Fig. 4.1
a PillCamESO2. b , c , d Current ESO ingestion protocol as per text
4.2 Ingestion Protocol
Small-bowel and colon capsules are typically ingested whilst the patient is sitting upright and water is given to aid passage of the capsule through the gastro-oesophageal junction (GEJ). Due to the inherent function of the oesophagus to act as efficient conduit into the stomach, it was necessary to develop a modified ingestion protocol for ECE. Normal solid oesophageal transit time is between 4 and 8 s [ 2 ] and, as such, too rapid to allow enough number of images for meaningful interpretation. Table 4.1 details the original ingestion protocol used in clinical trials and early ECE/ESO1 studies. This protocol resulted in highly variable oesophageal transit times between 6 and 1,200 s [ 1 , 3 ] with a mean oesophageal transit time of 189 s. It should be noted that use of a real-time viewer is essential during ECE studies.
Table 4.1
Original ECE ingestion protocol
Protocol stage | Duration | Angle of elevation |
|---|---|---|
1. Ingestion of 100 ml water whilst in upright position | 5 s | N/A |
2. Ingestion of capsule in supine position with 20 ml water | 2 min | 0º |
3. First elevation of back position | 2 min | 30º |
4. Second elevation of back position | 2 min | 60º |
5. Sip of water | 2 min | 60º |
6. Sit upright | 20 min | 90º |
Pendlebury et al. [ 4 ] further modified this protocol in an attempt to enhance imaging of the gastric lumen. An extra stage between stage 5 and 6 was added involving the patient lying flat and then rolling onto their left and right sides for 2 min each. The patient was then allowed to sit upright whilst the test completed. This group also employed the use of prokinetics (Metoclopramide or Domperidone) to increase visualisation of the duodenum during ECE studies, however results were mixed.
Unfortunately effective image visualisation of the oesophagus and GEJ remained an issue prompting Gralnek et al. [ 1 , 5 ] to develop a simplified ingestion protocol (SIP). This protocol has the patient ingesting the capsule whilst lying in the right lateral position. Sips of 15 ml of water are given every 30 s from a syringe (or via a straw) until the capsule enters the stomach (Fig. 4.1 b–d).
SIP improved on the original protocol by significantly extending oesophageal transit time (mean 3 m 45 s vs. 0 min 38 s; p = 0.0001) and improving visualisation of the GEJ but with no change in the number of GEJ frames captured [ 1 ]. De Jonge et al. (2008) [ 6 ] reported that SIP increased complete visualisation rate of the GEJ in a group of patients with Barrett’s and reflux oesophagitis when compared to the original protocol (93 vs. 68 % p = 0.04) and improved sensitivity in the diagnosis of these disorders. SIP has now been adopted as the protocol of choice for ECE in clinical centres [ 1 , 7 ].
4.3 Clinical Use of Oesophageal Capsule Endoscopy
4.3.1 Barrett’s Oesophagus Screening
Gastro-oesophageal reflux disease (GERD ) is a common problem [ 8 ] and a risk factor for developing Barrett’s oesophagus which occurs in 5–15 % of patients with symptomatic GERD [ 7 ]. Barrett’s oesophagus is associated with 0.5 % annual incidence of high-grade dysplasia or oesophageal adenocarcinoma [ 7 , 9 ]. Therefore, efforts to curb its incidence have been focused on the use of selective screening of high risk (for developing Barrett’s oesophagus) individuals [ 10 ]. Although there are currently no controlled trials to examine the validity of this surveillance strategy, esophagogastroscopy (EGD) remains the gold standard for the examination of the oesophagus [ 7 ]. EGD though is invasive, often uncomfortable and may be poorly tolerated; moreover, sedation carries risks that are not negligible, especially with increasing age [ 1 , 11 , 12 ].
Therefore, ECE seems to be a prime alternative candidate for this task. Several studies have been conducted since the first report of the use of PillCam®ESO1 in the diagnosis of oesophageal disorders that involved a small group 17 patients with GERD symptomatology [ 3 , 13 ]. The first systematic assessment was a meta-analysis in 2009 [ 14 ]. This was carried out in order to calculate the pooled sensitivity /specificity of ECE for the diagnosis of Barrett’s oesophagus. Subgroup analyses were performed based on the reference standard used. Nine studies, comprising a total of 618 patients, were included. In the majority of included studies, PillCam®ESO1 was used [ 15 – 19 ], whilst in one study [ 20 ] the current model (ESO2) was utilised. The PillCam® SB on a string was also used in another study [ 21 ]. Allowing for this heterogeneity, the pooled sensitivity and specificity for the diagnosis of Barrett’s oesophagus was 77 and 86 %, respectively. In subgroup analyses, when EGD and histopathology-confirmed intestinal metaplasia was taken as reference standard, pooled sensitivity/specificity of ECE was 78/90 % and 78/73 %, respectively. Furthermore, ECE was found to be safe and had a high rate of patient preference. However, on the basis of ECE moderate sensitivity/specificity for the diagnosis of Barrett’s, the authors concluded that in patients with GERD , conventional EGD remains the modality of choice for evaluation of suspected Barrett’s (Fig. 4.2 a–d).
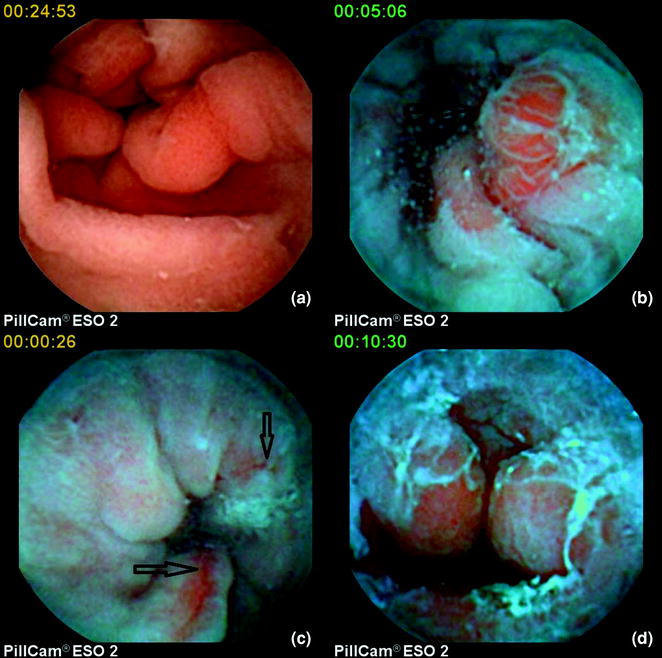

Fig. 4.2
a Normal gastro-oesophageal junction ( GEJ) (under white light ), b inflammatory nodule at the GEJ (under blue mode ), c oesophagitis (under blue mode ), d fibrous ring and bleeding at the GEJ (under blue mode )
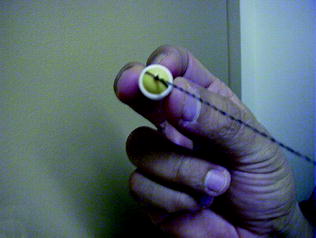
Interestingly, despite the fact that oesophageal inspection with single-head viewing capsules is disappointing [ 22 – 24 ], due to the rapid movement of the capsule and low capture frame rate together with the upright swallowing protocol, the string-capsule endoscopy devised by Ramirez et al. [ 21 ] and later modified by Liao et al. [ 25 ], allow converting a purely passive process to an operator-controlled procedure (Fig. 4.3 ) [ 26 , 27 ]. With this simple modification, dwell time in the oesophagus is increased and real-time monitoring of the images on a standard real-time viewer is feasible. Furthermore, after high-level of glutaraldehyde disinfection [ 25 ] or disposal of the removable latex sheath, the capsules can be reused multiple times, thus allowing significant cost-effectiveness (Fig. 4.4 ). Despite the aforementioned advantages, it should be noted that tethered capsule endoscopy has not received official approval (FDA or CE), therefore its use cannot be recommended.
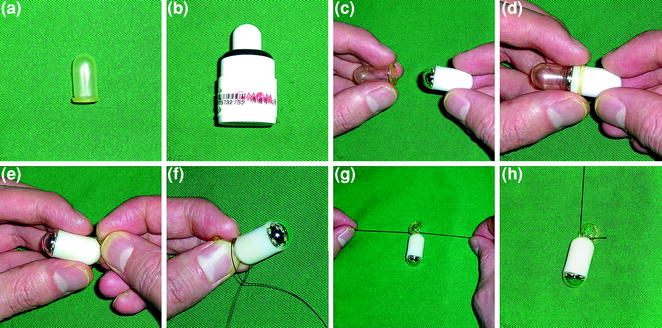

Fig. 4.4
The procedure of sleeve string-capsule endoscopy. a . The latex sleeve. b The OMOM® capsule endoscope. c , d , e Enclosing the capsule with the sleeve. f , g , h The string attachment. Reprint with permission from Gastrointest Endosc [ 25 ]
4.4 Oesophageal Varices Screening
The most common cause of portal hypertension is liver cirrhosis . It is estimated that cirrhosis accounts for more than 25,000 deaths and 373,000 hospital discharges in 1998 [ 28 ]. The rise in portal pressure is associated with the development of collateral circulation (varices) [ 29 ]. Cirrhotic patients develop varices at a rate of 8 % per year [ 29 ]. The risk of variceal rupture/haemorrhage increases with the size of varices [ 29 ]. The average mortality of the first episode of variceal bleeding is ~50 % [ 30 ]. Since primary prophylactic treatment (band ligation or non-selective beta-blockade) in cirrhotic patients with medium/large size varices reduces the risk of bleeding and mortality, current guidelines stress the importance of variceal screening [ 29 ]. The gold standard in the diagnosis of varices is EGD. Endoscopic screening is recommended for every 1–3 years, depending with the size of varices on index endoscopy [ 3 , 30 ].
In 2006, pilot studies from the States and France [ 3 , 31 , 32 ] reported high sensitivity and specificity (81–100 % and 89–100 %, respectively) in the detection of varices. Since then, an increasing evidence base allowed 2 meta-analyses. In the first meta-analysis (2010), Lu et al. included seven studies [ 33 ], all comparing the yield of ECE and EGD in patients diagnosed or suspected of having oesophageal varices . All were performed with the first ESO model (PillCam®ESO1). In a total of 446 patients, the pooled sensitivity and specificity of ECE for detecting oesophageal varices was 85.8 and 80.5 %, respectively. In subgroup analyses, the pooled sensitivity/specificity in patients undergoing oesophageal varices screening and in those under surveillance was 82.7/54.8 % and 87.3/84.7 %, respectively. A year later, Guturu et al. [ 34 , 35 ] presented a meta-analysis of 9 studies, comprising a total of 631 patients, comparing the use of ECE and EGD in screening and surveillance of oesophageal varices. In 619 patients (they were 12 capsule failures), the pooled sensitivity and specificity of PillCam®ESO 1 (as compared to conventional EGD) was 83 and 85 %, respectively. Pooled likelihood ratios (LR) for the detection of oesophageal varices by ECE were also calculated. It is accepted that LR > 5 and < 0.2 give strong diagnostic evidence. The pooled positive likelihood and negative likelihood ratios were 4.09 and 0.25, respectively. Therefore, the authors concluded that ECE can only be an acceptable alternative in certain situations, but it cannot be recommended as replacement option for conventional EGD (Fig. 4.5 ).
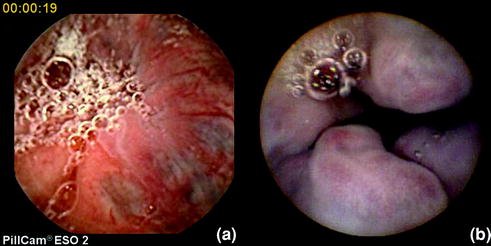

Fig. 4.5
Varices as seen with ESO
The string/tethered PillCam®SB seem to perform similarly well to its ESO counterpart. In a feasibility study [ 36 ] by Ramirez et al., 30 patients with clinical liver cirrhosis were enroled, 19 for surveillance and 11 for screening purposes. The procedure was safe (no strings were disrupted and no capsule was lost). The mean recording time was 5.8 min, the accuracy 96.7 % with only minimal discomfort. The majority (83.3 %) of patients preferred string-capsule endoscopy to EGD. A follow-up study was published in 2012 [ 37 ]; 100 patients (33 for screening and 67 for surveillance) were enroled. The sensitivity and specificity of tethered capsule endoscopy for clinically significant varices was 82 and 90 %, respectively with a PPV of 84 % and NPV of 89 %.
4.5 Contraindications and Complications
It should not be forgotten that ECE, despite its reduced battery life, has to traverse the rest of the GI tract to be excreted. Therefore, the same contraindication s that apply for small-bowel capsule endoscopy e.g. known and/or suspected critical bowel stenosis, pregnancy and/or prior complex abdominal surgery, apply for ECE. Moreover, ECE is contraindicated in patients with oesophageal achalasia and/or known oesophageal diverticulae [ 38 – 40 ]. Furthermore, the patients with known oropharyngeal dysfunction (due to high risk of aspiration) should be discouraged. Such group includes often elderly patients with oesophageal dysmotility problems. To date, unlike SBCE , there is no recorded case of aspiration of PillCam®ESO but this may be related to the frequency of its use [ 41 ]. Of course, some of the above contraindications are obsolete, in case the tethered capsule is used.
4.6 Alternatives
Minimally invasive alternatives for examining the oesophagus and the stomach include the trans-nasal endoscopy with ultrathin scopes and/or single use endoscopes [ 42 , 43 ] (Fig. 4.6 ). Moreover, the advent of a magnetically controlled capsule will theoretically allow an accurate (wireless) examination of the upper digestive tract [ 44 – 47 ]. Swain et al. [ 44 ] modified a capsule endoscope to include neodymium-iron-boron magnets. The capsule’s magnetic switch was replaced with a thermal one that turns on by hot water. One imager was removed from the PillCam® colon and the available space was used to house the magnets. In the first-in-human study, a handheld external magnet used to manipulate this capsule in the oesophagus of a volunteer. The capsule was swallowed and observed in the oesophagus by using a gastroscope. Capsule images were viewed on a real-time viewer. The capsule was manipulated in the oesophagus for 10 min. Furthermore, Hale et al. [ 45 ] showed that examination of the upper gastrointestinal tract is feasible in a porcine model using a magnet and positional change. Using a handheld magnet, Mirocam Navi (Intromedic Ltd), positional changes and a “real-time” viewer hey showed satisfactory marker recognition (Fig. 4.7 ).