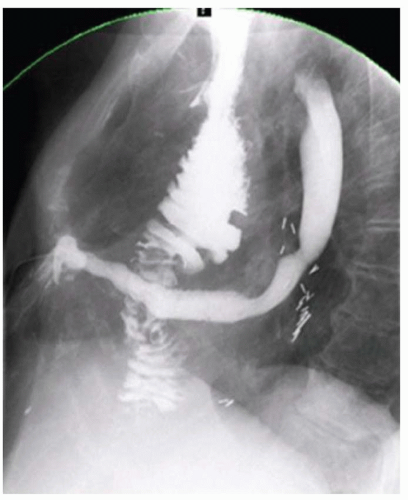
FINDINGS Initial chest radiograph (A) obtained in the emergency department demonstrates a retrocardiac air-fluid level. Differential diagnosis for this finding includes air-fluid level in a hiatal hernia versus in the pleural space due to esophageal rupture. A chest CT was performed (not shown) which demonstrated pneumomediastinum along with fluid and air in the left pleural space. Given CT findings and clinical history, there was a high level of concern for esophageal rupture. The patient underwent a swallow study which showed a somewhat linear collection of contrast material arising from and extending away from the left side of the distal esophagus (B).
DIFFERENTIAL DIAGNOSIS Boerhaave syndrome, Mallory-Weiss tear.
DIAGNOSIS Boerhaave syndrome.
DISCUSSION Boerhaave syndrome is defined as rupture of the esophageal wall due to vomiting. A proposed mechanism is failure of normal relaxation of the cricopharyngeus muscle during vomiting leading to increased intraesophageal pressure and rupture. The resultant esophageal tear is usually oriented longitudinally, 1 to 4 cm in length, and located in the left lateral wall of the distal esophagus just proximal to the esophagogastric junction.
At plain film, pneumomediastinum, left pleural fluid, and subcutaneous emphysema may be seen. These same findings are also visible at chest CT. In the appropriate clinical setting, these findings are highly suggestive of Boerhaave syndrome. Esophagography is the definitive imaging test. Treatment includes surgical repair and antibiotic therapy. Untreated, mortality is assumed to be 100%. With treatment, mortality rates are reduced to <20%.
A Mallory-Weiss tear differs from Boerhaave syndrome as a Mallory-Weiss tear only involves the esophageal mucosa. In other words, with a Mallory-Weiss tear no esophageal leak will be seen at fluoroscopy. Mallory-Weiss tears are difficult to diagnose with esophagography. Like the esophageal perforations associated with Boerhaave syndrome, Mallory-Weiss tears usually involve the distal esophagus.
Boerhaave syndrome is named after physician Hermann Boerhaave (1668 to 1783) based on his detailed description of the condition in a 1724 manuscript.
Questions for Further Thought
1. What contrast agent(s) should be used to evaluate for a possible esophageal rupture?
View Answer
1. Water-soluble contrast material should be used initially, after confirming that the patient is not allergic to iodine. Rationale for beginning with water-soluble contrast material is that if barium leaks into the mediastinum it will not be resorbed and may result in mediastinitis. By comparison, water-soluble contrast material will be resorbed if it leaks into the mediastinum. The goal is to first exclude a large leak with water-soluble contrast material.
If the initial water-soluble contrast material images are negative for leak (as in this case, images not shown), the patient should drink thin barium. Rationale is that barium (as in this case) sometimes demonstrates leaks that are not seen with water-soluble contrast material. One theory for why barium is more effective than water-soluble contrast material for demonstrating leaks is that patients will take larger gulps of barium as it is less foul-tasting than water-soluble contrast material. These larger gulps of barium distend the esophagus more than small sips of water-soluble contrast material and may demonstrate a leak not visible with water-soluble contrast material. Additionally, barium is denser than water-soluble contrast material and is therefore better seen at fluoroscopy.
2. What contrast agent(s) should be used to evaluate for possible aspiration?
View Answer
2. If concern is for aspiration, the patient should consume small sips of thin barium. Rationale is that aspiration of water-soluble contrast material can result in a severe pneumonitis or pulmonary edema, whereas barium is more inert. If the patient is observed to aspirate barium, the patient should be instructed to cough up as much as possible.
Reporting Requirements
1. Immediately contact the clinical team regarding the chest radiograph to convey concern regarding possible air in the pleural space indicating an esophageal perforation. Chest CT is a reasonable recommendation following the chest radiograph to confirm that the retrocardiac air is in the pleural space rather than a hiatal hernia.
2. Immediately contact the clinical team following the esophagram to inform them of the esophageal rupture.
What the Treating Physician Needs to Know
1. This patient has a perforated esophagus.
2. Cardiothoracic surgery (or thoracic surgery) consultation is needed.
Answers
1. Water-soluble contrast material should be used initially, after confirming that the patient is not allergic to iodine. Rationale for beginning with water-soluble contrast material is that if barium leaks into the mediastinum it will not be resorbed and may result in mediastinitis. By comparison, water-soluble contrast material will be resorbed if it leaks into the mediastinum. The goal is to first exclude a large leak with water-soluble contrast material.
If the initial water-soluble contrast material images are negative for leak (as in this case, images not shown), the patient should drink thin barium. Rationale is that barium (as in this case) sometimes demonstrates leaks that are not seen with water-soluble contrast material. One theory for why barium is more effective than water-soluble contrast material for demonstrating leaks is that patients will take larger gulps of barium as it is less foul-tasting than water-soluble contrast material. These larger gulps of barium distend the esophagus more than small sips of water-soluble contrast material and may demonstrate a leak not visible with water-soluble contrast material. Additionally, barium is denser than water-soluble contrast material and is therefore better seen at fluoroscopy.
2. If concern is for aspiration, the patient should consume small sips of thin barium. Rationale is that aspiration of water-soluble contrast material can result in a severe pneumonitis or pulmonary edema, whereas barium is more inert. If the patient is observed to aspirate barium, the patient should be instructed to cough up as much as possible.
REFERENCES
1. Kanne JP, Rohrmann CA Jr, Lichtenstein JE. Eponyms in radiology of the digestive tract: historical perspectives and imaging appearances. Part 1. pharynx, esophagus, stomach and intestine. Radiographics 2006;26:129-142.
2. Teh E, Edwards J, Duffy J, Beggs D. Boerhaave’s syndrome: a review of management and outcome. Interact CardioVasc Thorac Surg 2007;6:640-643.
CASE 1.12 CLINICAL HISTORY 32-year-old man with HIV and odynophagia.
FINDINGS Air-contrast barium swallow demonstrates three large, approximately 3-cm ulcers in the mid-esophagus.
DIFFERENTIAL DIAGNOSIS HIV ulcer, cytomegalovirus (CMV) ulcer, herpes simplex virus (HSV), pill-induced ulcer.
DIAGNOSIS CMV ulcer.
DISCUSSION Large ulcers are defined as ulcers greater than 1 cm. The differential diagnosis of large ulcers in an HIV-positive patient includes CMV and HIV infection. HSV may also present as ulcers at fluoroscopy, but HSV ulcers are typically less than 1 cm in size. Pill-induced ulcers are also typically small in size.
At fluoroscopy, CMV disease of the esophagus may manifest as large ulcers or a more diffuse esophagitis. In the HIV population, CMV infections usually occur in patients with advanced immunosuppression and CD4 counts <50. Since the introduction of highly active antiretroviral therapy (HAART), CMV end-organ infection has become quite rare with 6 cases per 100 person-years reported in recent series. Of these acquired immunodeficiency syndrome (AIDS) patients who experience end-organ CMV disease, approximately 5% to 10% will develop CMV esophagitis. Such patients typically present with fever, odynophagia, nausea, and chest pain.
As treatment of CMV esophagitis normally requires peripherally inserted central catheter line placement and a 21- to 28-day course of intravenous foscarnet or ganciclovir, endoscopy with tissue sampling is usually performed to distinguish between a CMV ulcer and an HIV ulcer.
Question for Further Thought
1. What would be the next step to confirm the diagnosis?
Reporting Requirement
1. Describe the size and location of ulcers.
What the Treating Physician Needs to Know
1. That a large ulcer in an HIV-positive patient may be caused by HIV disease or CMV.
Answer
1. Endoscopy and biopsy would be the steps to establish a diagnosis.
REFERENCES
1. Rubesin SE, Levine MS. Differential diagnosis of esophageal disease on esophagography. Appl Radiol 2001;10:11-21.
2. Balthazar EJ, Megibow AJ, Hulnick DH. Cytomegalovirus esophagitis and gastritis in AIDS. Am J Roentgenol 1985;144: 1201-1204.
3. Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Morb Mortal Wkly Rep 2009;58:1-198.
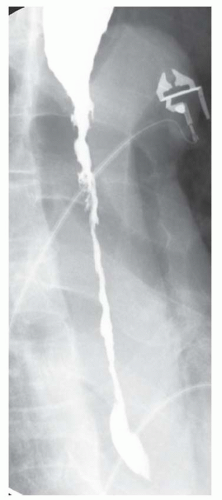
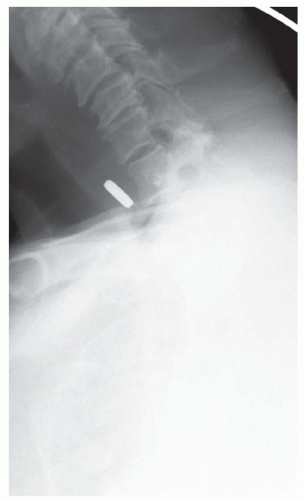
CASE 1.13 CLINICAL HISTORY 76-year-old woman with dysphagia.
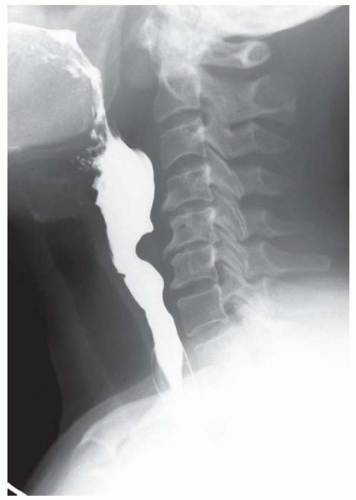
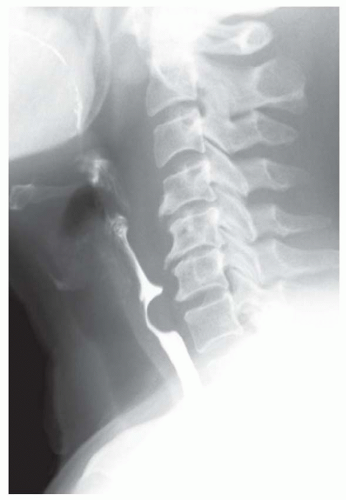
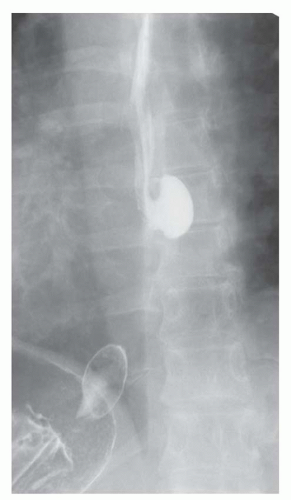
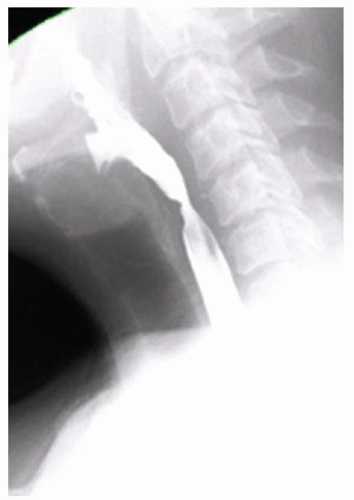
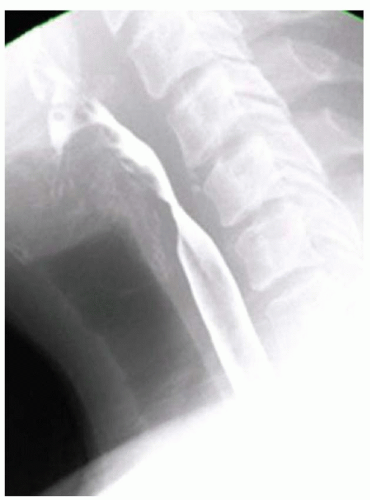
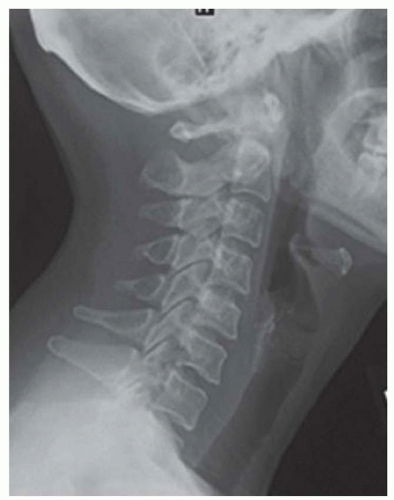
FINDINGS Fluoroscopic images from a single-contrast barium swallow (A, B) demonstrate a smooth impression on the posterior aspect of the proximal cervical esophagus at approximately the C5-C6 level. The esophageal lumen is narrowed by less than 50%, and there is no ballooning of the upstream pharynx.
DIFFERENTIAL DIAGNOSIS Esophageal web, prominent cricopharyngeal bar.
DIAGNOSIS Prominent cricopharyngeal bar.
DISCUSSION The cricopharyngeus muscle is a major component of the upper esophageal sphincter and is contracted in its basal state. Usually, the cricopharyngeus muscle relaxes when an individual swallows and is not visible at barium swallow. However, in individuals where the cricopharyngeus muscle fails to normally relax it is visible as an impression along the posterior wall of the proximal cervical esophagus, a condition sometimes referred to as cricopharyngeal achalasia.
The incidence of cricopharyngeal bars increases with age. Prominent cricopharyngeal bars are thought to, in general, be asymptomatic. Ballooning of the pharynx upstream from the bar may indicate that a bar is, in fact, symptomatic.
A prominent cricopharyngeal bar can be distinguished from an esophageal web as prominent bars are located posteriorly, whereas webs are usually located anteriorly.
Questions for Further Thought
1. Are most prominent cricopharyngeal bars asymptomatic or symptomatic?
2. What is the usual treatment of a prominent cricopharyngeal bar?
View Answer
2. Most bars are asymptomatic and therefore not treated. In rare cases, if the bar is thought to be the etiology of the patient’s symptoms, treatment options include injection of botulinum toxin into the cricopharyngeus muscle, dilatation of the bar, and myotomy.
Reporting Requirements
1. Describe the presence of the bar.
2. Describe if there is any evidence that the bar is symptomatic (e.g., ballooning of the upstream pharynx or hold-up of compressed barium tablet at the level of bar with reproduction of patient symptoms).
What the Treating Physician Needs to Know
1. Most cricopharyngeal bars are asymptomatic.
2. If the bar appears to be causing symptoms in the patient you are examining.
Answers
1. Asymptomatic.
2. Most bars are asymptomatic and therefore not treated. In rare cases, if the bar is thought to be the etiology of the patient’s symptoms, treatment options include injection of botulinum toxin into the cricopharyngeus muscle, dilatation of the bar, and myotomy.
REFERENCES
1. Leonard R, Kendall K, McKenzie SM. UES opening and cricopharyngeal bar in nondysphagic elderly and non elderly adults. Dysphagia 2004;19:182-191.
2. Dantas RO, Cook IJ, Dodds WJ, et al. Biomechanics of cricopharyngeal bars. Gastroenterology 1990;99:1269-1274.
3. Wang AY, Kadkade R, Kahrilas PJ, Hirano I. Effectiveness of esophageal dilation for symptomatic cricopharyngeal bar. Gastrointest Endosc 2005;61:148-152.
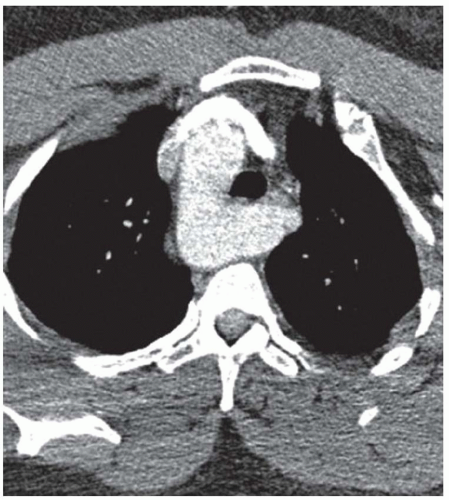
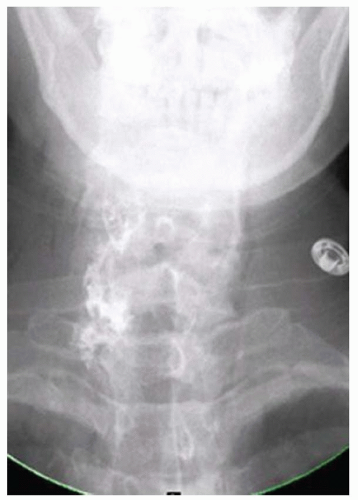
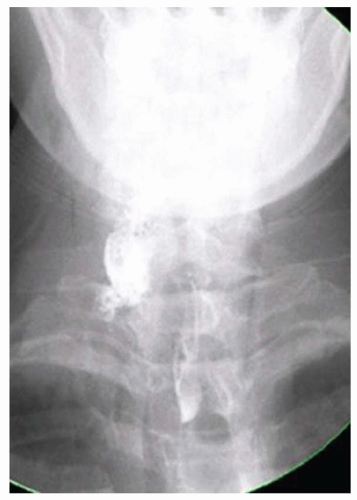
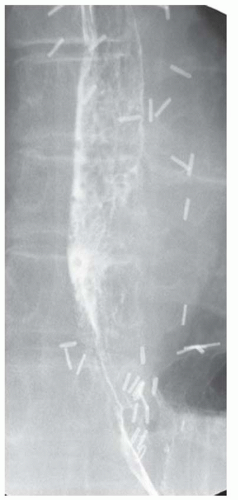
CASE 1.14 CLINICAL HISTORY 35-year-old woman with dysphagia.
FINDINGS Lateral image from a barium swallow (A) demonstrates a smooth impression on the posterior aspect of the proximal esophagus. Frontal chest radiograph (B) demonstrates mass effect along the right side of the trachea. Contrast- enhanced CT image (C) demonstrates a diverticulum of Kommerell and an aberrant left subclavian artery arising from a right-sided aortic arch. This vessel passes posterior to the trachea and esophagus.
DIFFERENTIAL DIAGNOSIS BASED ON BARIUM SWALLOW Submucosal mass, extrinsic compression.
DIAGNOSIS Extrinsic compression on esophagus due to aberrant left subclavian artery, also right-sided aortic arch.
DISCUSSION The above area of mass effect seen at barium swallow has a “marble under the rug” appearance indicating that it is a submucosal mass or extrinsic process. The smoothness of the mucosa also excludes a mucosally based process.
The key to making this diagnosis is an awareness of the possibility of an aberrant subclavian artery running behind
the esophagus. Chest CT would be the next best step in this case to confirm and better delineate the patient’s vascular anatomy. Mistakenly recommending EGD with biopsy in this case could result in catastrophic bleeding.
the esophagus. Chest CT would be the next best step in this case to confirm and better delineate the patient’s vascular anatomy. Mistakenly recommending EGD with biopsy in this case could result in catastrophic bleeding.
The two most common vascular rings reported in adults are double aortic arch and right-sided aortic arch with aberrant left subclavian artery. Patients with these conditions may present with symptoms related to the respiratory track (e.g., dyspnea on exertion or stridor) or with dysphagia. Some patients may be asymptomatic.
Questions for Further Thought
1. What is dysphagia lusoria?
View Answer
1. The term dysphagia lusoria originates from the Latin word lusus naturae which means “freak of nature” and is most commonly defined as compression of the esophagus due to an aberrant right subclavian artery running behind the esophagus.
2. What would be treatment options for the above-described patient?
View Answer
2. If a vascular ring is asymptomatic, no treatment is warranted. The above patient reported symptoms of dysphagia and shortness of breath. At barium swallow, there was no impairment to the transit of liquid barium or the barium tablet. However, because of her reported symptoms and concern for eventual aneurysm rupture, the patient underwent surgical repair which included repairing the diverticulum of Kommerell with an aortic graft and then anastomosing the left subclavian artery to the aortic graft.
Reporting Requirements
1. Inform the ordering clinician of the abnormal study.
2. Recommend chest CT to evaluate for vascular ring.
What the Treating Physician Needs to Know
1. The extrinsic esophageal mass could be a vascular structure, and therefore EGD with biopsy should not be performed as the next step in diagnosis.
2. This patient may be asymptomatic or experience symptoms related to the respiratory or GI tracts.
Answers
1. The term dysphagia lusoria originates from the Latin word lusus naturae which means “freak of nature” and is most commonly defined as compression of the esophagus due to an aberrant right subclavian artery running behind the esophagus.
2. If a vascular ring is asymptomatic, no treatment is warranted. The above patient reported symptoms of dysphagia and shortness of breath. At barium swallow, there was no impairment to the transit of liquid barium or the barium tablet. However, because of her reported symptoms and concern for eventual aneurysm rupture, the patient underwent surgical repair which included repairing the diverticulum of Kommerell with an aortic graft and then anastomosing the left subclavian artery to the aortic graft.
REFERENCES
1. Kent PD, Poterucha TH. Aberrant right subclavian artery and dysphagia lusoria. N Engl J Med 2002;346:1637.
2. Grathwohl KW, Afifi AY, Dillard TA, Olson JP, Heric BR. Vascular rings of the thoracic aorta in adults. Am Surg 1999;65: 1077-1083.
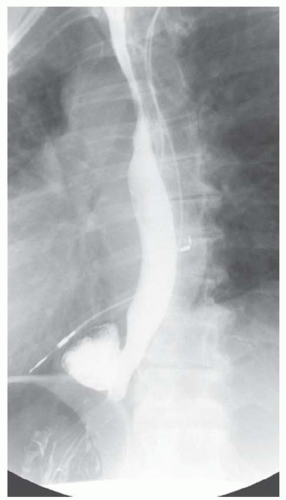
CASE 1.15 CLINICAL HISTORY 68-year-old man with dysphagia.
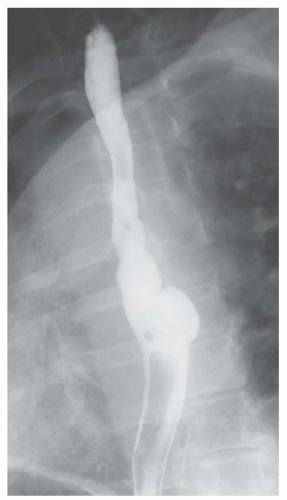
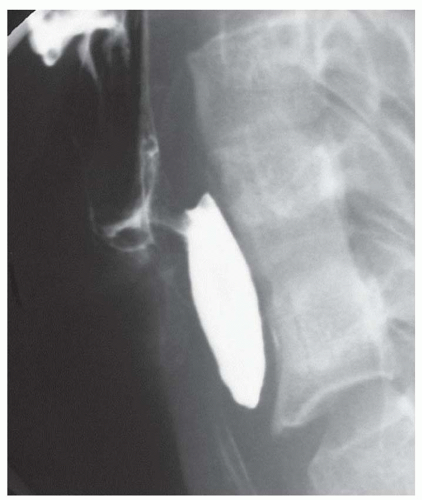
FINDINGS Oblique (A) and frontal (B) fluoroscopic images from a barium swallow demonstrate an approximately 4-cm outpouching arising from the left aspect of the distal esophagus. This outpouching was seen to fill with contrast material and empty several times during the study.
DIFFERENTIAL DIAGNOSIS Epiphrenic diverticulum, perforation.
DIAGNOSIS Epiphrenic diverticulum.
DISCUSSION An epiphrenic diverticulum is defined as an esophageal diverticulum that occurs within 10 cm of the diaphragm. Average size is 4 to 7 cm though diverticula as large as 14 cm have been reported. These pulsion diverticula result from herniation of the esophageal mucosa and submucosa through the musculature of the esophageal wall.
An epiphrenic diverticulum can be distinguished from a perforation as contrast material will be seen filling and emptying from a diverticulum, whereas contrast material will continue to accumulate at large sites of free perforation.
The etiology of epiphrenic diverticula is thought to be an underlying motility disorder such as achalasia, diffuse esophageal spasm, nutcracker esophagus, or a hypertensive lower esophageal sphincter. An esophageal motility disorder has been documented at barium swallow and/or manometry in 75% to 100% of patients with epiphrenic diverticula in recent series.
As an underlying motility disorder is thought to be the etiology of epiphrenic diverticula in most patients, the operating surgeon may perform a myotomy (to improve esophageal emptying) and a partial fundoplication procedure (to reduce the likelihood of reflux resulting from the myotomy) at the time of repair of the epiphrenic diverticulum.
Questions for Further Thought
1. What are common symptoms in patients with epiphrenic diverticula?
View Answer
1. Patients with epiphrenic diverticula may present with dysphagia, regurgitation of undigested food, chest pain, and/or weight loss.
2. What is the usual management of an epiphrenic diverticulum?
View Answer
2. Symptomatic epiphrenic diverticula are usually resected via a diverticulectomy with either an open or laparoscopic approach. Whether or not asymptomatic epiphrenic diverticula should be resected is a source of debate in the literature. Advocates of resection argue that a diverticulum can result in silent aspiration with significant morbidity and even mortality due to aspiration pneumonia. Advocates of “leaving alone” asymptomatic diverticula point to the potential for morbidity and even mortality due to surgery (which involves dissecting in the posterior mediastinum) in a patient who may never become symptomatic.
Reporting Requirements
1. Describe the size of the diverticulum and distance of the diverticulum from the esophagogastric junction.
2. Look for more than one epiphrenic diverticulum as 15% of patients will have two epiphrenic diverticula.
3. Report whether the patient has esophageal dysmotility in addition to the epiphrenic diverticulum.
What the Treating Physician Needs to Know
1. That the patient has an epiphrenic diverticulum.
2. Whether the patient also has episodes of gastroesophageal reflux or a hiatal hernia, both of which could impact operative management.
Answers
1. Patients with epiphrenic diverticula may present with dysphagia, regurgitation of undigested food, chest pain, and/or weight loss.
2. Symptomatic epiphrenic diverticula are usually resected via a diverticulectomy with either an open or laparoscopic approach. Whether or not asymptomatic epiphrenic diverticula should be resected is a source of debate in the literature. Advocates of resection argue that a diverticulum can result in silent aspiration with significant morbidity and even mortality due to aspiration pneumonia. Advocates of “leaving alone” asymptomatic diverticula point to the potential for morbidity and even mortality due to surgery (which involves dissecting in the posterior mediastinum) in a patient who may never become symptomatic.
REFERENCE
1. Soares R, Herbella FA, Prachand VN, Ferguson MK, Patti MG. Epiphrenic diverticulum of the esophagus. From pathophysiology to treatment. J Gastrointest Surg 2010;14:2009-2015.
CASE 1.16 CLINICAL HISTORY 56-year-old man undergoing preoperative evaluation prior to lung transplant.
FINDINGS Fluoroscopic images from an air-contrast barium swallow (A, B) demonstrate numerous fine transverse folds circumferentially involving the esophageal wall. These folds appeared intermittently during the study. The patient was asymptomatic when these folds were visible.
DIFFERENTIAL DIAGNOSIS Strictures from reflux esophagitis, feline esophagus.
DIAGNOSIS Feline esophagus.
DISCUSSION Feline esophagus refers to the pattern of fine transverse folds spaced 1 to 2 mm apart and involving the entire circumference of the esophagus. The transient nature of this finding and the fine, evenly spaced appearance of the mucosal folds distinguishes feline esophagus from strictures. A similar fold pattern occurs in the distal esophagus of cats.
When the esophagus shortens, the squamous mucosa undulates resulting in a “feline esophagus” appearance. Feline esophagus is a transient phenomenon and is thought to produce no symptoms. Feline esophagus may be seen as an isolated finding or in patients who also demonstrate GERD and hiatal hernias. Whether there is a true association between these conditions is unclear.
Question for Further Thought
1. What is the treatment for feline esophagus?
Reporting Requirements
1. Describe in the body of the report that intermittently during the study transverse folds were identified, in incidental finding.
2. Report whether gastroesophageal reflux events were observed.
3. Report if the patient has a hiatal hernia.
What the Treating Physician Needs to Know
1. Feline esophagus itself has no clinical significance, but this esophageal fold pattern has been reported to occur more frequently in patients with gastroesophageal reflux and/or a hiatal hernia.
Answer
1. No treatment is warrant for an isolated finding of feline esophagus.
REFERENCES
1. Williams SM, Harned RK, Kaplan P, Consigny PM. Work in progress: transverse striations of the esophagus: association with gastroesophageal reflux. Radiology 1983;146:25-27.
2. Samadi F, Levine MS, Rubesin SE, Katzka DA, Laufer I. Feline esophagus and gastroesophageal reflux. Am J Roentgenol 2010;194:972-976.
3. Furth EE, Rubesin SE, Rose D. Radiologic-pathologic conferences of the Hospital of the University of Pennsylvania: Feline esophagus. Am J Roentgenol 1995;164:900.
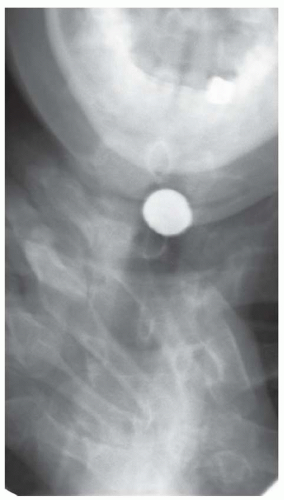
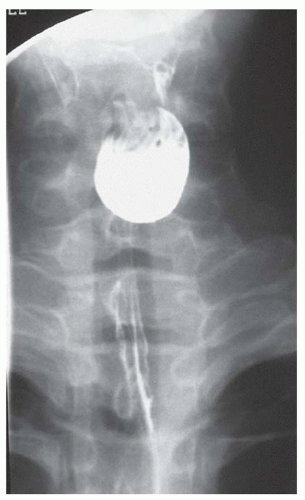
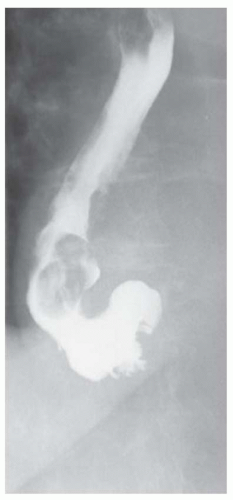
CASE 1.17 CLINICAL HISTORY 67-year-old woman with dysphagia and experiencing episodes of regurgitation of a large sausage-like mass which she could not expel.
FINDINGS Air-contrast barium swallow study demonstrates multiple smooth, expansile, sausage-shaped masses (A, B). Contrast material coats all sides of the masses, and the masses were mobile during real-time imaging.
DIFFERENTIAL DIAGNOSIS Fibrovascular polyps, leiomyomas.
DIAGNOSIS Fibrovascular polyps.
DISCUSSION Fibrovascular polyps are rare benign tumors. These tumors usually arise near the level of the cricopharyngeus muscle and gradually elongate over a period of years as they are dragged inferiorly due to esophageal peristalsis.
Patients sometimes present with weight loss and retrosternal pain. Polyp ulceration can result in bleeding. The patient may experience regurgitation of a lobulated mass with a broad stalk. In some cases, airway obstruction and asphyxia have been reported.
At fluoroscopy, fibrovascular polyps appear as intraluminal filling defects. An intraluminal location is suggested by the presence of contrast material coating all sides of the mass in multiple projections. Also, as fibrovascular polyps are usually tethered by a stalk, they often appear mobile at barium swallow. By comparison, leiomyomas typically do not appear polypoid but more often have a “marble under the rug” appearance.
Question for Further Thought
1. What are treatment options for fibrovascular polyps?
View Answer
1. Depending on the size of the polyp, it may be amenable to endoscopic excision. Alternatively, a thoracotomy may be required to remove larger polyps.
Reporting Requirements
1. Describe the size and location of the lesions.
2. Attempt to characterize the lesions as intraluminal in location which favors a diagnosis of fibrovascular polyps.
What the Treating Physician Needs to Know
1. This patient has intraluminal esophageal masses that are most likely fibrovascular polyps.
2. Surgical resection likely will be necessary to alleviate patient symptoms.
Answer
1. Depending on the size of the polyp, it may be amenable to endoscopic excision. Alternatively, a thoracotomy may be required to remove larger polyps.
REFERENCES
1. Levine MS, Rubesin SE. Diseases of the esophagus: diagnosis with esophagography. Radiology 2005;237:414-427.
2. Morin FD, Bret PM, Gret P, Palayew MJ. General case of the day: Fibrovascular polyp of the esophagus. Radiographics 1992;12:845-847.
3. Jang GC, Clouse ME, Fleischner FG. Fibrovascular polyps: a benign intraluminal tumor of the esophagus. Radiology 1969;92: 1196-1200.
CASE 1.18 CLINICAL HISTORY 43-year-old woman with dysphagia.
FINDINGS Single-contrast image (A) demonstrates a 2-cm contour abnormality in the middle third of the esophagus. The contour abnormality has the appearance of a “marble under a rug” with respect to the esophageal lumen. The esophageal mucosa is smooth, best seen in the air-contrast image (B).
DIFFERENTIAL DIAGNOSIS Submucosal mass (-omas: lipoma, leiomyoma, neuroma, fibroma, neurofibroma), extrinsic compression (lymph node, vascular structure, duplication cyst).
DIAGNOSIS Submucosal mass (granular cell tumor).
DISCUSSION The smooth mucosa and “marble under a rug” appearance make a mucosal abnormality extremely unlikely. A submucosal mass rather than an area of extrinsic compression can be favored by noting the normal outer contour of the esophagus at this level. By comparison, areas of extrinsic compression often distort the outer esophageal contour.
As a radiologist, you have done your job by identifying the abnormality as a submucosal mass for which CT (to evaluate for a fat containing lipoma) or EUS with possible tissue sampling could be performed as the next step to establish the diagnosis.
Granular cell tumors of the esophagus are rare tumors and are thought to be of neurogenic origin. Diagnosis is made based on endoscopic biopsy. Granular cell tumors have been reported in a variety of locations, including the skin, larynx, breast, and vulva. The “granularity” of these tumors is caused by accumulation of lysosomes in the cytoplasm.
When located in the esophagus, granular thecomas most frequently occur in the distal esophagus and are usually small at the time of detection with 75% <1 cm in size in one series. In many patients with small granular cell tumors, these tumors are thought to be an incidental finding at the time of endoscopy rather than the actual cause of the patient’s dysphagia. Granular cell tumors are not thought to produce dysphagia until they reach at least 1 cm in size.
A literature search for granular cell tumors of the esophagus reveals a smattering of case reports and case series. Given the relative rarity of this tumor, the best treatment
strategy is yet to be defined. In general, once the diagnosis of a granular cell tumor is made with endoscopic biopsy, periodic surveillance with endoscopy or fluoroscopy is thought to be adequate to assess for size stability. The optimal surveillance frequency is yet to be defined. If tumor removal is desired, endoscopic and surgical removal are options.
strategy is yet to be defined. In general, once the diagnosis of a granular cell tumor is made with endoscopic biopsy, periodic surveillance with endoscopy or fluoroscopy is thought to be adequate to assess for size stability. The optimal surveillance frequency is yet to be defined. If tumor removal is desired, endoscopic and surgical removal are options.
Question for Further Thought
1. What is the management of esophageal granular cell tumors?
View Answer
1. Esophageal granular cell tumors are such rare tumors that appropriate management is somewhat controversial. These benign tumors are thought to have no malignant potential. Therefore, a conservative approach with periodic (often yearly) surveillance to confirm size stability is suggested in patients with small (<1 cm), asymptomatic tumors.
Reporting Requirements
1. Describe the location of the abnormality.
2. Recommend EUS with possible tissue sampling or cross-sectional imaging (to evaluate for lipoma) for further evaluation.
What the Treating Physician Needs to Know
1. The patient has a submucosal esophageal mass.
2. Whether the mass is obstructing based on transit of liquid barium and the 12.5-mm compressed barium tablet past the level of the mass.
Answer
1. Esophageal granular cell tumors are such rare tumors that appropriate management is somewhat controversial. These benign tumors are thought to have no malignant potential. Therefore, a conservative approach with periodic (often yearly) surveillance to confirm size stability is suggested in patients with small (<1 cm), asymptomatic tumors.
REFERENCES
1. Goldblum JR, Rice TW, Zuccaro G, Richter JE. Granular cell tumors of the esophagus: a clinical and pathologic study of 13 cases. Ann Thorac Surg 1996;62:860-865.
2. Voskull JH, van Dijk MM, Wagenaar SS, van Vilet AC, Timmer R, van Hees PA. Occurrence of esophageal granular cell tumors in The Netherlands between 1998 and 1994. Dig Dis Sci 2001;46: 1610-1614.
CASE 1.19 CLINICAL HISTORY 42-year-old woman with newly diagnosed HIV infection and CD4 count of 0 presents with epigastric pain.
FINDINGS Single-contrast swallow study demonstrates a large, approximately 3-cm outpouching of contrast material along the distal third of the esophagus (A, B).
DIFFERENTIAL DIAGNOSIS Candidiasis, CMV ulcer, HSV ulcer, HIV ulcer.
DIAGNOSIS HIV ulcer (idiopathic esophageal ulceration).
DISCUSSION In the current era of HAART for HIV disease, compliant HIV-positive patients presenting with odynophagia are more likely to have symptoms caused by GERD rather than an opportunistic infection.
Of those patients who have an opportunistic infection as the etiology of odynophagia, Candida is the most common etiology followed by CMV, HSV type 1 or 2, and mycobacterial tuberculosis infection. Idiopathic ulcers or “HIV ulcers” are defined as ulcers that are negative on biopsy for known viral and fungal agents with HIV-like viral particles visible at electron microscopy.
Esophageal candidiasis appears as extensive confluent areas of mucosal irregularity. CMV appears as a large ulcer(s) or small ulcer(s) in the mid- to distal esophagus and is indistinguishable from an HIV ulcer at esophagography. HSV appears as multiple small (<1 cm) ulcers at esophagography.
Large ulcers are defined as ulcers greater than 1 cm. Endoscopy and biopsy with brushings and culture are needed to distinguish a CMV ulcer from an HIV ulcer. If biopsies and cultures are negative, the ulcer is termed “idiopathic esophageal ulceration” in the gastroenterology literature and an “HIV ulcer” in the radiology literature.
Determining whether an ulcer is caused by HIV itself or an opportunistic infection is critical because HIV ulcers are treated with HAART and sometimes steroids whereas opportunistic infections are treated with tailored medicines such as ganciclovir for CMV ulcers and fluconazole for candidiasis.
Question for Further Thought
Reporting Requirements
What the Treating Physician Needs to Know
1. A large ulcer in an HIV-positive patient may be caused by HIV disease or CMV.
2. Endoscopy with biopsy and culture is needed to distinguish between an HIV ulcer and a CMV ulcer.
Answer
1. Endoscopy and biopsy.
REFERENCES
1. Levine MS, Loercher G, et al. Giant, human immunodeficiency virus-related ulcers in the esophagus. Radiology 1991;180:323-326.
2. Werneck-Silva AL, Prado IB. Role of upper endoscopy in diagnosing opportunistic infections in human immunodeficiency virus-infected patients. World J Gastroenterol 2009;15:1050-1056.
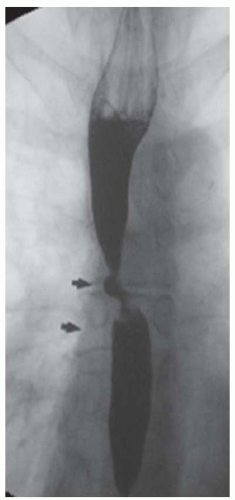
CASE 1.20 CLINICAL HISTORY 56-year-old man with dysphagia.
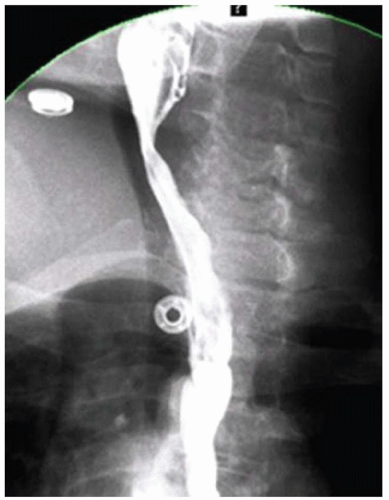
FINDINGS Fluoroscopic images (A, B) from an air-contrast barium swallow demonstrate a circumferential narrowing of the distal esophagus. Also note small sliding-type hiatal hernia.
DIFFERENTIAL DIAGNOSIS Ring, stricture.
DIAGNOSIS Lower esophageal ring (Schatzki ring).
DISCUSSION The ring visible in both of the above images is referred to as a Schatzki ring. The above ring is occurring at the level of the B ring. The B ring marks the esophagogastric junction, is a mucosal ring, and is covered by squamous epithelium along the upper surface and columnar epithelium along the undersurface.
In image A above, a second ring is visible a few millimeters above the Schatzki ring. This more cranial ring is termed an “A ring.” The A ring is a muscular contraction at the junction of the tubular and vestibular esophagus and is covered with squamous epithelium on both sides.
The etiology of lower esophageal rings is uncertain. Chronic ingestion of medications that damage esophageal mucosa and chronic GERD have been posited as causative factors.
Rings may be asymptomatic or symptomatic depending on the remaining luminal diameter of the esophagus. When performing a barium swallow, it is important to administer a compressed barium tablet, evaluate whether the tablet becomes lodged at the level of the ring, and report whether the patient experienced symptoms when the tablet was lodged. If symptomatic, rings may be treated with mechanical or balloon dilatation along with medical management of GERD if present.
Schatzki rings are named after radiologist Richard Schatzki who described ring-associated dysphagia in 1953.
Questions for Further Thought
1. What esophageal luminal diameter is thought to be associated with symptoms of dysphagia?
View Answer
1. Mucosal rings less than 13 mm in diameter are usually symptomatic. Rings more than 20 mm in diameter are usually asymptomatic, and between 13 and 20 mm is a gray area.
2. What is the steakhouse syndrome?
View Answer
2. Steakhouse syndrome refers to esophageal obstruction due to an ingested foreign body that becomes stuck at the level of an esophageal ring. Patients may present with dysphagia and chest pain.
Reporting Requirements
What the Treating Physician Needs to Know
1. Most lower esophageal rings are asymptomatic.
2. Rings that impede transit of the 12.5-mm compressed barium tablet are more likely to be symptomatic.
Answers
1. Mucosal rings less than 13 mm in diameter are usually symptomatic. Rings more than 20 mm in diameter are usually asymptomatic, and between 13 and 20 mm is a gray area.
2. Steakhouse syndrome refers to esophageal obstruction due to an ingested foreign body that becomes stuck at the level of an esophageal ring. Patients may present with dysphagia and chest pain.
REFERENCES
1. Jalil S, Castell DO. Schatzki’s ring: a benign cause of dysphagia in adults. J Clin Gastroenterol 2002;35:295-298.
2. Schatzki R, Gary JE. Dysphagia due to a diaphragm-like localized narrowing in the lower esophagus (lower esophageal ring). Am J Roentgenol 1953;70:911-922.
CASE 1.21 CLINICAL HISTORY 66-year-old woman with dysphagia.
FINDINGS Frontal film from a single-contrast barium swallow demonstrates an approximately 5-cm area of narrowing (A) in the mid-esophagus. The mucosa itself appears primarily smooth in the area of narrowing (A). Noncontrast CT scan (B) demonstrates a soft-tissue mass completely encasing the esophagus (B) and also surrounding the left lower lobe bronchus.
DIFFERENTIAL DIAGNOSIS Bronchogenic carcinoma, esophageal cancer, lymphoma.
DIAGNOSIS Bronchogenic carcinoma.
DISCUSSION When a mass-like area is identified at barium swallow, the mucosal surface should be evaluated. Smooth mucosa suggests a submucosal or an extrinsic process as in this case. By comparison, mucosal irregularity suggests a mucosa-based process.
If a process is characterized as submucosal or extrinsic, cross-sectional imaging or EUS with tissue sampling may be the next step to try to determine the etiology. By comparison, endoscopy with tissue sampling is usually the next step for mucosal processes. Malignant extrinsic pathologies that can narrow the esophagus include bronchogenic carcinoma and lymphoma.
Questions for Further Thought
1. How would a tissue diagnosis be obtained in this patient?
View Answer
1. Options for tissue diagnosis in this patient would include bronchoscopy or EGD/EUS with biopsy.
2. What stage is lung cancer that directly invades the esophagus?
3. What treatment options are available for this patient?
View Answer
3. Treatment options would include placement of a percutaneous gastrostomy tube for feeding purposes (as was done for this patient).
Reporting Requirements
1. Describe the location of the abnormality.
2. Indicate high level of concern for malignancy.
What the Treating Physician Needs to Know
1. This patient has a large, likely extrinsic mass that is highly suspicious for malignancy.
2. Chest CT may be helpful to further characterize the mass.
3. Whether the mass is obstructing based on transit of liquid barium and the 12.5-mm compressed barium tablet past the level of the mass.
Answers
1. Options for tissue diagnosis in this patient would include bronchoscopy or EGD/EUS with biopsy.
2. Lung cancer that directly invades the esophagus is designated as T4 disease.
3. Treatment options would include placement of a percutaneous gastrostomy tube for feeding purposes (as was done for this patient).
REFERENCE
1. DiPerna CA, Wood DE. Surgical management of T3 and T4 lung cancer. Clin Cancer Res 2005;11:5038s-5044s.
CASE 1.22 CLINICAL HISTORY 57-year-old woman with history of multiple esophageal surgeries and leakage from right chest.
FINDINGS Lateral chest radiograph from an esophagram and fistulagram. Prior to this image being obtained, the patient ingested water-soluble contrast material by mouth. The patient’s right chest wall skin defect was also cannulated, and sterile water-soluble contrast material was injected.
DIAGNOSIS Colonic interposition; also fistula from right chest wall to blind-ending esophageal remnant.
DISCUSSION After esophagectomy, options for a replacement conduit include stomach, jejunum, and colon. The location of the conduit may be orthotopic, retrosternal, left or right chest, or subcutaneous. The anastomosis may be thoracic or cervical.
This patient had a remote history of a colonic interposition in the 1970s for a benign esophageal stricture. The patient developed a stricture at the esophagocolonic anastomosis, and the colonic interposition was revised with a new colonic interposition. The patient then developed a fistulous communication between her skin and the remaining blind-ending esophageal remnant.
Historically, colon was the most frequently used conduit. Presently, stomach is the most frequently used conduit. Current indications for selecting a colonic rather than a gastric conduit include history of prior gastrectomy and gastric involvement by tumor. If a colonic interposition is performed, the right colon is used more frequently than the left colon.
Short-term complications following colonic interposition include anastomotic leak. Long-term complications include stricture. Strictures more frequently form at the esophagocolonic anastomosis rather than the colonic-small bowel anastomosis.
Questions for Further Thought
1. What contrast agent should be used if there is concern for an esophageal leak into the mediastinum?
View Answer
1. Water soluble. Rationale: water-soluble contrast material will be resorbed if it leaks into the mediastinum. On the other hand, barium is inert, is not resorbed, and can trigger an inflammatory response. Keep in mind that barium should be used if there is concern for aspiration as water-soluble contrast material can result in a severe pneumonitis and pulmonary edema if aspirated. Also remember that water-soluble contrast material contains iodine, so confirm that the patient does not have an iodine allergy before administering water-soluble contrast material. If the patient is allergic to iodine, depending on the severity of the reaction the swallow study can be performed following a steroid prep.
2. What contrast agent should be used to evaluate a fistula?
View Answer
2. The safest contrast agent to use is a sterile, water-soluble contrast agent. Iodinated contrast material used for intravascular opacification at CT satisfies both of these criteria. As above, confirm that the patient is not allergic to iodine before administering iodinated contrast material.
Reporting Requirement
1. Notify the ordering physician of the presence of a fistula.
What the Treating Physician Needs to Know
1. The patient’s colonic conduit is intact without leak or stricture.
2. There is a fistulous communication between the patient’s right chest and the esophageal remnant.
Answers
1. Water soluble. Rationale: water-soluble contrast material will be resorbed if it leaks into the mediastinum. On the other hand, barium is inert, is not resorbed, and can trigger an inflammatory response. Keep in mind that barium should be used if there is concern for aspiration as water-soluble contrast material can result in a severe pneumonitis and pulmonary edema if aspirated. Also remember that water-soluble contrast material contains iodine, so confirm that the patient does not have an iodine allergy before administering water-soluble contrast material. If the patient is allergic to iodine, depending on the severity of the reaction the swallow study can be performed following a steroid prep.
2. The safest contrast agent to use is a sterile, water-soluble contrast agent. Iodinated contrast material used for intravascular opacification at CT satisfies both of these criteria. As above, confirm that the patient is not allergic to iodine before administering iodinated contrast material.
REFERENCE
1. Davis PA, Law S, Wong J. Colonic interposition after esophagectomy for cancer. Arch of Surg 2003;138:303-308.
CASE 1.23 CLINICAL HISTORY 72-year-old man with dysphagia.
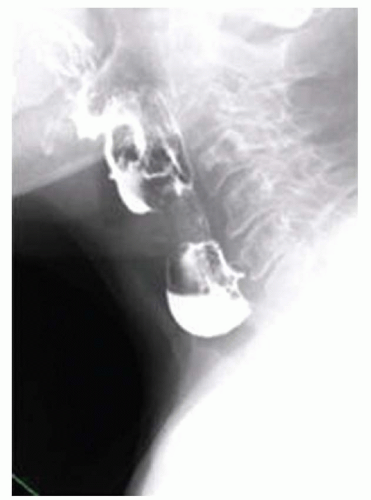
FINDINGS Fluoroscopic images from an air-contrast barium swallow (A, B, C) demonstrate an approximately 3-cm outpouching with smooth margins arising from the middle third of the esophagus. Contrast material remains in the outpouching after the neighboring esophageal lumen at the same level has cleared.
DIFFERENTIAL DIAGNOSIS Pulsion diverticulum, traction diverticulum.
DIAGNOSIS Features of both, most likely a pulsion diverticulum.
DISCUSSION Diverticula are divided into pulsion and traction diverticula. Pulsion diverticula are thought to result from a dysmotility disorder. For example, failure of normal relaxation of the cricopharyngeus muscle is thought to contribute to the formation of both Zenker and Killian-Jamieson diverticula. Failure of normal relaxation of the lower esophageal sphincter is thought to contribute to the formation of epiphrenic diverticulum. Pulsion diverticula most commonly involve the proximal or distal esophagus.
By comparison, traction diverticula are due to an adjacent inflammatory or infectious process that distorts esophageal anatomy. Traction diverticula most commonly occur in the mid-esophagus and are thought to be associated with, for example, inflammation of adjacent mediastinal lymph nodes. Traction diverticula also may occur in the cervical esophagus secondary to inflammation from cervical spine hardware.
Pulsion diverticula (unlike traction diverticula) do not contain muscle in their walls and therefore do not empty with the remainder of the esophagus. Traction diverticula would be expected to clear with the remainder of the esophagus. Pulsion diverticula are more rounded in shape, whereas traction diverticula often are more triangular in shape.
The above diverticulum has features of both a pulsion and a traction diverticulum as it is located in the mid-esophagus which is a common location for traction diverticula. However, as the diverticulum does not empty with the remainder of the esophagus it was favored to be a pulsion diverticulum.
Questions for Further Thought
1. Is there a management difference for traction versus pulsion diverticula?
View Answer
1. A variety of surgical approaches have been described for removing and repairing symptomatic diverticula. At the present time, surgical techniques are similar for traction and pulsion diverticula.
2. What symptoms may be associated with diverticula?
View Answer
2. Symptoms that may be associated with diverticula include dysphagia, regurgitation, and aspiration. Many diverticula are asymptomatic.
Reporting Requirements
1. Describe the location and size of the diverticulum.
2. If possible, describe whether the diverticulum appears to be a pulsion or traction-type diverticulum.
What the Treating Physician Needs to Know
1. The location and size of the diverticulum.
Answers
1. A variety of surgical approaches have been described for removing and repairing symptomatic diverticula. At the present time, surgical techniques are similar for traction and pulsion diverticula.
2. Symptoms that may be associated with diverticula include dysphagia, regurgitation, and aspiration. Many diverticula are asymptomatic.
REFERENCES
1. Pinto do Nascimento FA, Lemme EMO, Costa MMB. Esophageal diverticula: pathogenesis, clinical aspects and natural history. Dysphagia 2006;21:198-205.
2. Khan N, Ismail F, van de Werke IEA. Oesophageal pouches and diverticula: a pictorial review. S Afr J Surg 2012;50:71-75.
3. Alexander RE, Silber J, Myssiorek D. Staged surgical management of hypopharyngeal traction diverticulum. Ann Otol Rhinol Laryngol 2008;117:731-733.
CASE 1.24 CLINICAL HISTORY 42-year-old man with dysphagia; history of radiation for head and neck cancer.
FINDINGS Lateral view (A, B) from a barium swallow demonstrates a focal short-segment narrowing of the proximal cervical esophagus.
DIFFERENTIAL DIAGNOSIS Benign or malignant stricture.
DIAGNOSIS Benign stricture due to radiation.
DISCUSSION Etiologies of benign strictures include reflux esophagitis, infectious esophagitis, caustic ingestion, medication-induced esophagitis, and radiation-induced esophagitis.
Radiation often is used to treat locally advanced head and neck cancers. The radiation field may be large due to a desire to treat the tumor bed as well as regional lymph nodes. Dysphagia is a common symptom following head and neck chemoradiation occurring in up to 50% of patients. This dysphagia may be due to xerostomia, mucositis, or a mechanical stricture. Such strictures are not only uncomfortable but can also lead to significant malnutrition.
Strictures due to head and neck radiation can be difficult to treat as they are often refractory to dilatation. Radiation also can damage other adjacent structures resulting in an impaired swallowing mechanism. Such proximal esophageal strictures often are not amenable to stenting as placing a stent proximal to the cricopharyngeus muscle is contraindicated since the patient would feel a foreign body sensation if a stent were placed in this location.
Question for Further Thought
1. What is the definition of a short-segment stricture?
Reporting Requirement
1. Describe the location and approximate length of the stricture.
What the Treating Physician Needs to Know
1. Development of a stricture following radiation therapy may indicate a radiation-induced stricture. However, in questionable cases, EGD should be performed to exclude malignancy.
Answer
1. Short-segment strictures are usually defined as being less than 2 cm in length.
REFERENCES
1. Baron TH. Management of benign esophageal strictures. Gastroenterol Hepatol 2011;7:46-49.
2. Hu H-T, Shin JH, Kim JH, Park J-H, Sung K-B, Song H-Y. Fluoroscopically guided balloon dilation for pharyngoesophageal stricture after radiation therapy in patients with head and neck cancer. Am J Roentgenol 2010;194:1131-1136.
CASE 1.25 CLINICAL HISTORY 52-year-old woman with soft-tissue fullness seen along the distal esophagus on a chest CT performed for shortness of breath.
FINDINGS Barium swallow demonstrates a contour abnormality along the distal esophagus (A, B). The esophageal mucosa is smooth, and the contour abnormality has a “marble under the rug” appearance.
DIFFERENTIAL DIAGNOSIS Submucosal mass (-omas: lipoma, leiomyoma, neuroma, fibroma, neurofibroma), extrinsic compression (lymph node, vascular structure, duplication cyst).
DIAGNOSIS Leiomyoma.
DISCUSSION The smooth mucosal surface of the above lesion indicates that it is most likely a submucosal or extrinsic process. By comparison, a mucosa-based process such as esophageal carcinoma would be expected to demonstrate mucosal irregularity. The “marble under the rug” appearance of the above mass suggests that it is a submucosal process. However, the deformation of the external contour of the esophagus means that an extrinsic process cannot be fully excluded.
When an esophageal abnormality is thought to be submucosal or extrinsic, EUS with tissue sampling or cross-sectional imaging may be the most appropriate next step to distinguish between a submucosal process and extrinsic compression. By comparison, EGD is usually performed as the next step to further evaluate mucosal pathology.
Leiomyomas are the most common esophageal submucosal mass. These tumors may be symptomatic, more commonly occur in the lower two-thirds of the esophagus, and often can be removed by enucleation rather than esophageal resection.
Question for Further Thought
1. What would be the next step to establish the diagnosis?
View Answer
1. CT or MR can help clarify the space of a lesion as being submucosal versus extrinsic to the esophagus and can confidently diagnose some lesions such as lipomas obviating the need for tissue sampling. EUS with tissue sampling may be necessary to establish most other diagnoses.
Reporting Requirements
1. Describe the location of the abnormality.
2. Since this lesion is most likely a submucosal abnormality, EUS with tissue sampling or cross-sectional imaging (CT or MR) could be performed for further evaluation.
What the Treating Physician Needs to Know
1. The patient has a submucosal esophageal mass.
2. Whether the mass is obstructing based on transit of liquid barium and the 12.5-mm compressed barium tablet past the level of the mass.
Answer
1. CT or MR can help clarify the space of a lesion as being submucosal versus extrinsic to the esophagus and can confidently diagnose some lesions such as lipomas obviating the need for tissue sampling. EUS with tissue sampling may be necessary to establish most other diagnoses.
REFERENCES
1. Levine MS, Rubesin SE. Diseases of the esophagus: diagnosis with esophagography. Radiology 2005;237:414-427.
2. Mutrie CJ, Donahue DM, Wain JC, et al. Esophageal leiomyoma: a 40-year experience. Ann Thorac Surg 2005;79: 1122-1125.
CASE 1.26 CLINICAL HISTORY 54-year-old man with dysphagia.
FINDINGS Fluoroscopic images from a single-contrast barium swallow (A, B) demonstrate a long-segment fixed narrowing of the mid- and distal esophagus.
DIFFERENTIAL DIAGNOSIS Esophageal stricture, esophageal cancer.
DIAGNOSIS Long-segment esophageal stricture.
DISCUSSION The smooth margins of this area of fixed narrowing favor a benign stricture rather than malignancy. EGD should be recommended to distinguish a benign from a malignant stricture in most cases.
Esophageal strictures result from long-standing esophageal inflammation leading to fibrosis. Strictures can be classified based on their location as well as whether they are long-segment or short-segment strictures. Historically, GERD was the etiology of the majority of esophageal strictures in the United States and could produce long-segment or short-segment strictures. Due to improved medical management options for GERD, reflux-induced strictures have declined in frequency.
Other potential etiologies of long-segment strictures include caustic ingestion and radiation. Patients with longstanding indwelling nasogastric tubes can also develop long-segment distal esophageal strictures due to the tube preventing closure of the lower esophageal sphincter with resultant reflux of acidic gastric contents into the distal esophagus. Scleroderma patients also may develop a nonfunctioning lower esophageal sphincter due to smooth muscle atrophy with resultant spontaneous gastroesophageal reflux.
Whether or not a 12.5-mm compressed barium tablet passes through a stricture can be used to assess the esophageal luminal diameter in the area of the stricture.
Questions for Further Thought
1. Name three etiologies of short-segment esophageal strictures.
View Answer
1. Reflux esophagitis, medication-induced, and esophageal cancer are etiologies of short-segment strictures.
2. What is the usual treatment for a benign esophageal stricture?
View Answer
2. First-line treatment of a benign esophageal stricture is endoscopic dilatation using either a bougie (a cylindrical instrument used for dilatation) or a balloon. Stents may be placed in patients whose strictures are refractory to dilatation.
Reporting Requirements
1. Describe the length and location of the stricture.
2. Based on stricture length and location, suggest the potential causative etiologies.
3. Recommend EGD to exclude malignancy in most cases.
What the Treating Physician Needs to Know
1. Whether the stricture appears benign or malignant. If uncertain, EGD should be recommended for further evaluation.
Answers
1. Reflux esophagitis, medication-induced, and esophageal cancer are etiologies of short-segment strictures.
2. First-line treatment of a benign esophageal stricture is endoscopic dilatation using either a bougie (a cylindrical instrument used for dilatation) or a balloon. Stents may be placed in patients whose strictures are refractory to dilatation.
REFERENCES
1. Luedtke P, Levine MS, Rubesin SE, Weinstein DS, Laufer I. Radiologic diagnosis of benign esophageal strictures: a pattern approach. Radiographics 2003;23:897-909.
2. Baron TH. Management of benign esophageal strictures. Gastroenterol Hepatol 2011;7:46-49.
CASE 1.27 CLINICAL HISTORY 72-year-old woman with neck mass and poor oral intake.
FINDINGS Frontal oblique image from a barium swallow (A) demonstrates rightward deviation of the cervical esophagus. Coronal reformatted image from a contrast-enhanced neck CT (B) demonstrates marked enlargement of the thyroid gland with the right lobe measuring 7.5 cm in transverse dimension and the left lobe measuring 9.0 cm in transverse dimension. The nodular appearance of the thyroid gland is in keeping with a multinodular goiter. There was no obstruction to the transit of a 12.5-mm compressed barium tablet.
DIAGNOSIS Mass effect on cervical esophagus due to enlarged thyroid gland.
DISCUSSION The barium swallow demonstrates a mass-like area of narrowing along the left aspect of the cervical esophagus. The smooth appearance of the esophageal mucosa indicates that this is a submucosal or extrinsic process. Neck CT demonstrates that the patient’s neck mass is a large thyroid goiter. Goiter is defined as enlargement of the thyroid gland. Patients with goiter may be hypothyroid, euthyroid, or hyperthyroid. Thyroid goiter can result in compression of the trachea and/or esophagus. Symptomatic narrowing is more commonly caused by thyroiditis than by a colloid goiter.
High levels of thyroid-stimulating hormone (TSH) result in goiter. TSH levels increase in the setting of thyroid hormone deficiency or TSH receptor agonists (e.g., tumors producing human chorionic gonadotropin [hCG]). In the United States, most goiters are caused by autoimmune thyroiditis. Worldwide, most goiters are caused by iodine deficiency.
Questions for Further Thought
1. What are treatment options for goiter?
View Answer
1. Medical management (levothyroxine suppressive therapy) and surgical resection are treatment options for goiter.
2. What pregnancy-related hormone can result in thyroid enlargement?
Reporting Requirements
1. Inform the ordering clinician of the presence of esophageal narrowing.
2. Inform the ordering clinician that there was no obstruction to transit of the 12.5-mm compressed barium tablet.
What the Treating Physician Needs to Know
1. The patient’s enlarged thyroid is narrowing but not obstructing the esophagus.
2. Endocrine and surgical consults are warranted.
Answers
1. Medical management (levothyroxine suppressive therapy) and surgical resection are treatment options for goiter.
2. hCG can result in thyroid enlargement.
REFERENCE
1. Alfonso A, Christoudias G, Amarudding Q, Herbsman H, Gardner B. Tracheal or esophageal compression due to benign thyroid disease. Am J Surg 1981;142:350-354.
CASE 1.28 CLINICAL HISTORY 72-year-old man with dysphagia.
FINDINGS Fluoroscopic images from a barium swallow (A, B) demonstrate an approximately 3-mm fixed, smooth indentation along the anterior aspect of the cervical esophagus. The 12.5-mm compressed barium tablet became lodged at this location (C, D). Note the patient’s hand over the throat in D, a response to feeling that the tablet was “stuck” in his throat.
DIFFERENTIAL DIAGNOSIS Esophageal web, prominent cricopharyngeal bar, stricture.
DIAGNOSIS Esophageal web.
DISCUSSION Esophageal webs are thin mucosal folds and can be circumferential or partially circumferential. Esophageal webs most commonly arise in the cervical esophagus anteriorly at the level of the cricopharyngeus muscle (C5-C6). Most esophageal webs are asymptomatic. Findings at barium swallow indicating that a web may be symptomatic include (1) the 12.5-mm compressed barium tablet becoming lodged at the site of the web with reproduction of patient symptoms and/or (2) observing a jet of liquid barium passing through the web.
The pathophysiology of esophageal webs is controversial. One theory is that webs may represent a remnant of embryologic development in which the esophagus fails to normally recanalize. A possible link between esophageal webs and iron-deficiency anemia has been posited but not conclusively proven.
A cervical esophageal web can be distinguished from a prominent cricopharyngeus muscle as webs arise anteriorly or are circumferential, whereas prominent cricopharyngeal muscles arise posteriorly. An esophageal web can be differentiated from a stricture by its thin, shelf-like appearance and the 90-degree angle it forms with the lumen of the esophagus.
Questions for Further Thought
2. What is the usual treatment of an esophageal web?
View Answer
2. Most webs are asymptomatic and therefore not treated. First-line treatment for a symptomatic web is behavioral modification such as instructing the patient to more fully chew the food and cut up large pills. If patients are still symptomatic even with behavioral modifications, dilatation with a mechanical bougie can be performed.
Reporting Requirements
1. Describe the location of the web.
2. Describe if the compressed barium tablet becomes lodged at the web and reproduces the patient’s symptoms.
What the Treating Physician Needs to Know
1. Most esophageal webs are asymptomatic.
2. If the web appears to be causing symptoms in the patient you are examining.
Answers
1. Asymptomatic.
2. Most webs are asymptomatic and therefore not treated. First-line treatment for a symptomatic web is behavioral modification such as instructing the patient to more fully chew the food and cut up large pills. If patients are still symptomatic even with behavioral modifications, dilatation with a mechanical bougie can be performed.
REFERENCES
1. Grant PD, Morgan DE, Scholz FJ, et al. Pharyngeal dysphagia: what the radiologist needs to know. Curr Probl Diagn Radiol 2009;38:17-32.
2. Chisholm M, Adran GM, Callender ST, et al. Iron deficiency and autoimmunity in post-cricoid webs. Q J Med 1971;40:421-433.
CASE 1.29 CLINICAL HISTORY 56-year-old man with dysphagia.
FINDINGS Lateral view (A) from a barium swallow demonstrates an approximately 4-cm collection of contrast material located posterior to the esophagus with the cranial portion of the collection arising at approximately the level of the C5-C6 disk space. Frontal film (B) demonstrates the collection to be located along the midline.
DIFFERENTIAL DIAGNOSIS Zenker diverticulum, perforation.
DIAGNOSIS Zenker diverticulum.
DISCUSSION Zenker diverticula originate just proximal to the cricopharyngeus muscle. These diverticula occur when a portion of the esophageal wall herniates through the space between the inferior pharyngeal constrictor and the cricopharyngeus muscle. The etiology of Zenker diverticula is unclear although failure of normal relaxation of the cricopharyngeus muscle has been postulated. With smaller diverticula, the hypopharynx and esophagus remain in line. However, as these diverticula enlarge, they push the lumen of the esophagus anteriorly resulting in dysphagia. Pooling of contrast material in a Zenker diverticulum can be a source of aspiration.
A Zenker diverticulum can be distinguished from a perforation as contrast material will readily fill and empty from a diverticulum, whereas contrast material usually will continue to accumulate at a site of perforation. Diverticula usually demonstrate a smooth, rounded morphology, whereas sites of perforation are more irregular in shape. Zenker diverticula are named after pathologist Friedrich Alber von Zenker (1925 to 1898).
Questions for Further Thought
1. What are common symptoms in patients with Zenker diverticula?
View Answer
1. Dysphagia, regurgitation of undigested food hours after eating, halitosis, and aspiration are relatively common complications of Zenker diverticula. Bleeding, fistula formation, and development of squamous cell carcinomas in the diverticulum are less common complications.
2. What are current treatment options for Zenker diverticula?
View Answer
2. Small (<2 cm) diverticula in asymptomatic patients often are not treated. Surgical options for larger, symptomatic diverticula include diverticulectomy.
Reporting Requirements
1. Report the approximate size of the diverticulum.
2. Report if the diverticulum narrows the esophageal lumen.
What the Treating Physician Needs to Know
1. Zenker diverticula can be asymptomatic if small but are usually symptomatic when large.
Answers
1. Dysphagia, regurgitation of undigested food hours after eating, halitosis, and aspiration are relatively common complications of Zenker diverticula. Bleeding, fistula formation, and development of squamous cell carcinomas in the diverticulum are less common complications.
2. Small (<2 cm) diverticula in asymptomatic patients often are not treated. Surgical options for larger, symptomatic diverticula include diverticulectomy.
REFERENCE
1. Grant PD, Morgan DE, Scholz FJ, Canon CL. Pharyngeal dysphagia: what the radiologist needs to know. Curr Probl Diagn Radiol 2009;38:17-32.
CASE 1.30 CLINICAL HISTORY 54-year-old woman with history of dysphagia.
FINDINGS Lateral image from a barium swallow (A) demonstrates an approximately 4-cm collection of contrast material projecting over the esophagus at the level of the C5 and C6 vertebral bodies. Frontal image (B) demonstrates the collection of contrast material to project to the right of the esophagus.
DIFFERENTIAL DIAGNOSIS Killian-Jamieson diverticulum, Zenker diverticulum.
DIAGNOSIS Killian-Jamieson diverticulum.
DISCUSSION Both Killian-Jamieson and Zenker diverticula are pulsion diverticula that are located in the cervical esophagus and hypopharynx, respectively. Killian-Jamieson diverticula can be distinguished from Zenker diverticula based on location. Killian-Jamieson diverticula project lateral to the esophagus on a frontal film and project over the esophagus on a lateral film. By comparison, Zenker diverticula project over the esophagus on a frontal film and posterior to the esophagus on a lateral film. Also, Killian-Jamieson diverticula originate below the cricopharyngeus muscle, whereas Zenker diverticula originate just above this muscle. Treatment options for symptomatic patients include surgical resection.
Questions for Further Thought
1. What is the Killian-Jamieson space?
View Answer
1. The Killian-Jamieson space is defined as the space located inferior to the cricopharyngeus muscle and lateral to the longitudinal muscle of the esophagus. Killian-Jamieson diverticula originate through this space.
2. What is Killian’s dehiscence?
View Answer
2. Killian’s dehiscence is defined as the posterior muscular gap between the inferior pharyngeal constrictor and the cricopharyngeus muscle. Zenker diverticula occur through Killian’s dehiscence.
3. Who was Killian?
View Answer
3. Gustave Killian (1860 to 1921) was a German ENT surgeon and is credited with being a founder of bronchoscopy.
Reporting Requirement
1. Describe the size and location of the diverticulum.
What the Treating Physician Needs to Know
1. The size of the Killian-Jamieson diverticulum.
Answers
1. The Killian-Jamieson space is defined as the space located inferior to the cricopharyngeus muscle and lateral to the longitudinal muscle of the esophagus. Killian-Jamieson diverticula originate through this space.
2. Killian’s dehiscence is defined as the posterior muscular gap between the inferior pharyngeal constrictor and the cricopharyngeus muscle. Zenker diverticula occur through Killian’s dehiscence.
3. Gustave Killian (1860 to 1921) was a German ENT surgeon and is credited with being a founder of bronchoscopy.
REFERENCES
1. Rubesin SE, Levine MS. Killian-Jamieson diverticula: radiographic findings in 16 patients. Am J Roentgenol 2001;177:85-89.
2. Chea CT, Siow SL, Khor TW, Nik Azim NA. Killian-Jamieson diverticulum: the rarer cervical esophageal diverticulum. Med J Malaysia 2011;66:73-74.
CASE 1.31 CLINICAL HISTORY 26-year-old man with recent gunshot wound and pneumomediastinum seen on chest CT.
FINDINGS Initial image (A) demonstrates a bullet projecting to the right of the mid-esophagus. Contrast material is present in the proximal to mid-esophagus with a small amount of contrast material extending to the left of the mid-esophagus (A). Subsequent image obtained slightly more inferiorly (B) demonstrates contrast material moving more distally in the left mainstem bronchus and also moving distally within the esophagus.
DIFFERENTIAL DIAGNOSIS Tracheoesophageal fistula, laryngotracheal aspiration.
DIAGNOSIS Tracheoesophageal fistula due to gunshot wound.
DISCUSSION The word fistula comes from the Latin word for pipe and is defined as an abnormal communication between two structures or between a structure and the skin surface. Acquired tracheoesophageal fistulas most commonly are due to malignancy, iatrogenic complications, or trauma. Esophageal cancer followed by lung cancer are the malignancies that most commonly result in tracheoesophageal fistulas.
Traumatic tracheoesophageal fistulas are most commonly caused by motor vehicle collisions and resultant impact between the chest and steering wheel. Though such fistulas may be present immediately in the setting of extreme trauma, more frequently these fistulas take 3 to 10 days to develop due to evolving tissue necrosis. Operative mortality when such fistulas are repaired is as high as 15%.
The majority of iatrogenic tracheoesophageal fistulas result from pressure necrosis due to the inflated cuff of an endotracheal tube. Endoscopy, esophageal stenting, and esophagectomy with gastric pull-through also may be complicated by the development of a tracheoesophageal fistula.
Esophagography is approximately 70% sensitive for the detection of tracheoesophageal fistulas. Endoscopy is considered to be the most sensitive test for diagnosing tracheoesophageal fistulas.
Question for Further Thought
1. What contrast agent should be used in a patient with suspected tracheoesophageal fistula?
View Answer
1. Barium should be used if there is concern that a fistula communicates with the tracheobronchial tree because water-soluble contrast material could lead to potentially lethal pulmonary edema due to osmolality effects.
Reporting Requirements
1. Describe the level of the fistula.
2. Immediately contact the treating physician.
What the Treating Physician Needs to Know
1. Immediately notify the treating physician of the presence of a tracheoesophageal fistula.
2. The location, width, and direction of the tracheoesophageal fistula should be reported if possible.
Answer
1. Barium should be used if there is concern that a fistula communicates with the tracheobronchial tree because water-soluble contrast material could lead to potentially lethal pulmonary edema due to osmolality effects.
REFERENCES
1. Diddee R, Shaw IH. Acquired tracheo-oesophageal fistula in adults. Contin Educ Anaesth Crit Care Pain 2006;6: 105-108.
2. Pickhardt PJ, Bhalla S, Balfe DM. Acquired gastrointestinal fistulas: classification, etiologies and imaging evaluation. Radiology 2002;224:9-23.
3. Reich SB. Production of pulmonary edema by aspiration of water-soluble nonabsorbable contrast media. Radiology 1969;92: 367-370.
CASE 1.32 CLINICAL HISTORY 78-year-old woman with dysphagia; history of melanoma diagnosed 5 years previously.
FINDINGS Images from a single-contrast swallow study demonstrate an approximately 4-cm lobulated, nonobstructing mass in the distal esophagus (A-C).
DIFFERENTIAL DIAGNOSIS Esophageal malignancy, fibrovascular polyp, -omas (lipoma, leiomyoma, neuroma, fibroma, neurofibroma), metastatic disease.
DIAGNOSIS Metastatic melanoma.
DISCUSSION The above solid mass has a nonspecific imaging appearance, and EGD with biopsy was required for diagnosis. The relatively smooth appearance of the mucosa makes an esophageal adenocarcinoma or squamous cell cancer less likely. Fibrovascular polyps more commonly originate in the proximal esophagus rather than the distal esophagus. Lipomas and other submucosal masses tend to have a smooth mucosal appearance similar to this case; however, such neoplasms are often not as multilobular.
Metastatic disease to the esophagus from a distant primary malignancy is uncommon. In an autopsy study of patients who expired due to a variety of malignancies, 6% of patients had metastatic implants located in the esophagus. These
implants were most commonly submucosal in location as in this case.
implants were most commonly submucosal in location as in this case.
Breast and lung cancers are the malignancies that most commonly result in distant metastases to the esophagus. Esophageal metastases caused by cancers of the colon, prostate, ovary, kidney, and rectum have been reported.
Presentation of metastases to the esophagus may be quite delayed with a 16-year delay between initial cancer diagnosis and esophageal metastasis diagnosis reported in one series. Treatment and prognosis depend on the rate of tumor progression. Tumors with long latency may be aggressively treated with esophagectomy. By comparison, rapidly progressing tumors may be treated with palliative stenting.
Question for Further Thought
1. Can primary melanomas occur in the esophagus?
View Answer
1. Yes. Primary melanomas can occur in the esophagus. Approximately 4% of normal esophagi contain submucosal melanocytes. Primary esophageal melanoma accounts for <1% of primary esophageal malignancies.
Reporting Requirements
1. Report the presence of a large lobulated mass in the distal esophagus.
2. The mass is not obstructing.
3. EGD with biopsy will likely be required for diagnosis.
What the Treating Physician Needs to Know
1. The patient has a large esophageal mass which is suspicious for malignancy.
2. EGD with biopsy will be required for diagnosis.
Answer
1. Yes. Primary melanomas can occur in the esophagus. Approximately 4% of normal esophagi contain submucosal melanocytes. Primary esophageal melanoma accounts for <1% of primary esophageal malignancies.
REFERENCES
1. Simchuk EJ, Low DE. Direct esophageal metastasis from a distant primary tumor is a submucosal process: a review of six cases. Dis Esophagus 2001;14:247-250.
2. Mizobuchi S, Tachimori Y, Kato H, Watanabe H, Nakanishi Y, Ochiai A. Metastatic esophageal tumors from distant primary lesions: report of three esophagectomies and study of 1835 autopsy cases. Jpn J Clin Oncol 1997;27:410-414.
3. Vashi PG, Gupta D, Tan B. Colon carcinoma with unusual metastasis to the esophagus manifesting as multiple nodules and dysphagia: management with systemic chemotherapy. Case Rep Gastroenterol 2012;6:484-488.
4. Naomoto Y, Perdomo JA, Kamikawa Y, et al. Primary malignant melanoma of the esophagus: report of a case successfully treated with pre- and post-operative adjuvant hormone-chemotherapy. Jpn J Clin Oncol 1998;28:758-761.
CASE 1.33 CLINICAL HISTORY 58-year-old man with dysphagia.
FINDINGS Frontal radiographs (A, B) from a barium swallow demonstrate rounded lateral pharyngeal outpouchings that promptly filled with contrast material and emptied.
DIFFERENTIAL DIAGNOSIS Lateral pharyngeal diverticula, Killian-Jamieson diverticula.
DIAGNOSIS Lateral pharyngeal diverticula.
DISCUSSION Lateral outpouchings at the level of the pharynx are characteristics of lateral pharyngeal diverticula. These diverticula may be unilateral or bilateral. The anatomic location of lateral pharyngeal diverticula has been variously described as being through the thyrohyoid membrane anterior to the thyrohyoid ligament and between the superior and middle pharyngeal constrictors. These diverticula are also sometimes called pharyngoceles, pharyngeal pouches, or hypopharyngeal ears.
Incidence of lateral pharyngeal diverticula increases with age. There is also a strong male predominance. Large lateral pharyngeal diverticula have been described in players of wind instruments, presumably due to prolonged episodes of increased intrapharyngeal pressure. Asking the patient to increase intrapharyngeal pressure by puffing out his/her cheeks can increase the conspicuity of these lateral pharyngeal diverticula.
Question for Further Thought
1. Are lateral pharyngeal diverticula associated with dysphagia?
View Answer
1. Whether lateral pharyngeal diverticula result in dysphagia is a source of controversy in the literature. Some authors describe these structures as being asymptomatic. Other authors contend that such diverticula can result in dysphagia and other symptoms such as regurgitation of undigested food and argue for removal.
Reporting Requirement
1. This patient has small bilateral lateral pharyngeal diverticula.
What the Treating Physician Needs to Know
1. The significance of lateral pharyngeal diverticula is controversial. Referral to an otolaryngologist may be warranted to attempt to correlate symptoms with imaging findings and discuss management options.
Answer
1. Whether lateral pharyngeal diverticula result in dysphagia is a source of controversy in the literature. Some authors describe these structures as being asymptomatic. Other authors contend that such diverticula can result in dysphagia and other symptoms such as regurgitation of undigested food and argue for removal.
REFERENCES
1. Curtis DJ, Cruess DF, Crain M, Sivit C, Winters C Jr, Dachman AH. Lateral pharyngeal outpouchings: a comparison of dysphagic and asymptomatic patients. Dysphagia 1988;2:156-161.
2. Norris CW. Pharyngoceles of the hypopharynx. Laryngoscope 1979;89:1788-1807.
CASE 1.34 CLINICAL HISTORY 38-year-old man with HIV disease and dysphagia.
FINDINGS Air-contrast barium swallow (A, B) demonstrates numerous relatively parallel, linear outpouchings of contrast material arising from the esophageal lumen. These outpouchings are oriented perpendicular to the long axis of the esophagus.
DIFFERENTIAL DIAGNOSIS Intramural pseudodiverticulosis ulcers.
DIAGNOSIS Intramural pseudodiverticulosis.
DISCUSSION Esophageal intramural pseudodiverticulosis is a rare condition that is present in fewer than 1% of swallow studies. The pathogenesis of pseudodiverticulosis is unknown. At histology, dilated submucosal glands and excretory ducts are visible. Pseudodiverticula are thought to reflect contrast material within these dilated excretory ducts.
At barium swallow, pseudodiverticulosis is characterized by numerous small contrast-filled outpouchings located parallel to each other but perpendicular to the esophageal lumen. The outpouchings sometimes appear flask-shaped, though not in the above case. These outpouchings may involve the esophagus diffusely or segmentally, and a relatively equal distribution of segmental disease along the proximal, mid-, and distal esophagus has been described.
Intramural pseudodiverticulosis has been described in association with a number of conditions, including strictures, reflux esophagitis, esophageal candidiasis, acid injury, and esophageal cancer. Patients with esophageal pseudodiverticulosis often present with dysphagia or chest pain though symptoms are usually attributed to conditions such as reflux that accompany pseudodiverticulosis rather than the outpouchings themselves.
Questions for Further Thought
1. What is the treatment of esophageal intramural pseudodiverticulosis?
View Answer
1. Treatment is not directed specifically at the pseudodiverticula but rather at the associated condition such as reflux esophagitis or candidiasis.
2. Are these pseudodiverticula expected to resolve with treatment?
View Answer
2. Pseudodiverticula usually resolve with successful treatment of the accompanying condition.
Reporting Requirements
1. Report the location of esophageal pseudodiverticulosis.
2. Suggest that EGD may be necessary to determine the underlying etiology.
What the Treating Physician Needs to Know
1. Esophageal intramural pseudodiverticulosis is associated with a variety of usually benign conditions.
2. EGD may be necessary to diagnose the underlying condition.
Answers
1. Treatment is not directed specifically at the pseudodiverticula but rather at the associated condition such as reflux esophagitis or candidiasis.
2. Pseudodiverticula usually resolve with successful treatment of the accompanying condition.
REFERENCES
1. Levine MS, Moolten DN, Herlinger H, Laufer I. Esophageal intramural pseudodiverticulosis: a reevaluation. Am J Roentgenol 1986;147:1165-1170.
2. Levine MS, Rubesin SE. Diseases of the esophagus: diagnosis with esophagography. Radiology 2005;237:414-427.
3. Chon YE, Hwang S, Jung KS, et al. A case of esophageal intramural pseudodiverticulosis. Gut Liver 2011;5:93-95.
CASE 1.35 CLINICAL HISTORY 48-year-old man with HIV disease, dysphagia, and chest pain.
FINDINGS Air-contrast barium swallow demonstrates a diffusely abnormal appearance of the esophageal mucosa. Numerous plaque-like areas are seen with thin linear areas of contrast material in between these plaque-like areas (A, B).
DIFFERENTIAL DIAGNOSIS Candidiasis, glycogenic acanthosis, reflux esophagitis, superficial esophageal malignancy.
DIAGNOSIS Candidiasis.
DISCUSSION Esophageal candidiasis is common in HIV-positive patients and is most commonly seen when the CD4 count is <200. C. albicans is the most common causative species and usually responds to a 14- to 21-day course of oral or intravenous fluconazole. Esophageal candidiasis is sometimes asymptomatic but usually results in odynophagia.
At physical examination, oropharyngeal candidiasis (also known as “thrush”) appears as white plaques that can be scraped off. Esophageal candidiasis has a similar appearance at EGD. At fluoroscopy, esophageal candidiasis may initially manifest as raised plaque-like areas as in this case. With more severe disease, the esophagus may develop a “shaggy” appearance. Other conditions that lead to immunocompromise (e.g., diabetes) or stasis (e.g., achalasia or obstructing masses) also can result in candidiasis.
Glycogenic acanthosis may also result in raised plaquelike areas. Glycogenic acanthosis is a benign and usually asymptomatic condition characterized by intracellular glycogen accumulation. This condition most commonly affects elderly patients. Reflux esophagitis may also result in nodular or plaque-like raised areas, most commonly located in the distal esophagus. Superficial esophageal cancer can also appear as raised, plaque-like areas.
Questions for Further Thought
1. Is endoscopy required to confirm esophageal candidiasis prior to treatment in AIDS patients with oral thrush?
View Answer
1. No. If oral thrush is present and esophageal symptoms are reported, esophageal candidiasis is assumed. Endoscopy is usually unnecessary and is only performed if such patients do not respond to treatment for candidiasis.
2. How can plaques be distinguished from ulcers at fluoroscopy?
View Answer
2. Plaques are raised areas. At esophagography, contrast material outlines the edges of plaques. On the other hand, ulcers are depressed areas. At esophagography, contrast material is present centrally within an ulcer.
Reporting Requirements
1. Describe the presence of diffuse plaque-like areas throughout the esophagus.
2. Suggest that clinical correlation be made for possible candidiasis.
What the Treating Physician Needs to Know
1. In an HIV-positive patient with oral thrush, the above appearance can be assumed to be due to esophageal candidiasis.
2. In other clinical scenarios, EGD with tissue sampling may be necessary to establish a diagnosis prior to treatment.
Answers
1. No. If oral thrush is present and esophageal symptoms are reported, esophageal candidiasis is assumed. Endoscopy is usually unnecessary and is only performed if such patients do not respond to treatment for candidiasis.
2. Plaques are raised areas. At esophagography, contrast material outlines the edges of plaques. On the other hand, ulcers are depressed areas. At esophagography, contrast material is present centrally within an ulcer.
REFERENCES
1. Rubesin SE, Levine MS. Differential diagnosis of esophageal disease on esophagography. Appl Radiol 2001;30:11-21.
2. Bianchi Porro G, Parente F, Cermuschi M. The diagnosis of esophageal candidiasis in patients with acquired immune deficiency syndrome: is endoscopy always necessary? Am J Gastroenterol 1989;84:143-146.
3. Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Morb Mortal Wkly Rep 2009;58:1-198.
CASE 1.36 CLINICAL HISTORY 63-year-old man with fevers and tachycardia 3 days following esophagectomy for Barrett esophagus.
FINDINGS Water-soluble contrast study demonstrates contrast material in the esophageal lumen and a large volume of contrast material spilling into the right hemothorax (A, B).
DIFFERENTIAL DIAGNOSIS Anastomotic leak.
DIAGNOSIS Anastomotic leak.
DISCUSSION Leaks are diagnosed at fluoroscopy by identifying an extraluminal collection of contrast material that increases in volume over time and does not peristalse away.
Anastomotic leaks following esophagectomy occur in 4% to 13% of patients. Leaks increase patient morbidity and are associated with increased rates of anastomotic stricture formation. Treatment options include surgical repair, conservative management with supportive care, and covered stent placement to attempt to seal off the leak site. Risk factors that correlate with the development of postoperative leaks include diabetes and increased age. High-volume surgeons in high-volume centers have been reported to have relatively lower rates of leak.
A variety of surgical techniques are used for esophageal resection. Variables include whether a cervical or thoracic anastomosis is performed and the extent of lymphadenectomy (e.g., cervical, thoracic, and celiac nodal stations). The stomach is most commonly used as the replacement conduit though some surgeons prefer to use colon.
Questions for Further Thought
1. What contrast agent should be used when evaluating for leak following esophagectomy?
View Answer
1. Water-soluble contrast material should be used initially to rule out a large leak. Spill of large volumes of barium into the mediastinum or pleural space should be avoided since barium will not be resorbed and can trigger inflammation. If the initial water-soluble study is negative, barium should be administered as leaks are sometimes visible with barium even when the initial water-soluble contrast images are negative.
2. List other complications that can occur following esophagectomy.
View Answer
2. Anastomotic stricture, dumping syndrome, pneumonia, and vocal cord paralysis due to recurrent laryngeal nerve injury are other potential complications following esophagectomy.
Reporting Requirement
1. Contact the ordering physician and inform him/her of the anastomotic leak.
What the Treating Physician Needs to Know
1. The location of the leak and a subjective assessment of the volume of the leak (e.g., large versus small) are helpful data points for the surgeon.
2. If leaked contrast material appears to exit via a surgically placed drain.
Answers
1. Water-soluble contrast material should be used initially to rule out a large leak. Spill of large volumes of barium into the mediastinum or pleural space should be avoided since
barium will not be resorbed and can trigger inflammation. If the initial water-soluble study is negative, barium should be administered as leaks are sometimes visible with barium even when the initial water-soluble contrast images are negative.
barium will not be resorbed and can trigger inflammation. If the initial water-soluble study is negative, barium should be administered as leaks are sometimes visible with barium even when the initial water-soluble contrast images are negative.
2. Anastomotic stricture, dumping syndrome, pneumonia, and vocal cord paralysis due to recurrent laryngeal nerve injury are other potential complications following esophagectomy.
REFERENCES
1. D’Amico TA. Outcomes after surgery for esophageal cancer. Gastrointest Cancer Res 2007;5:188-196.
2. Schuchert MJ, Abbas G, Nason KS, et al. Impact of anastomotic leak on outcomes after transhiatal esophagectomy. Surgery 2010;148:838-840.
3. Hunerbein M, Stroszczynski C, Moesta KT, Schlag PM. Treatment of thoracic anastomotic leaks after esophagectomy with self-expanding plastic stents. Ann Surg 2004;240:801-807.
CASE 1.37 CLINICAL HISTORY 59-year-old woman with dysphagia.
FINDINGS Air-contrast barium swallow demonstrates a short-segment narrowing of the proximal esophagus with minimal adjacent mucosal irregularity.
DIFFERENTIAL DIAGNOSIS Benign stricture, malignant stricture.
DIAGNOSIS Benign stricture.
DISCUSSION GERD is the most common cause of esophageal stricture. Other etiologies of esophageal stricture include infection, pill-related injury, malignancy, radiation-induced, congenital, caustic ingestion, and autoimmune disorders.
Patients with strictures often present with dysphagia, initially to solids and sometimes later to liquids.
Question for Further Thought
1. List medications associated with medication-induced strictures.
View Answer
1. More than 100 different medications have been reported to cause pill-induced esophagitis. Some of the more commonly implicated medications include bisphosphonates, nonsteroidal anti-inflammatory drugs (NSAIDs), and antibiotics including doxycycline.
Reporting Requirements
1. Report the location and length of the stricture.
2. If there is associated mucosal irregularity, suggest EGD to evaluate for malignancy.
What the Treating Physician Needs to Know
1. This patient has a short-segment esophageal stricture in the proximal to mid-esophagus.
Answer
1. More than 100 different medications have been reported to cause pill-induced esophagitis. Some of the more commonly implicated medications include bisphosphonates, nonsteroidal anti-inflammatory drugs (NSAIDs), and antibiotics including doxycycline.
REFERENCE
1. Pace F, Antinori S, Repici A. What is new in esophageal injury (infection, drug-induced, caustic, stricture, perforation)? Curr Opin Gastroenterol 2009;25:372-379.
CASE 1.38 CLINICAL HISTORY 34-year-old woman with sensation of something “stuck” in her throat after swallowing a fish bone.
FINDINGS Initial anteroposterior (A) and lateral (B) softtissue neck radiographs did not demonstrate a radiopaque foreign body. A 2 cm × 3 cm fish bone fragment was found in the mid- to distal esophagus at subsequent EGD. The following day, the patient’s throat discomfort worsened and subcutaneous emphysema developed. Water-soluble contrast material swallow study demonstrates an irregular collection of contrast material (C) along the right aspect of the pharynx that increased in subsequent images (D) indicating a perforation.
DIFFERENTIAL DIAGNOSIS Pharyngeal perforation.
DIAGNOSIS Pharyngeal perforation caused by foreign body (fish bone).
DISCUSSION Fish bones are thought to be the most commonly ingested bone fragments. When fish heads are consumed, large irregularly shaped pieces of bone may be ingested. A sharp pricking sensation with swallowing is the most sensitive and specific symptom of a lodged fish bone. The majority of lodged fish bones are located in the oral cavity or pharynx.
Plain films are only 32% to 39% sensitive and 72% to 91% specific for the detection of fish bones located in the pharynx and cervical esophagus. The low sensitivity reflects the fact that the majority of fish bone fragments are radiolucent. The <100% specificity reflects the fact that cartilaginous calcifications may be mistaken for bone fragments. By comparison, CT is more sensitive for the identification of fish bones.
Given the limited sensitivity of plain films for the detection of fish bones, some authors advocate performing CT on all patients with suspected retained fish bones and other authors advocate performing endoscopy on all patients with the sensation of a retained fish bone.
Question for Further Thought
1. Are most beef and chicken bones radiopaque?
View Answer
1. Unlike fish bones that are most often NOT radiopaque, the vast majority of beef and chicken bones are radiopaque.
Reporting Requirements
1. No radiopaque foreign body is seen in the initial neck soft-tissue radiographs.
2. As radiographs are of limited sensitivity for detection of fish bones, CT or endoscopy should be considered.
What the Treating Physician Should Know
1. The majority of fish bones are radiolucent and, therefore, invisible on radiographs.
2. CT is more sensitive than plain film for fish bones.
3. If strong clinical concern for a lodged bone fragment and imaging studies are negative, endoscopy should be performed.
Answer
1. Unlike fish bones that are most often NOT radiopaque, the vast majority of beef and chicken bones are radiopaque.
REFERENCES
1. Lue AJ, Fang WD, Manolidis S. Use of plain film radiography and computed tomography to identify fish bone foreign bodies. Otolaryngol Head Neck Surg 2000;123:435-438.
2. Ngan JHK, Fok PJ, Lai ECS, Branicki FJ, Wong J. A prospective study on fish bone ingestion: experience of 358 patients. Ann Surg 1990;211:459-462.
3. Hunter TB, Taljanovic MS. Foreign bodies. Radiographics 2003;23:731-757.
CASE 1.39 CLINICAL HISTORY 72-year-old man with history of odynophagia and lung cancer.
FINDINGS Air-contrast barium swallow demonstrates marked irregularity of the esophageal mucosa with raised plaque-like areas outlined by contrast material as well as small ulcerations indicated by rounded and linear collections of contrast material (A, B).
DIFFERENTIAL DIAGNOSIS Esophagitis, esophageal cancer.
DIAGNOSIS Radiation-induced esophagitis.
DISCUSSION Acute radiation esophagitis occurs approximately 2 to 4 weeks following the administration of at least 5,000 cGy to the mediastinum. The risk of developing radiation-induced esophagitis increases with concurrent chemotherapy and also with increased radiation dose.
Making the diagnosis of radiation esophagitis can help the patient as symptoms may be ameliorated with the administration of proton pump inhibitors and viscous lidocaine. Bland diet as well as avoidance of coffee, alcohol, and acidic foods may also decrease symptoms. Radiation-induced strictures are a delayed complication that may occur 4 to 8 months following the cessation of radiotherapy.
Other etiologies of esophagitis include reflux, infection (e.g., C. albicans, HSV, CMV, and HIV), drug-induced esophagitis, and caustic ingestion. Clinical history is helpful in determining the etiology of esophageal mucosal irregularity, and EGD with tissue sampling may be required in some cases. Disease confined to the radiation port is highly suggestive of radiation esophagitis.
Question for Further Thought
1. What skin conditions are associated with esophagitis?
View Answer
1. Epidermolysis bullosa dystrophica and pemphigoid are skin conditions associated with esophagitis. Both conditions can result in blister formation in the skin and mucous membranes.
Reporting Requirements
1. Report findings of esophagitis.
2. Evaluate for the presence or absence of an associated stricture.
What the Ordering Clinician Needs to Know
Answer
1. Epidermolysis bullosa dystrophica and pemphigoid are skin conditions associated with esophagitis. Both conditions can result in blister formation in the skin and mucous membranes.
REFERENCES
1. Berkey FJ. Managing the adverse effects of radiation therapy. Am Fam Phys 2010;15:381-388.
2. Singh AK, Lockett MA, Bradley JD. Predictors of radiation-induced esophageal toxicity in patients with small-cell lung cancer treated with three-dimensional conformal radiotherapy. Int J Radiation Oncol Biol Phys 2003;55:337-341.
3. Levine MS, Rubesin SE. Diseases of the esophagus: diagnosis with esophagography. Radiology 2005;237:414-427.
CASE 1.40 CLINICAL HISTORY 86-year-old woman with dysphagia.
FINDINGS Oblique images (A, B) from a barium swallow demonstrate contrast material within the esophageal lumen. Additionally, a linear and somewhat undulating area of contrast material is seen anterior to the esophagus. (Note vertebral bodies posteriorly.)
DIFFERENTIAL DIAGNOSIS Aspiration, tracheoesophageal fistula.
DIAGNOSIS Aspiration.
DISCUSSION Aspiration of contrast material is defined as the contrast material passing into the trachea below the level of the true vocal cords. Based on the above two images, it is difficult to distinguish between a fistula and aspiration. Evaluation of the larynx is required to evaluate for aspiration. The lateral projection is most helpful to evaluate for aspiration as barium passing from the pharynx through the vocal cords will be demonstrated. By comparison, contrast material will extend directly from the esophagus to the trachea in the setting of a tracheoesophageal fistula. Undulations seen above in the linear area of contrast material anterior to the esophagus are due to tracheal cartilage.
Distinguishing between a tracheoesophageal fistula and aspiration is critically important. Treatment for a tracheoesophageal fistula usually includes surgery. Treatment of aspiration involves maneuvers such as “chin-tuck” and “head-tilt” to try to diminish the volume of aspirated material.
If there is clinical concern for aspiration, barium should be used rather than water-soluble contrast material. Water-soluble contrast material is contraindicated in the setting of possible aspiration due to the risk of severe pulmonary edema due to osmolality effects. If aspiration is observed, the patient should be encouraged to cough up as much barium as is possible.
Questions for Further Thought
1. What is the difference between penetration and aspiration?
View Answer
1. Penetration is defined as contrast material entering the airway but not extending below the level of the vocal cords.
2. What are potential etiologies of aspiration?
View Answer
2. Impaired swallowing due to stroke is a major cause of aspiration. Other neuromuscular disorders may also result in aspiration.
Reporting Requirement
1. Report if aspiration is “silent.” Aspiration is considered to be “silent” if the aspiration event does not trigger the choking reflex and coughing in the patient.
What the Treating Physician Needs to Know
1. Contact the treating physician to report the presence of aspiration.
2. Aspiration puts the patient at risk for aspiration pneumonia.
Answers
1. Penetration is defined as contrast material entering the airway but not extending below the level of the vocal cords.
2. Impaired swallowing due to stroke is a major cause of aspiration. Other neuromuscular disorders may also result in aspiration.