VCE device
Manufacturer
Year introduced
FDA approved
Dimensions
Angle of view
Max images per second
Single/dual optical dome
Battery life
Small bowel
Pillcam SB I (M2A)
Given Imaging LTD, Yokneam, Israel
2001
Yes
11 × 26 mm
140
2
Single
8 h
Pillcam SB 2
Given Imaging LTD, Yokneam, Israel
2007
Yes
11 × 26 mm
156
2
Single
8 h
EndoCapsule
Olympus, Tokyo, Japan
2007
Yes
11 × 26 mm
145
2
Single
8 h
OMOM
Jinshan S and T Co., Chongqing, China
2004
No
13 × 27.9 mm
140
2
Single
8–16 h
MiroCAM
IntroMedic, Seoul, Korea
2005
Yes
11 × 24 mm
140
3
Single
11 h
Esophagus
Pillcam ESO
Given Imaging LTD, Yokneam, Israel
2004
Yes
11 × 26 mm
140
14
Dual
20 min
Pillcam ESO 2
Given Imaging LTD, Yokneam, Israel
2007
Yes
11 × 26 mm
169
18
Dual
30 min
Pillcam ESO 3
Given Imaging LTD, Yokneam, Israel
2011
Yes
11 × 26 mm
169
35
Dual
30 min
Colon
Pillcam Colon
Given Imaging LTD, Yokneam, Israel
2006
No
31 × 11 mm
156 × 2
Dual
Unspecified
Pillcam Colon 2
Given Imaging LTD, Yokneam, Israel
2009
Yes
31.5 × 11.6 mm
172 × 2
35
Dual
Unspecified
Indications for Small Bowel Video Capsule Endoscopy (VCE)
There are several well-established indications for small bowel video capsule endoscopy (VCE) , including obscure GI bleeding, investigation of Crohn’s disease, and evaluation of small bowel tumors and hereditary polyposis syndromes. The use of VCE in the evaluation of celiac disease remains under investigation.
Obscure GI Bleeding
Obscure GI bleeding (OGIB) is defined as recurrent episodes of clinically evident GI bleeding (i.e., melena, hematochezia, or hematemesis), positive fecal occult blood testing, or chronic iron deficiency anemia (IDA) despite negative upper and lower endoscopies. Obscure GI bleeding may include either overt or occult obscure GI bleeding. Overt GI bleeding indicates the visible presence of blood in the vomitus or stool, as opposed to occult bleeding, which presents as normal appearing stools with either positive fecal occult blood testing or unexplained iron deficiency anemia. OGIB is by far the most common indication for VCE. Numerous studies have demonstrated the superiority of VCE in identifying a source of OGIB compared to the other methods of small bowel evaluation, with the exception of double balloon enteroscopy (DBE), which has a similar diagnostic yield [5–8]. Based on a multicenter meta-analysis published by Kamalaporn et al., agreement between VCE and DBE was 74 % for angioectasias and approximately 95 % for all other lesions such as polyps, tumors, and ulcerations [9] (Figs. 1,2). Given the invasive and time-intensive nature of double balloon enteroscopy (DBE), VCE is a more reasonable first-line study for the evaluation of OGIB.
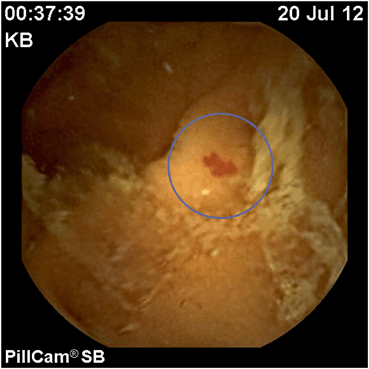
Fig. 1
Nonbleeding small bowel angioectasia, jejunum
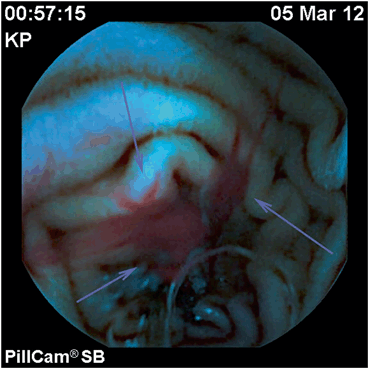
Fig. 2
Active bleeding, small bowel angioectasia
A retrospective review of 260 OGIB cases revealed diagnostic yields of 60 % in cases of overt obscure bleeding versus 46 % in patients with occult obscure bleeding [10]. In cases of overt obscure bleeding, capsule endoscopy has the highest diagnostic yield within the first 48 h following hospitalization [11]. Negative VCE studies are also clinically important as these have been associated with rebleeding rates of less than 5 % compared to patients with a positive study, in whom rebleeding rates approach 50 % [12]. In a study by Apostolopoulos et al., 57 % of 51 ambulatory patients with IDA referred for VCE were found to have likely sources of bleeding [13]. Since VCE is purely diagnostic, further evaluation or definitive therapy usually requires single or double balloon enteroscopy, surgery, or angiography.
Crohn’s Disease
In the setting of known or suspected Crohn’s disease, VCE may be helpful in establishing or confirming the diagnosis, assessing the extent and severity of small bowel involvement, determining mucosal response to therapy, and evaluating recurrent disease following surgery (Fig. 3). The use of VCE may also be considered in patients diagnosed with ulcerative colitis with atypical clinical features and in cases of indeterminate colitis [14]. Studies have yet to show significant difference in diagnostic yield of VCE compared to the current imaging modalities [15]. VCE’s ability to directly visualize small bowel mucosa potentially may represent a novel means of monitoring disease activity and response to treatment. It is unclear whether VCE is superior to traditional imaging when evaluating recurrent disease [16] although this was suggested in a study published by Pons Beltron et al., which showed higher rates of recurrence in postsurgical patients monitored with VCE compared to endoscopic evaluation of the colon and neoileum [17]. Several scoring systems have been developed to aid the physician in quantifying disease severity although none have yet been adopted for widespread use. Recent studies have failed to show a significant correlation with mucosal healing and clinical symptom improvement, and as such, the importance of mucosal healing in the treatment of Crohn’s disease remains under investigation [18].
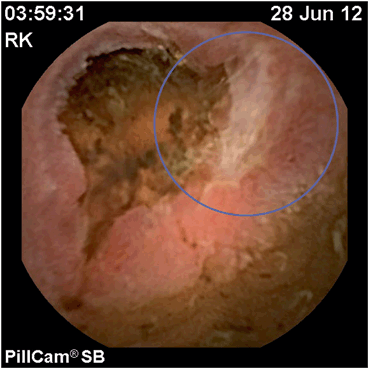
Fig. 3
Ulceration of distal ileum consistent with recurrent Crohn’s disease
VCE has significant limitations in assessing Crohn’s disease. Although very good at visualizing small bowel ulcerations, VCE cannot readily distinguish between ulcers associated with Crohn’s disease or due to other etiologies such as nonsteroidal anti-inflammatory drug (NSAID) use. For this reason, it has been suggested that patients undergoing VCE for evaluation of Crohn’s disease should avoid NSAIDs for at least 1–2 months prior to the exam [19]. VCE is also unable to evaluate extraluminal manifestations of Crohn’s disease such as abscess or fistulas. Due to the concerns of capsule retention in Crohn’s disease patients, the evaluation of small bowel patency with Small Bowel Follow Through Radiologic Examination (SBFT) Computerized Tomography (CT), or a patency capsule is generally performed prior to capsule endoscopy [20, 21]. Several scoring systems have been developed over the years in an attempt to standardize the evaluation of small bowel Crohn’s disease. The first such scoring system was developed by Kornbluth et al. in 2004 which examined five parameters: erythema, edema, nodularity, ulcers, and stenosis [22]. The scoring index introduced by Gralnek et al. in 2005, divided the small bowel into thirds and evaluated each segment for villous edema, ulcers, and stenosis [23]. Finally, the capsule endoscopy Crohn’s disease activity index (CECDAI) is the most recent scoring system which was introduced by Gal et al. in 2008 [24]. The CECDAI divides the small bowel into two sections (proximal and distal) and scores each section depending on the most severe disease present, the extent of disease, and the presence of strictures (Table 2). A multicenter, double-blind, prospective study published by Niv et al. in 2012 validated CECDAI and advocated its use in future studies [25].
Table 2
Capsule endoscopy-related Crohn’s disease activity index (CECDAI
Segment | A: Inflammation (0–5) | B: Extent (0–3) | C: Stricturing (0–3) | CECDAI score |
|---|---|---|---|---|
Proximal | 0 = None | 0 = None | 0 = None | A1 × B1 + C1 |
(section 1) | 1 = Mild/moderate | 1 = Focal | 1 = Single | |
2 = Severe | 2 = Patchy | 2 = Multiple | ||
3 = Ulcer < 5 mm | 3 = Diffuse | 3 = Obstructing | ||
4 = Ulcer 5–20 mm | ||||
5 = Ulcer > 20 mm | ||||
Distal | 0 =None | 0 = None | 0 = None | A2 × B2 + C2 |
(section 2) | 1 =Mild/moderate | 1 = Focal | 1 = Single | |
2 = Severe | 2 = Patchy | 2 = Multiple | ||
3 = Ulcer < 5 mm | 3 = Diffuse | 3 = Obstructing | ||
4 = Ulcer 5–20 mm | ||||
5 = Ulcer > 20 mm | ||||
Section 1 + section 2 = Total |
At present, VCE has a complementary role in the diagnosis of Crohn’s disease and is typically used to identify patients with possible inflammatory disease of the small bowel when the traditional imaging and endoscopic modalities have failed to make a definite diagnosis. On the other hand, VCE in the evaluation of recurrent disease and grading of disease severity does not have a clearly defined role currently and further studies are needed.
Hereditary Polyposis Syndromes and Small Bowel Tumors
Although there are only a few published studies regarding VCE and polyposis syndromes, VCE would appear to be an excellent tool in the detection of small bowel polyps. VCE is superior to barium contrast studies and magnetic resonance imaging (MRI) for polyps less than 15 mm. Advantages of MRI include more accurately determining polyp location and, in cases of large polyps, better estimation of polyp size when compared to VCE [21, 26].Patients with familial adenomatous polyposis (FAP) should undergo complete GI tract evaluation at the time of diagnosis, although the presence of upper GI adenomas prior to colonic disease is rare. Lifetime development of duodenal adenomas is high (60–90 %) and the risk of duodenal or periampullary malignancy is estimated at 5–12 %. Due to limited visualization of the ampulla on VCE, esophagogastroduodenoscopy (EGD) with a side-viewing instrument in addition to forward-viewing scope is mandatory. The endoscopic surveillance intervals and the treatment approaches are determined by the Spigelman staging (a classification system based on the number, size, histology, and grade of dysplasia of duodenal polyps). The role for more distal small bowel screening is still unclear. Jejunal and ileal adenomas develop in approximately 40 and 20 % of FAP patients, respectively, with very rare transformation to adenocarcinoma. Hence, there are no consensus guidelines for small-bowel surveillance past the duodenum, and no guidelines as to what lesions would warrant DBE with biopsy or polypectomy . One suggested approach is to perform VCE in patients with stage III-IV duodenal polyposis with subsequent small bowel enteroscopy for biopsy and/or removal of high-risk polyps [27–29].
Patients with Peutz-Jeghers syndrome (PJS) are at increased risk for a variety of cancers, including the small bowel (13 % of all cancers in PJS patients). A routine screening of the small bowel with VCE is recommended every 2–3 years, with subsequent DBE and polypectomy if polyps are detected. No routine screening of the small bowel is currently recommended in other polyposis syndromes [27, 28].
Small bowel tumors in the absence of polyposis syndrome were thought to be fairly uncommon, but increased use of VCE has led to an increment in their detection: between 2.4 and 9.6 % of patients undergoing VCE for OGIB have been found to have a small bowel tumor. Of these, the majority are gastrointestinal stromal tumors (GIST), followed by adenocarcinomas, carcinoids, lymphomas, and sarcomas [30–36].
Celiac Disease
Although celiac disease can be suspected on clinical grounds or by serologic assays, the gold standard for diagnosis remains small bowel biopsy with histological changes of villous atrophy and increased intraepithelial lymphocytes. Two studies examining patients with suspected celiac disease based on positive serological tests compared conventional EGD with duodenal biopsies to VCE. One found sensitivities for VCE of 85–87.5 % and specificities between 90–100 % [35, 37]. The second study examined the diagnostic yield of VCE in patients with biopsy proven celiac disease and reported a sensitivity and specificity of 92 and 100 %, respectively. The obvious limitation of VCE in diagnosing celiac disease is the inability to detect microscopic changes of celiac disease, which can be present without evident macroscopic mucosal changes. Moreover, typical macroscopic changes of celiac disease such as scalloping can also be seen in a variety of other conditions such as amyloidosis, eosinophilic enteritis, giardiasis, and human immunodeficiency virus (HIV) enteropathy. For these reasons, VCE is not routinely used in the diagnosis of celiac disease [37–39].
One instance where VCE may provide additional diagnostic benefit is in patients with suspected or refractory celiac disease. Although celiac disease usually involves the proximal duodenum, there have been cases where the fourth portion of the duodenum and proximal jejunum are the only affected areas. These more distal changes can be observed on VCE and may help determine if this subset of patients would benefit from a push enteroscopy and histologic confirmation. In refractory celiac disease, VCE may help exclude the presence of ulcerative jejunoileitis and enteropathy associated T-cell lymphoma (EATL) [40, 41].
Esophageal Capsule Endoscopy
The role of video capsule endoscopy in assessing the esophagus remains an issue under ongoing investigation . EGD remains the gold standard for evaluation of the esophagus due to the ability to perform endoscopic interventions, carefully inspect and interrogate areas of interest, and insufflate to permit optimal visualization. EGD is also relatively quick, well-tolerated, widely available, low cost, and is associated with minimal complication rates. Despite the advantages of EGD, VCE is an attractive option for the examination of the esophagus in those at high risk for anesthesia-related complications and those who prefer noninvasive means of diagnostic evaluation. The most studied indications for VCE include screening and surveillance of varices, screening for Barrett’s esophagus, and evaluation of reflux esophagitis.
In 2004, Given Imaging Ltd. developed the PillCam ESO®, which was the first wireless video capsule specifically designed for noninvasive evaluation of the esophagus. This capsule was similar in size to the intestinal capsule (11 × 26 mm) but equipped with two optical domes instead of one, which captured 14 images per second (7 from each optical dome) and had an operational time of 20 min. In 2007, the PillCam ESO 2® was released, which increased the angle of view from 140 ° to 169 °, increased the frame rate to 18, improved image quality, and added the ability to adjust illumination in real time. The PillCam ESO 3®, which boasts an increased frame rate of 35 images per second, received FDA approval in 2011. Ingestion protocol for these devices involve the patient lying on their right side and taking sips of water every 15 s for 3 min following ingestion of the capsule [42, 43].
Studies comparing VCE to EGD have focused primarily on the difference in detection rates of Barrett’s esophagus and esophageal varices. There has been significant heterogeneity among studies; when compared to EGD as the gold standard, VCE detection rates of Barrett’s esophagus and erosive esophagitis have ranged from 60–100 % and 50–90 %, respectively [44–47]. There appears to be similar heterogeneity in VCE rates of variceal detection, ranging from 68 to 100 %, depending on the study [48–53]. A meta-analysis conducted by Lu et al. examined over 440 patients and reported sensitivity and specificity rates of 86 and 81 % when comparing VCE to EGD, with sub-group analysis showing markedly lower specificity of 55 % in the screening-only population [54]. One study compared EGD to the use of a video capsule attached to a string, allowing for longer evaluation of the esophagus and reported > 95 % agreement in detecting varices [55]. At present, there is insufficient data to recommend the use of VCE as an alternative to EGD for evaluation of the esophagus. Although VCE is largely preferred by patients involved in comparison studies, the wide variability in specificity seen across numerous studies raises questions about whether it can be adopted as standard practice. Much of the variability likely resides in the novelty of such wireless VCE evaluations and relative lack of expertise in interpreting the studies. As the functionality of these devices continues to improve and their use becomes more widespread, further studies will determine whether wireless VCE can find a niche in everyday practice .
Colon Capsule Endoscopy
Colon capsule endoscopy (CCE) , approved by the US FDA in 2014 for screening for colorectal cancer in patients with incomplete traditional colonoscopy, represents the newest modality in offering a minimally invasive means of screening for colorectal cancer and its precursor lesions.
The first PillCam Colon® (Given Imaging Ltd.) was introduced in 2006. It was a 31 × 11 mm video capsule with two optical domes allowing for an angle of view of 156 °. It acquired images at the rate of four images per second (two from each dome) and had an operational time of 10 h. At the start of an exam, the capsule would transmit images for 3 min, after which it would enter a “sleep mode” for 105 min to save battery life. A second generation model, the PillCam Colon 2® (See Figure), is slightly larger at 31.5 × 11.6 mm but has an increased angle of view to 172 ° (344 ° total viewing). Rather than having a “sleep mode,” the newer PillCam Colon will obtain 14 images per minute until small bowel is detected, after which the capsule records images using an adaptable frame rate to preserve battery life; while motionless, the capsule takes four images per second, but once motion is detected, the number of images per second automatically increases to 35. Additionally, the liquid crystal display (LCD) on the external recording device can prompt the patient to continue the preparation protocol, once the capsule has moved into the small intestine. The viewing software includes tools such as a polyp size estimator [56].
In the USA, Pillcam Colon 2 has been approved for detection of colon polyps in patients after an incomplete optical colonoscopy. Potential future applications may be similar to those for diagnostic colonoscopy, such as colorectal cancer screening in average risk individuals, surveillance in patients with prior colorectal adenomas or cancer, as well as diagnostic evaluation of colorectal symptoms (in patients at high risk for cardiopulmonary complications due to sedation or in subjects with significant comorbidities). Colon capsule endoscopy provides an advantage over CT colography in that it does not involve exposure to radiation and may be used in patients who have had an incomplete colonoscopy [57]. Since it is a minimally invasive, diagnostic-only examination, anticoagulants need not be stopped. There are, however, several limitations of colon capsule endoscopy, including the inability to perform interventions such as biopsy or snare, inability to insufflate, wash, or suck, which limits the quality of the inspection, and incomplete examinations resulting from extended examination duration and insufficient battery life .
As there is no means to clean the colon wall during a capsule endoscopy, an exceptionally well-prepped colon is required for adequate visualization. Numerous colon cleansing protocols combined with prokinetics have, therefore, been suggested, since traditional colon preparations achieve complete examinations in only 20 % of patients—an unacceptably low rate. The first modified colon preparation used by Schoofs et al. [58] involved a clear liquid diet the day prior to the exam, followed by 3 L of polyethylene glycol solution. Patients then had to drink an additional liter of Polyethlene Glycol (lavage solution) (PEG) over an hour to be finished 1 h prior to capsule ingestion. Patients were given domperidone (not FDA approved in the USA) 15 min prior to capsule ingestion, and then were made to ingest sodium phosphate with a liter of water both 2 and 4 h post ingestion. After 8.5 h, the patient administers a bisacodyl suppository. Several studies showed sodium phosphate was integral for capsule transit and expulsion within the 10-h exam time [59, 60].A newer protocol developed by Eliakim et al. [61] included a more balanced split dose PEG preparation (2 L the evening before and the morning of the exam), lower dose of sodium phosphate boosters, and domperidone only if the capsule failed to pass from the stomach after 1 h. Under the protocol described by Schoofs (often referred to as the “Belgium Regimen”), capsule excretion rates within 10 h were reported to be between 83 and 100 %, with a median of 92 % (fairly close to the > 95 % benchmark recommended for complete screening colonoscopies). In the Eliakim et al. study, the excretion rate at 8 h was 81 %, at which time a colonoscopy was performed as part of the study design. Even with strict adherence to the vigorous colon preparations used for CCE, approximately 20 % of exams are deemed inadequate due to insufficient visualization.
A more recent regimen was suggested in 2011 following a pilot study consisting of 60 prospectively enrolled patients. These patients followed a split regimen of PEG administration (2 L the night prior to the test and 2 L on the morning of the procedure) and a 45 mL dose of sodium phosphate. Four senna tablets and a low-residue diet 2–5 days before capsule ingestion were also proposed. CCE excretion rate, colon cleansing, and accuracy were assessed [62]. At CCE, bowel preparation was rated as good in 78 % of patients, fair in 20 %, and poor in 2 %. CCE excretion rate occurred in 83 % of patients .
A large meta-analysis published by Rokkas et al. [63] examined polyp detection rates between the PillCam Colon® and traditional colonoscopy. Compared to colonoscopy, CCE detection of polyps greater than 5 mm had sensitivity and specificity of 68 and 82 %, respectively. Two studies comparing the PillCam Colon 2® to conventional colonoscopy reported much higher sensitivity in detecting polyps greater than 5 mm at 84–89 % with a specificity of 64–76 %. For polyps larger than 1 cm, sensitivity of 88 % and specificity of 89–95 % were reported [64]. Both studies examined populations of patients with known colon disease. Given the lower prevalence of polyps in an average risk individual, sensitivity is likely to be lower in a screening population. Further studies examining polyp detection rates with new generation colon VCE devices are needed. In another multicenter study that included 320 patients, out of 19 cancers detected by colonoscopy, CCE identified 14 (sensitivity 74 %, specificity 74 %) [65]. In patients with unremarkable findings on CCE, a repeat screening test is recommended in 5 years, unless the quality of the prep was inadequate [66].
Similarly, studies examining the use of capsule endoscopy to assess disease severity in ulcerative colitis compared to colonoscopy have had mixed results and further studies are needed [67].
Is CCE cost-effective for use in the general population? This has not yet been determined. However, its safety profile does appear to be excellent, with no major complications reported in any study so far. Only minor limitations have been reported—in the study by Eliakim et al., 2 of 126 patients (1.6 %) were unable to swallow the capsule . In cases such as these, the capsule could be introduced into the stomach or duodenum via a capsule delivery system.
Potential future areas for research include the assessment of efficacy and limitations in colorectal screening, its use to investigate signs and symptoms, assessment of risks (e.g., capsule retention due to the large capsule size), cost analysis compared to traditional colonoscopy, and determination of optimal bowel preparation methods for the procedure [68].
Future of Wireless Video Capsule Endoscopy
Despite tremendous technological advances in wireless video capsule endoscopy , there will be continued opportunities for further refinement and improvement in the future.
Examples of these improvements could include development of smaller, less expensive, cost-effective, easier to swallow capsules, with less risk of capsule retention. Further improvement in the field of vision and use of dual-ended video capsule imaging devices may further reduce the risk of missed lesions. Enhancements in software to more reliably and rapidly detect actively bleeding or vascular lesions, enhanced software to reduce video length that identify “sameness,” as well as software enhancements to better identify significant non-bleeding lesions, such as small bowel tumors should be forthcoming. Use of flexible spectrum imaging color enhancement (FICE) or other imaging enhancement technology or other “electronic chromoendoscopic” techniques are on the horizon [69]. Better localization of lesions detected within the GI tract is desirable, and the ability to control the capsule to alter or regulate its speed of passage through the GI tract, perhaps with use of directional magnets, is technologically feasible [70, 71].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








