, Ivan Damjanov1 and Ryan M. Taylor2
(1)
University of Kansas School of Medicine, University of Kansas, Kansas City, KS, USA
(2)
Division of Gastroenterology and Hepatology, University of Kansas School of Medicine, Kansas City, KS, USA
Liver response to injury includes a relatively narrow spectrum of morphologic changes, and accordingly there are only several pathologic patterns that can be recognized microscopically. First of all one must determine if the liver biopsy is from a native liver or from a liver transplant. If it is native liver one must decide if the liver was biopsied for a mass lesion requiring a tumor diagnosis or a diffuse liver disease. Relevant patient data provided by the clinician is always welcome and encouraged to provide appropriate contextual review.
Diffuse liver diseases may be divided clinicopathologically in two major groups: acute liver diseases and chronic liver diseases (Suriawinata and Thung 2011). Another approach is to examine the liver biopsy and classify the disorder according to the predominant pattern of injury (Torbenson 2015). The third approach would combine these two schemes, and also add an etiologic dimension, separating the liver diseases into those whose causes can be determined, such as infections (e.g. viral, bacterial, parasitic), immune mediated diseases (e.g. autoimmune hepatitis, primary biliary cirrhosis), drug/toxin induced lesions, and biliary tract obstruction (Desmet and Rosai 2011). Since many patterns of liver injury could have more than one cause, and the same causative agents could produce several morphological patterns, it is impossible to devise a generally applicable approach or classification of liver diseases based on incontrovertible criteria that would be acceptable to all authorities in the field.
Our recommendation to the interpretation of liver biopsies is thus to avoid preordained schematic classifications and dogmatism; realize the limitations and actual value of morphologic findings; and try to be as flexible and open-minded as possible. To simplify the matters and to avoid unnecessary confusion we will try first to explain the common patterns of liver injury. In the next chapter we will then illustrate the common pathological findings and explain the significance of terms commonly used in hepatopathology. In the final chapters finally we shall illustrate the common clinical-pathologic entities, as they are seen during the microscopic examination of the liver biopsies.
Algorithmic Approach to Liver Biopsy Interpretation
Algorithmic approaches to diagnosis have been widely used in medicine. Popularity of such approaches may have several explanations. First of all it reflects the logical pathway to diagnosis and at its core it is nothing more than formalized, practice tested deductive reasoning. Second, the algorithms are a useful practical method that can be readily taught to trainees and students; and for them it is easy to follow the outlines and schemes presented in a logical manner. Third, the logical sequences forming the basis of algorithms can be easily translated into computer languages and adapted to electronic instruction and/or keeping records. Fourth, algorithmic approach forces all of us to be mentally disciplined and systematically cover all facts, reducing thus the subjectivity of diagnosis. Finally, algorithmic data are easily presented in synoptic form.
Pathologists have been using algorithms in daily practice and synoptic reporting of liver biopsies has been widely accepted by hepatologists and pathologists alike (Ludwig 1992). Algorithmic approaches have been advocated by the leading hepatopathologists (Lefkowitch 2010; Theise and Saxena 2015; Torbenson 2015), who all promote an eclectic, team based, evidence tested and rather flexible approach. One should note that the algorithms included in such an approach may begin with a microscopic observation (e.g., predominantly portal inflammation, or steatosis), or a clinical-laboratory diagnosis (e.g., serologically confirmed viral hepatitis C). This applies especially to algorithms developed for diffuse liver disease. Tumors, cysts and other mass liver lesions are not included in these schemes and need to be diagnosed using an entirely different approach. Radiologic and or laparoscopic findings are critical for the diagnosis of lesions in this second group. Clinical-pathologic correlation is essential for proper diagnosis of both diffuse liver diseases and mass lesions.
Standardized grading and staging of several liver diseases, such as chronic viral hepatitis C, primary biliary cirrhosis, and steatohepatitis is also based on algorithms, which are constructed on the basis of detailed descriptions defining each grade and stage. These tabular systems generate numerical parameters which are very convenient for the follow up the diseases under observation or the evaluation of treatment. We will discuss them in the chapter devoted to specific liver disease.
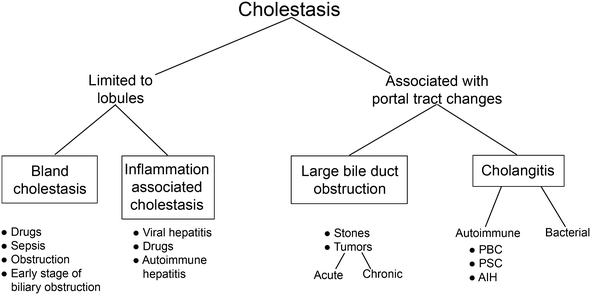
In this chapter we will present our approach to stepwise diagnosis of liver diseases based on pattern recognition advocated by essentially all major hepatology texts. Schematically our approach is presented in Fig. 1. However, before we proceed, it is worth repeating some of crucial caveats, as follows:
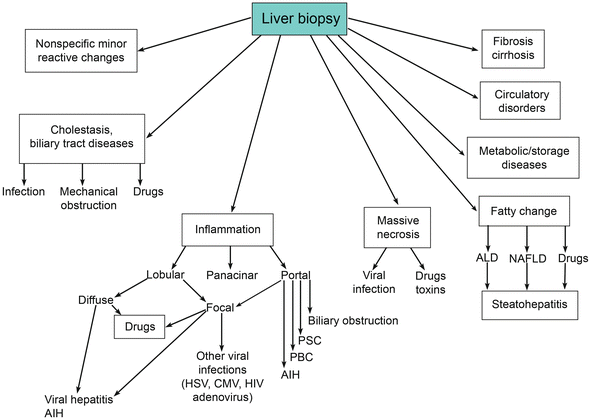

Fig. 1.
Patterns of liver injury
Liver biopsy may show several patterns of injury and accordingly it is imperative to recognize the predominant pattern, which could presumably represent the common denominator for all other findings. It is not uncommon to have more than one clinical scenario present in the same patient (e.g., hepatitis C patient with nonalcoholic fatty liver disease as an example).
Pathognomonic findings are uncommon, and often found only focally. It is still worth looking for them, like the PAS+ cytoplasmic inclusion in hepatocytes in AAT deficiency, or cytomegalovirus inclusions in CMV hepatitis.
Mimicry is common in hepatopathology and one should remember that many diseases may present in several forms, some of which mimic another liver diseases.
Drug induced liver injury (DILI) should be included in the differential diagnosis of most liver diseases, but especially if there are several overlapping patterns of injury, and if there is a significant discordance between the clinical/laboratory and pathology data.
Histologic Patterns of Liver Injury
Several histologic patterns can be recognized in the liver biopsy and accordingly the biopsy findings could be classified into several major categories as follows:
Minor nonspecific reactive change
Cholestasis
Inflammation
Massive necrosis
Fatty liver and related diseases
Accumulations and depositions
Circulatory disorders
Fibrosis and cirrhosis
Minor Nonspecific Reactive Changes
This group of changes includes several morphologic alterations none of which is diagnostic of a specific liver disease, individually or in aggregate (Burt et al. 2012). Such changes could involve hepatocytes, bile ducts, Kupffer cells, or hepatic vasculature. Torbenson (2015) lists them under the heading of “almost normal liver biopsy”. The biopsy findings are usually nonspecific and rarely if ever diagnostic (Table 1).
Table 1.
Minor nonspecific changes in liver biopsies
Portal tracts | Hepatocytes | Kupffer cells |
|---|---|---|
Portal infiltrates of lymphocytes | Hydropic change | Increased number and size |
Portal fibrosis | Single cell or group necrosis with or without inflammation | Cytoplasmic PAS+ granules |
Bile duct proliferation | Glycogen accumulation, nuclei or cytoplasm | |
Lipid accumulation (macro or microvesicular) | ||
Anisocytosis |
The most common “abnormality” is mild focal infiltration of some portal tracts with lymphocytes (Fig. 2). Since the normal portal tracts contains only a few lymphocytes, the assessment of portal lymphocytosis is subjective and essentially “in the eyes of the beholder”. We usually review all the portal tracts in the biopsy specimen and if only one or two out of 20 are infiltrated with lymphocytes, we tend to ignore that finding, especially if there are no clinical correlates to suggest chronic hepatitis. It is worth mentioning that infiltrates of portal lymphocytes occur quite often in the liver parenchyma around some other lesions, especially primary or metastatic carcinomas. Scattered lymphocytes or neutrophils may be seen inside the lobular parenchyma as well.
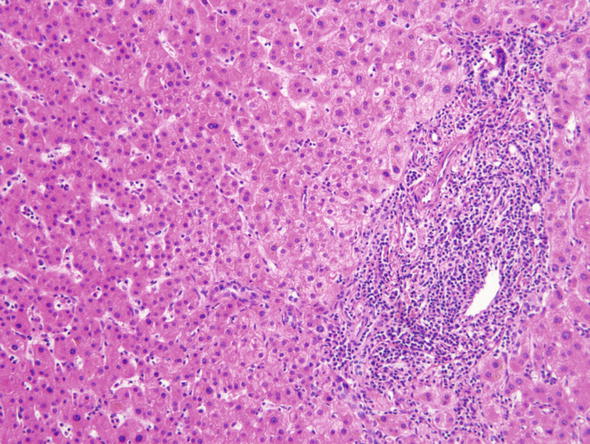

Fig. 2.
Minor nonspecific reactive changes. This microphotographs was taken from the liver parenchyma in the vicinity of a metastatic adenocarcinoma. Some portal tracts like the one shown here contained infiltrates of lymphocytes
∎ Hepatocytes may show foci or single cell hydropic change or prominent anisonucleosis (variation in size of nuclei) outside of the normal range (Fig. 3). Glycogen may accumulate in the nuclei of hepatocytes accounting for the clearing up of nuclei, which are then referred to as glycogenated nuclei (Fig. 4). In the cytoplasm of scattered hepatocytes glycogen can form of pseudogroundglass inclusions, which impart the cytoplasm a finely granular appearance (Fig. 5). This change may be caused by a variety of stimuli, such as drugs, hormones, diabetes and even chronic passive congestion. Fatty change, which usually varies from one lobule to another may present in form of small or large droplets, i.e., as microvesicular or macrovesicular steatosis (Fig. 6). Brown cytoplasmic granules, especially in elderly or chronically ill patients usually represent lipofuscin. It must be distinguished from hemosiderin, which may be demonstrated with the Perl iron stain.
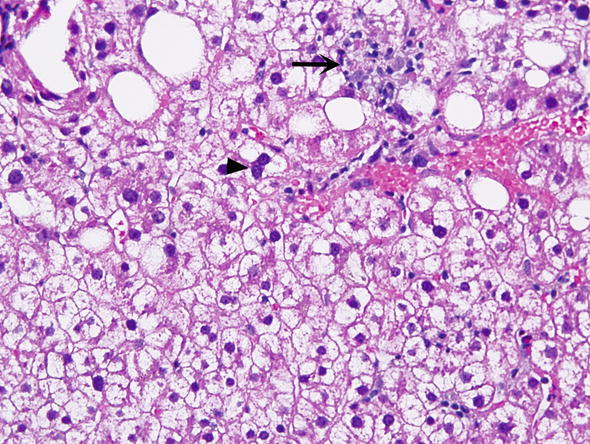
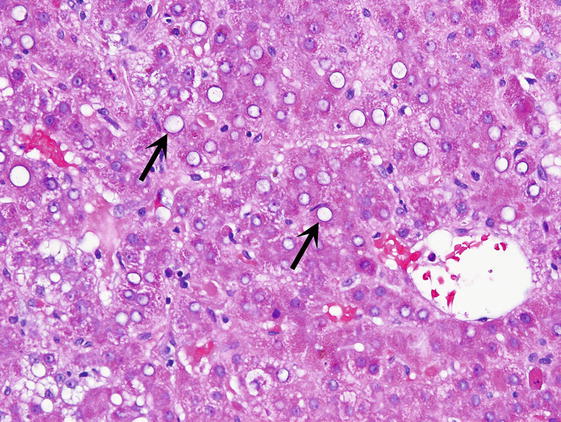
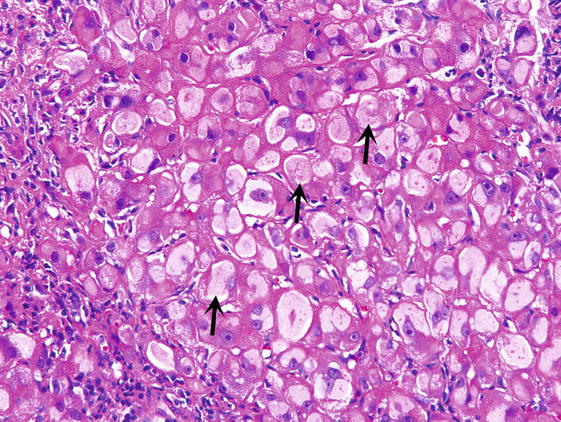
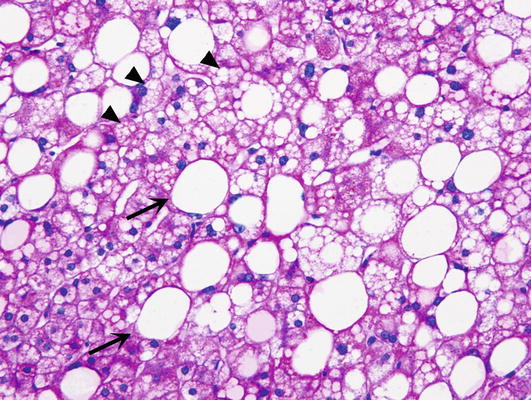

Fig. 3.
Minor nonspecific reactive changes. Hepatocytes have nuclei that vary in size (anisocytosis). Some hepatocytes have more than one nucleus (arrowhead). The cytoplasm of most hepatocytes appears clear and vacuolated. Kupffer cells are prominent. The arrow points to an aggregate of macrophages

Fig. 4.
Glycogenated nuclei. Hepatocytes have clear nuclei, which also vary in size (arrows)

Fig. 5.
Pseudogroundglass inclusions. The cytoplasm of affected hepatocytes has a finely granular appearance (arrows)

Fig. 6.
Steatosis. Macrovesicular steatosis is marked by large droplets filling the cytoplasm of hepatocytes, and displacing of the nucleus toward the periphery (arrow). Microvesicular steatosis presents in form of small vesicles in the cytoplasm of hepatocytes (arrowheads)
∎ Bile ducts may be increased in number in portal tracts (Fig. 7a). Such portal tracts may be infiltrated by neutrophils or lymphocytes. The increased number of bile ductules (cholangioles) may be associated with periductular fibrosis. It should be distinguished from ductular proliferation combined with acute pericholangitis and mild portal tract edema which is usually associated with mechanical biliary obstruction. Ductal and ductular proliferation and ductular reaction of biliary obstruction are best seen in slides immunohistochemically stained with antibodies to cytokeratin CK7 (Fig. 7b).
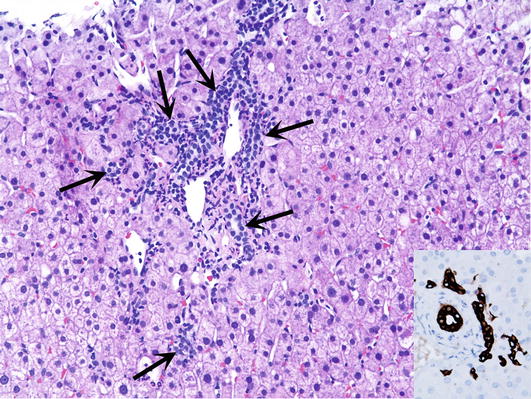

Fig. 7.
Ductal proliferation. (a) Portal tract contains an increased number of ducts (arrows) and lymphocytes. (b) Inset shows bile ducts and the ducts of Hering immunohistochemically staining with the antibody to cytokeratin CK7
∎ Inflammatory cells may be seen in the portal tracts or lobules of patients who have some systemic disease, but also in some that have no signs of inflammation. Most often one deals with lymphocytes (Fig. 8). An increased number of Kupffer cells cannot be distinguished from an infiltration of bone marrow derived macrophages. In surgically removed tissue or in needle biopsies performed during surgical operation the liver parenchyma may contain aggregates of neutrophils (Fig. 9). This surgical acute hepatitis is related to mechanical handling of the liver and should not be confused with changes related to bacterial infections.
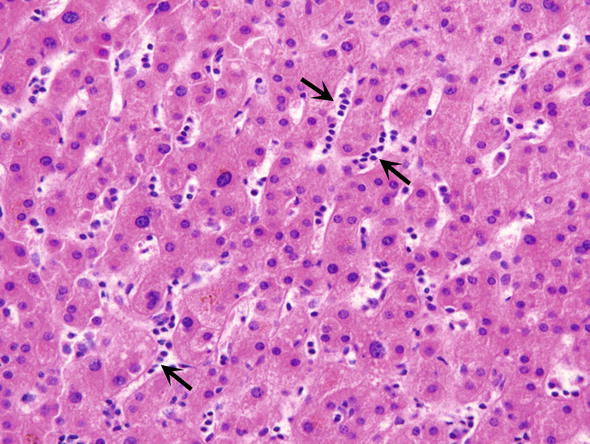
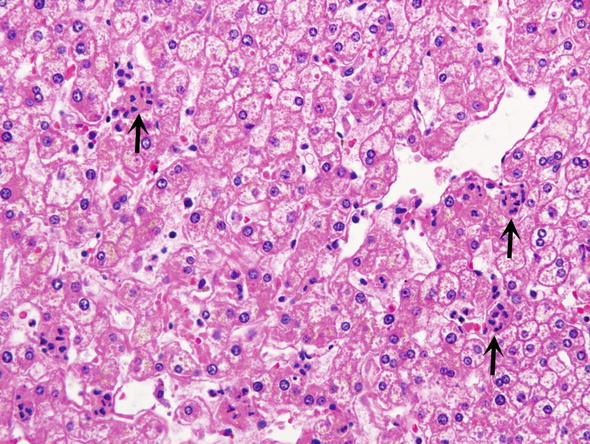

Fig. 8.
Intrasinusoidal lymphocytes. Some sinusoids contains lymphocytes arranged in rows (arrows), often referred to as infectious mononucleosis pattern

Fig. 9.
Surgical hepatitis. It is characterized by an accumulation of neutrophils which form small groups in the parenchyma (arrow)
∎ Kupffer cells may be increased in number and prominent due their abundant cytoplasm. The cytoplasm of these cells may contain granules of brown pigment (Fig. 10a). This glycolipid-rich pigment, called ceroid, stains with PAS and is resistant to digestion with diastase (Fig. 10b). It is usually seen as evidence of a past liver cell injury (especially in drug induced liver injury), in which the remnants of liver cell cytoplasm were taken up by phagocytosis into the cytoplasm of resident macrophages. Such findings are sometimes referred to as resolving hepatitis pattern (Torbenson 2015).
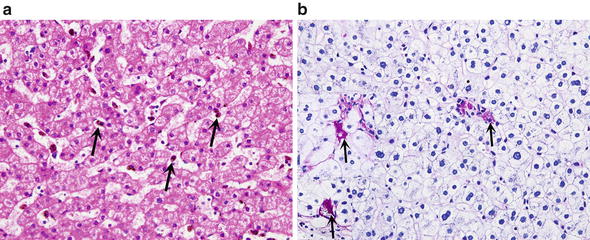

Fig. 10.
Kupffer cell reactions. (a) Reactive Kupffer cells have eosinophilic cytoplasm (arrows). (b) Ceroid laden Kupffer cells (arrows) contain in their cytoplasm PAS positive (red) granules that are resistant to predigestion with diastase (PAS staining with diastase predigestion)
∎ Sinusoids may show focal dilatation and congestion (Fig. 11). Furthermore these vascular spaces may contain a number of cells that are important for diagnosis. The pathology of hepatic sinusoids has been reviewed recently by Brunt et al. (2014).
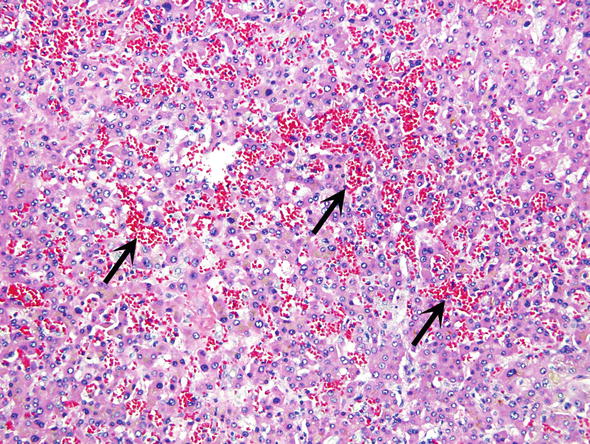

Fig. 11.
Sinusoidal dilatation and congestion. Backpressure from the hepatic vein has caused chronic passive congestion and filling of the sinusoids with blood (arrows)
The significance and the extent of circulatory changes cannot be always established from the limited sample obtained by needle biopsy. It is worth remembering that certain drugs, such as oral contraceptives and steroid hormones may cause sinusoidal dilatation and congestion. Additional clinical information provided such as echocardiogram showing congestive heart failure or hepatic vein outflow obstruction on ultrasound or cross sectional imaging would be relevant. Inside the sinusoids one may see reactive lymphocytes in various systemic viral diseases, such as infectious mononucleosis. In various leukemias sinusoids contain neoplastic white blood cells. In myelofibrosis the sinusoids may contain hematopoietic cells, indicative of extramedullary hematopoiesis.
∎ Clinical indications for the liver biopsy showing mild nonspecific and reactive changes usually include vary from one case to another. Most often the biopsy is performed because of mild chronic laboratory abnormalities. Liver enzymes may be only mildly altered, but in some cases there is prolonged elevation of AST, ALT, GGT or alkaline phosphatase. In some cases the abnormalities fluctuate or there are significant elevations of liver enzymes. In most instances the biopsy is performed only to reassure the patients that there is no serious liver disease.
∎ Laboratory findings indicative of a liver disease may be encountered in many patients with systemic infection or sepsis. Chronic pancreatic or biliary tract diseases, such as cholelithiasis, chronic cholecystitis or pancreatitis or cystic fibrosis may cause hepatic changes. Gastrointestinal diseases of unknown etiology, such as ulcerative colitis or Crohn’s disease, and some systemic autoimmune diseases such as systemic lupus erythematosus, and blood disorders such as sickle cell anemia may also affect the liver. Preoperative biopsies in candidates for heart or kidney transplantation may also show minor nonspecific changes. Table 2 contains a list of representative diseases that may cause non diagnostic minor reactive changes in the liver.
Table 2.
Common causes of minor nonspecific changes seen in liver biopsies
Sepsis or chronic infection |
Systemic diseases—autoimmune diseases |
Endocrine diseases (e.g., hypo or hyperthyroidism) |
Hemato/lymphoid diseases (e.g. sickle cell anemia, lymphoma, multiple myeloma) |
Genetic diseases (e.g., glycogenosis, cystic fibrosis, Wilson disease) |
Biliary/pancreatic diseases (e.g., cholelithiasis, chronic pancreatitis) |
Gastrointestinal chronic diseases (e.g., celiac disease, Crohn disease, ulcerative colitis) |
Drugs |
Peritumoral liver parenchyma |
It is worth remembering that the liver biopsy performed for mass lesions may miss the lesion seen on CT or MRI scanning. In such cases the pathologist may receive a sample of the adjacent liver parenchyma instead of a suspected tumor. These samples often show nonspecific reactive changes, including focal chronic inflammation, compression of the hepatocytes, bile stasis and fibrosis. It is important for the clinician to recognize the potential for missing the mass lesion on biopsy and if unexpected results return review to the case to ensure proper needle placement within the suspected mass or lesion or rebiopsy may need to be considered.
Cholestasis
Normal liver tissue does not contain any visible bile, and accordingly any bile that can be seen in the liver biopsy should be considered as pathological. The most common causes of cholestasis are listed in Table 3.
Table 3.
Common and less common causes of bland cholestasis
Drugs (steroids, oral contraceptives) |
Sepsis |
Early stages of large bile duct obstruction |
Benign recurrent intrahepatic cholestasis |
Genetic disorders |
Jaundice of pregnancy |
Thyroid diseases (hypo or hyperthyroidism) |
Paraneoplastic syndromes (lymphoma, carcinoma) |
In acute cholestasis, as encountered following short-lived bile duct obstruction, bile accumulates in the form of dot-like or short linear bile plugs in the dilated intercellular bile canaliculi. Retrograde pressure resulting from acute bile duct obstruction will be transmitted to the centrilobular area, and thus the bile plugs composed of yellow/brown bilirubin will first be seen in the centrilobular zone 3. Later on bile plugs may be seen in the entire lobule (Fig. 12). Bile may be seen inside the cytoplasm of liver cells and Kupffer cells. In chronic cholestasis bile plugs will be seen in the periportal zone 1. In addition to bilirubin these biliary plugs contain biliary salts (cholates) which may cause liver cell injury. Cholate stasis is thus associated with feathery degeneration of liver cells and regeneration of liver cells which form so called cholestatic hepatocellular rosettes around the centrally located bile filled canaliculi (Fig. 13). Destruction of liver cells accompanied by accumulation of bile in the extracellular spaces results in the formation of “bile lakes” (Fig. 14). In long term biliary obstruction bile may be seen in the lumen of biliary ducts, but this happens rarely. The most common cause of ductal cholestasis is sepsis, which may also cause intralobular cholestasis and can be seen as a sign of biliary tract infection. Bile may be seen in the lumen of distorted ducts in polycystic liver disease or parts of bile duct hamartoma included accidentally in the liver biopsy (Fig. 15). Ductal cholestasis is also seen in various congenital abnormalities of the biliary system in children.
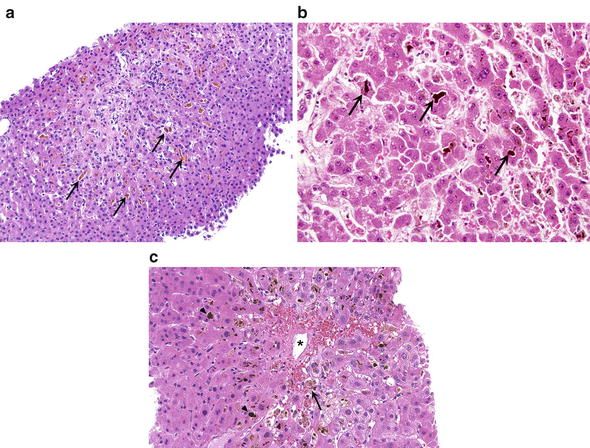
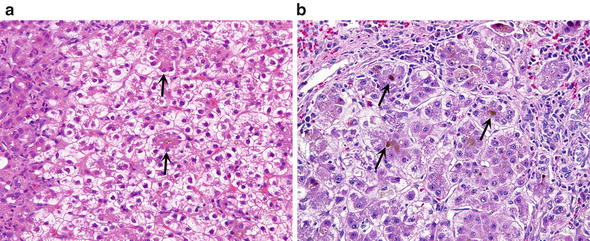
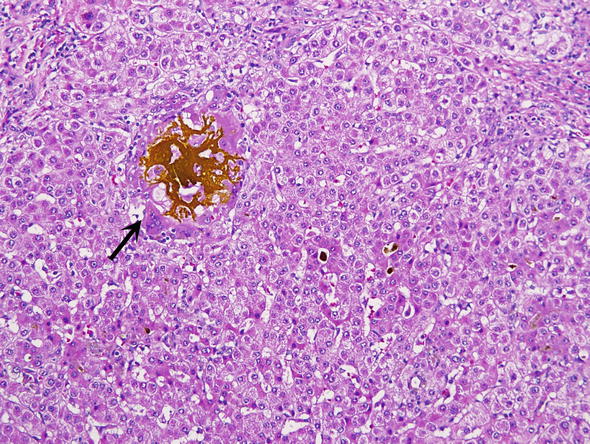
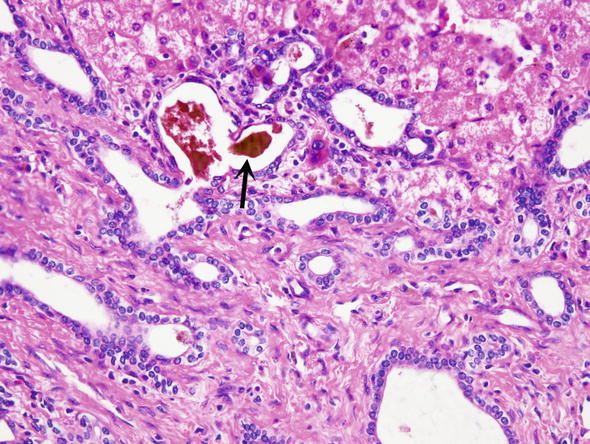

Fig. 12.
Cholestasis. (a) The intercellular bile canaliculi are dilated and some of them contain bile, which may be seen even at low magnification (arrows). (b) Dilated intercellular canaliculi contain bile plugs (arrows). (c) Accumulation of bile may be seen in Kupffer cells (arrow). Bile plugs are marked by arrowheads

Fig. 13.
Periportal cholestasis. (a) Canaliculi contain bile plugs in the center of hepatocellular rosettes (arrows). There is prominent clearing of the cytoplasm of the periportal hepatocytes. The portal tract is at the left margin of the figure. (b) Rosettes contain bile plugs in their centers, corresponding to dilated bile canaliculi (arrows)

Fig. 14.
Bile lake. Extravasated brownish yellow bile is surrounded by a foreign body giant cell reaction (arrow)

Fig. 15.
Ductal cholestasis. Brownish yellow bile is seen in the lumen of bile ducts in this bile duct hamartoma (arrow)
Box 1: Diagnostic Evaluation of Cholestatic Changes
1.
Determine if the cholestasis is associated with portal changes and thus related to bile ductal pathology, or limited to lobules, suggesting a primary hepatocellular injury.
2.
If the changes are limited to the lobular parenchyma determine if the cholestasis is bland or associated with inflammation.
3.
Bland cholestasis is most often related to a drug reaction or sepsis. It may also be found in early stages of jaundice related to large bile duct obstruction by tumors of gallstones. Other less common causes of bland intralobular cholestasis are listed in Table 1.
4.
Cholestasis associated with inflammation is most often caused by viral hepatitis, drugs or autoimmune hepatitis. On the basis of pathologic findings these three diseases cannot reliably be distinguished one from another. Clinical and laboratory/serologic data are thus essential for proper diagnosis. The timing of onset of cholestasis as noted in the patient may assist in the clinical diagnosis.
5.
If there are portal tract changes one must determine if these changes are related to large bile duct obstruction or portal bile duct injury.
6.
Large bile duct obstruction is most often caused by gallstones or tumors. It may be acute or chronic.
7.
Acute obstruction is usually diagnosed clinically in most instances and rarely biopsied. In acute large bile duct obstruction the portal tracts are edematous, contain lymphocytes and neutrophils. There is prominent bile ductular proliferation. Bile may be seen in the portal ducts and the newly formed ductules as well as in the lobule itself.
8.




Chronic obstruction of large bile ducts is characterized with bile ductular proliferation and fibrosis, accompanied by infiltrates lymphocytes and less commonly neutrophils. In some longstanding cases there is bile duct loss and fibrous obliteration of bile ducts. Cholate stasis predominates, hepatocellular rosettes are prominent and the entire lobule may show signs of cholestasis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree