Fig. 9.1
(a and b) Angiodysplasia in jejunum
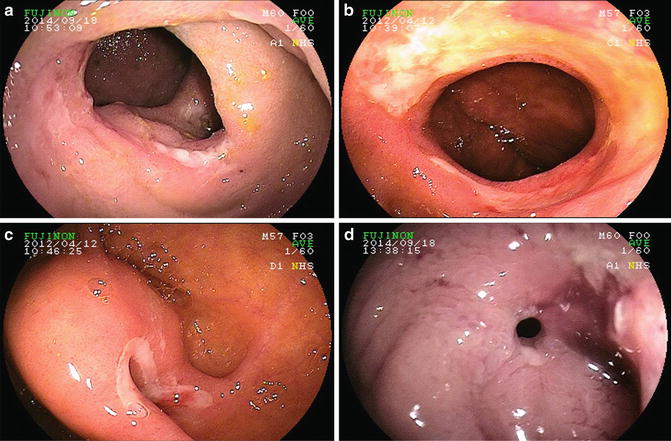
Fig. 9.2
(a) Ulcer due to ileal Crohn’s disease (CD). (b) Ulceration due to recurrent CD in neo-terminal ileum. (c) Ulcer at ileal–ileal anastomosis post-ileal resection. (d) Ileal stricture due to CD
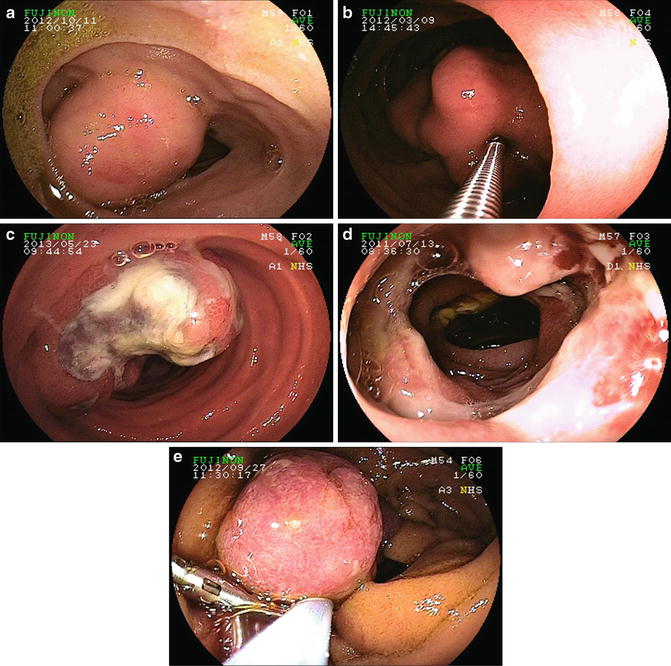
Fig. 9.3
(a) Ileal gastrointestinal stromal tumor (GIST). (b) GIST in jejunum. (c) Metastatic melanoma. (d) Enteropathy-associated T-cell lymphoma. (e) Juvenile polyp
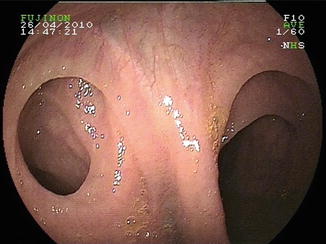
Fig. 9.4
Meckel’s diverticulum
Factors Predictive of a Positive Study
Given the time, cost, and potential adverse events related to VCE and DBE, it is important to understand the variables that are associated with an increased likelihood of a positive small bowel study in order to optimize the performance of these tests (see Table 9.1). Patients presenting with overt rather than with occult bleeding [11, 12], as well as those with ongoing overt bleeding rather than overt bleeding that has stopped [13, 14], are more likely to have a positive VCE. Similar superiority has been observed when DBE is performed for patients with overt bleeding who continue to bleed compared to those with inactive overt bleeding or occult bleeding [15]. Furthermore, there is a consistent pattern demonstrating increased diagnostic yield with earlier administration of VCE, speaking to the importance of the timely evaluation of patients with suspected small intestinal bleeding. The source of bleeding is more likely to be identified if VCE is performed within 2 weeks [14], within 1 week [16] and even more so within 48 h of the bleeding presentation, with the latter group having a particularly high diagnostic yield of 75 % [17]. In a study that examined patients admitted to hospital with an overt OGIB, those who underwent VCE within 3 days of admission had significantly higher diagnostic yields (44 % versus 28 %), increased rates of subsequent therapeutic intervention (19 % versus 7 %) and shorter lengths of hospital stay (6 versus 10 days) than those who had VCE beyond day 3 of hospitalization [18]. Furthermore, the patients who underwent delayed VCE in hospital had no better diagnostic or therapeutic rates compared to patients evaluated in an outpatient setting, suggesting that the benefit of inpatient workup for OGIB is lost if the necessary small bowel tests are not performed as soon as possible. All of this speaks to the importance of having OGIB protocols in place that enable referring physicians to have patients access tertiary centers performing small bowel endoscopy in a rapid fashion.
Table 9.1
Factors predictive of a positive small bowel study
Initial small bowel study for OGIB | Repeat study performed for recurrent bleeding |
|---|---|
• Active overt > inactive overt bleeding | • Previously positive BAE |
• Overt > occult presentation | • Decline in hemoglobin >4 g/dL |
• Short interval from bleeding episode to investigation | • Change in presentation from occult to overt bleeding |
• Severe anemia/increased blood transfusion requirements • Multiple bleeding episodes • Anterograde > retrograde BAE insertion | • Iron deficiency anemia with hemoglobin decrease to <10 g/dL |
In addition to the timing of the investigations, there are a number of clinical factors that predict the likelihood of a positive result on VCE or DBE, including more severe anemia [19–21], increased transfusion requirements [19, 22, 23], multiple bleeding episodes [24], and increased number of previous endoscopies [23], as well as some conflicting data regarding associated medical conditions such as chronic kidney disease [25]. From a purely technical perspective, the diagnostic and therapeutic yield of BAE is superior when performed via the anterograde rather than the retrograde approach, especially when done without the benefit of a previously abnormal VCE or computed tomography (CT) scan [26]. However, this study was performed in the USA, and its finding of a preponderance of proximal bleeding sources may not necessarily hold true in centers elsewhere in the world such as Asia where the distribution of small bowel pathology tends to be different. Lastly, it does not seem to matter whether BAE is performed in the morning or in the afternoon as the diagnostic and therapeutic rates do not differ [27].
Clinical Outcomes with Long-Term Follow-Up
Most of the small bowel endoscopy literature consists of studies looking at the diagnostic and/or therapeutic yields of VCE or BAE, with relatively little research defined by outcomes that are truly relevant to patient care such as bleeding cessation, rates of rebleeding, time to rebleeding, improvements in hemoglobin levels and transfusion requirements. Recently, there has been increasing attention paid to these types of clinical outcome studies with long-term follow-up.
VCE
As a purely diagnostic test, VCE cannot alter clinical outcomes but can at best localize bleeding lesions to direct subsequent treatments or can demonstrate the absence of significant pathology that could pose a risk for ongoing or future bleeding. This is perhaps best illustrated by studies reporting outcomes with VCE prior to the local availability of BAE. In a retrospective, single-center study of a prospectively maintained database of 95 patients in Seoul, South Korea who underwent VCE between 2003 and 2010 for predominantly overt OGIB, significant bleeding lesions were identified in 40 % of patients [28]. After a median follow-up of 24 months, the rebleeding rate in patients who had a positive VCE (37 %) was higher than in patients with a negative VCE (23 %), with a median time-to-rebleeding of 10 months. However, only four patients with a positive VCE underwent BAE due to the delayed introduction of BAE at this center, demonstrating the lack of benefit of finding a bleeding source on VCE if nothing is done to address the lesion. This mirrors the findings of other smaller studies that showed that without specific treatments to suspected bleeding sites, rates of rebleeding after long-term follow-up are the same in patients with positive and with negative VCE, but do decrease if therapeutic interventions are performed [29, 30]. In a single center study from Barcelona of 105 patients who underwent VCE for both occult and overt OGIB and who were followed for slightly less than 1 year, rebleeding occurred in 31 % of patients at a median of 157 days [31]. However, once again the rate of rebleeding was lower in patients who had received lesion-directed treatment compared to nonspecific treatment (21 % versus 36 %, respectively). In contrast, a large retrospective analysis of 696 patients who underwent VCE for OGIB at a single center in Rome, Italy between 2002 and 2011 demonstrated substantially lower rebleeding rates in patients with negative VCE studies [32]. After a median follow-up of 24 months, the rate of rebleeding was 16 % after negative VCE compared to 45 % after positive VCE (p = 0.00001), with the majority of rebleeding episodes occurring within the first year. However, it is unclear from this study how the patients with abnormal VCE were treated, and in particular, whether they underwent lesion-directed therapy by BAE or other means following their VCE. Two trends seem to emerge from these VCE studies assessing recurrence of bleeding over time:
1.
Significant lesions identified on VCE require subsequent targeted treatment by other modalities in order for the performance of VCE to confer clinical benefit.
2.
The negative predictive value for rebleeding of a normal VCE study is not as robust as was previously thought, since these more recent studies reveal rebleeding rates after negative VCE of 16–35 % [28–32], much higher than earlier studies that suggested rebleeding rates of only 5–11 % after negative VCE [8–10].
In fact, a large, multicenter study of 305 patients who underwent VCE for overt OGIB at 13 hospitals in South Korea from 2006 to 2009 found no decline in rebleeding rates after positive VCE [33]. Despite a diagnostic yield of 52 % in this study, only 12 % of patients received interventional therapies, perhaps due to the low prevalence of angiodysplasia (only found in 10 %) and relatively high rates of ulcerative lesions (26 %) in this population sample. Perhaps as a result, the overall rebleeding rate of 19 % at a mean follow-up of 39 months did not differ based on the presence of findings on VCE or on having received a therapeutic intervention. The significant predictors of rebleeding were angiodysplasia seen on VCE, duration of bleeding >3 months, and an inability to discontinue antiplatelet or anticoagulation drugs in the subgroup who had been taking these medications at the time of their initial bleeding presentation [33]. Therefore, it appears that finding a presumed bleeding source on VCE had little impact on long-term outcome in the Korean OGIB experience. In fact, a retrospective analysis of 125 patients who received VCE for OGIB at the Asan Medical Center in Seoul, South Korea between 2007 and 2009 showed that after 36-month follow-up, positive VCE leading to a specific treatment resulted in only marginally lower rebleeding rates compared to negative VCE without subsequent therapy (21 % versus 27 % respectively, p = 0.50) [34].
Similar challenges are highlighted by the outcomes from studies focusing on VCE performed solely for the investigation of iron deficiency anemia. A retrospective review of all VCEs performed for unexplained iron deficiency anemia at a single center in Ireland from 2009 to 2011 found a diagnostic yield of 71 %, with 53 % of patients having a source found within the small bowel and 18 % within the upper GI tract or right colon [35]. At a mean follow-up of 9 months, 42 % of patients had persistent anemia, split evenly between patients who had positive and negative VCE, meaning that a patient was no more likely to have resolution of anemia after having positive VCE findings compared to having a normal VCE. Furthermore, even among the patients in whom a positive VCE led to a specific change in treatment, the majority (61 %) remained anemic at follow-up. Despite this limitation, considerable clinical value may be achieved by performing VCE even if it does not lead to long-term resolution of the anemia, since an important additional goal of small bowel investigations is to exclude the presence of worrisome pathology. This may be particularly true in younger patients. A study from the UK looked at the presence of “sinister pathology,” defined as small bowel malignancy, significant Crohn’s disease inflammation, strictures, or Celiac disease, which was found on VCE performed for the investigation of iron deficiency anemia, and stratified the findings by age group [36]. They found that the prevalence of “sinister pathology” was 25 % in patients ≤40 years-old, but was only 7.5 % in patients >age 40, and that there was a general trend toward an increasing frequency of angioectasias and decreasing incidence of sinister pathology with increasing age. Therefore, even if the clinical outcome studies with VCE demonstrate that it is challenging to achieve long-term resolution of OGIB, particularly for vascular lesions, VCE is still valuable to identify or to exclude so-called sinister pathology, especially in younger patients.
If patients are no more likely to stop bleeding after receiving specific treatment for a culprit lesion identified on VCE than if no potential bleeding source is found, a cynical argument could be made for questioning the value of performing VCE at all for OGIB. However, such an interpretation would be a misread of the literature that we have just discussed. These studies are all retrospective analyses of prospective cohorts, but in no case were patients randomized to receive endoscopic/surgical treatments or not. In fact, there are substantial selection biases inherent in the retrospective nature of these studies that influence the apparent outcomes. It can almost certainly be assumed that patients with the most concerning lesions seen on VCE were the ones most likely to undergo therapeutic interventions. Therefore, it is a probable assumption that were these patients to have not received specific treatments, their rebleeding rates would have been much higher than that in patients with negative VCE. Indeed, such a pattern was observed in several of the previously discussed studies [28, 30]. What merits further examination is the fact that the rate of rebleeding after negative VCE remains so high, and why the rate of rebleeding after treatment of a likely bleeding lesion remains even higher, even though it may be considerably lower than what it might otherwise have been if it were never treated. To further examine these issues, we will turn our attention to clinical outcome studies with BAE.
BAE
As with most areas in the BAE literature, nearly all of the outcomes studies for OGIB relate to DBE. One of the first studies that looked at long-term follow-up after DBE was a telephone survey administered at Stanford and the University of Chicago [37]. At 12 months follow-up, 23 % of patients reported recurrent overt bleeding while 35 % required blood transfusions or iron therapy, and by 30 months 24 % of patients had overt bleeding and 18 % still needed transfusions or iron. This suggests that over half of patients experience rebleeding, with the majority occurring within the first year after the index DBE. In addition, patients with vascular lesions found on DBE were most likely to have ongoing overt or occult bleeding difficulties. However, only one-third of patients in the study population participated in follow-up, likely resulting in substantial selection and other biases that may have unduly influenced the apparent results. A stronger study is a retrospective analysis by Shinozaki et al. of 151 patients who underwent DBE for predominantly inactive overt OGIB in Japan [38]. The outcome of interest used in this study was “control of bleeding,” which was defined as the absence of overt bleeding, blood transfusions or iron therapy, and hemoglobin less than 10.0 g/dL. Overall, 63 % of patients had control of bleeding at a mean follow-up of 30 months. However, this result hides the large discrepancy in outcomes for patients with vascular small bowel lesions compared to patients with other, nonvascular sources of bleeding. Among patients with vascular lesions, 40 % had a recurrent overt bleed within 1 year of their DBE and only 40 % achieved “control of bleeding” at long-term follow-up. In contrast, 84 % of patients with tumors or polyps and 65 % with ulcers or erosions achieved long-term control of bleeding. The significant predictors of failure to achieve “control of bleeding” among the vascular subgroup were the presence of multiple versus single lesions, increased blood transfusion requirements prior to DBE, and the finding of “suspicious” rather than “definite” lesions on endoscopy, perhaps due to the increased probability that the identified lesion was not the true bleeding source [38]. Further evidence supporting the observation that vascular lesions in the small bowel are the most difficult to treat with long-term success was provided by a retrospective study of 147 consecutive patients who underwent single balloon endoscopy (SBE) for OGIB at Washington University from 2008 to 2010 [39]. More than 90 % of patients had small bowel pathology identified on VCE prior to undergoing enteroscopy, resulting in a diagnostic yield of 65 % on SBE, with 83 % of positive findings being vascular lesions (angioectasias and Dieulafoy lesions). Mean follow-up of 24 months was achieved for 110 patients (37 lost to follow-up), split evenly between those with overt and occult OGIB presentations. Recurrent bleeding was defined as overt bleeding signs; hospitalization for GI bleeding; any endoscopic, surgical or radiologic intervention for bleeding; or need for blood transfusion or iron therapy. The overall rebleeding rate was 45 %, with greater success among patients with positive findings on SBE (41 % rebleeding) compared to patients who had a normal SBE (56 % rebleeding). Interestingly, the rate of rebleeding among patients found to have vascular lesions on SBE did not significantly differ from that among patients without a bleeding source identified (48 % versus 56 %; p = 0.47), leading the authors to speculate that most patients with OGIB who have no bleeding source found on BAE likely have vascular lesions that have gone undetected. Strikingly, there was no rebleeding in the small group of patients found to have nonvascular bleeding sources. What is clear from these few clinical outcomes studies following BAE is that while a significant proportion of patients achieve long-term success, a considerable number have ongoing or recurrent bleeding problems, particularly those found to have vascular lesions in the small bowel.
Recognizing the challenge of achieving durable long-term success when treating small bowel vascular lesions, several studies have now specifically focused on clinical outcomes with OGIB patients in this category. The first was published by May et al. from Germany who reported long-term follow-up of 50 patients who received argon plasma coagulation (APC) to treat small bowel vascular lesions while undergoing DBE for predominantly overt OGIB [40]. Rebleeding was defined as overt melena or hematochezia or as an occult drop in hemoglobin >1.0 g/dL, and occurred in 46 % of patients after a long clinical follow-up period of 55 months. Despite the high rate of rebleeding in nearly half of patients, treatment with APC (see Video 9.1) made a positive impact on clinical outcomes by significantly improving hemoglobin levels and reducing blood transfusion requirements. The mean hemoglobin level improved from 7.6 g/dL prior to DBE to 11.0 g/dL at follow-up, while 60 % of patients were transfused a median of 9 units of blood prior to DBE and only 16 % were transfused a median of 2 units afterwards. A similar rebleeding rate was seen in a retrospective follow-up study from France that included 98 patients who received successful endoscopic therapy to vascular lesions in the small bowel while undergoing DBE for overt and occult OGIB [41]. At 3 years’ follow-up, 46 % of patients experienced recurrent overt bleeding or iron deficiency anemia with need for blood transfusions or iron therapy, with the majority found to be bleeding from the same type of vascular lesions within the same segment of the small bowel as seen on the index DBE. The significant predictors of rebleeding on multivariate regression were the total number of vascular lesions (p = 0.001) and the presence of valvular or arrhythmic heart disease (p = 0.007). An interesting, multicenter prospective follow-up study has recently been published, again from France, that included 183 patients with both overt and occult OGIB who were found to have angiodysplasia on VCE and subsequently went on to receive endoscopic therapy during DBE [42]. The lesions seen on VCE were classified according to the system previously proposed by Saurin et al. [43]: P0 = no bleeding potential; P1 = uncertain bleeding potential (e.g., mucosal red spots); P2 = high bleeding potential (e.g., classic angiodysplasia); P3 = actively bleeding lesion (see Table 9.2). The authors then defined P1 lesions as having a low likelihood of bleeding, and P2 and P3 lesions as having a high likelihood of bleeding [42]. Rebleeding was defined as overt bleeding signs, need for blood transfusion, or decline in hemoglobin >2 g/dL after excluding other potential causes. At 1-year follow-up, 35 % of patients had experienced rebleeding, with cardiac disease (hazard ratio 2.04; p < 0.01) and an overt bleeding presentation (hazard ratio 1.78; p = 0.03) predictive of rebleeding risk on multivariate regression. Perhaps the most unique finding from this study was the analysis demonstrating that patients who received endoscopic therapy during DBE to P1 lesions considered low likelihood for bleeding when seen on VCE had significantly increased rates of rebleeding compared to patients with high likelihood lesions (P2 and P3) treated during DBE (hazard ratio 1.87), with a longer time-to-rebleeding among the patients with high likelihood lesions. What this shows is that minor abnormalities identified on VCE are unlikely to represent the true bleeding source and therefore, the expected benefit of treating such lesions via DBE is likely to be limited. A limitation with this study is that the mean wait time from VCE to DBE was over 4 months. Since we know that the sooner that VCE or DBE is performed after a bleeding episode the greater the likelihood of finding significant bleeding lesions, and since this current study tells us that the benefit of endoscopic therapy during DBE is most realized when applied to higher risk lesions, any delay between bleeding presentation, localization of high likelihood bleeding lesion on VCE, and treatment of that lesion during DBE may be detrimental to clinical outcome.
Table 9.2




Classification of small bowel vascular lesions
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








