Predisposing factor
Percent of ACPO patientsa
Surgery
35–52
OB/gynecology
9.8–10
Abdominal/pelvic
9.3–10
Orthopedic
7–7.3
Other (urologic, thoracic, neurosurgery)
11.8
Trauma
11–11.3
Infection
10
Cardiac
10
Neurologic
9–9.3
Medical conditions
30
Medications
Electrolyte derangements
Liver or renal failure
Neoplasia
Alcohol abuse
Mechanical ventilation
Predisposing medical conditions include systemic or intra-abdominal infection, myocardial infarction and congestive heart failure, alcohol abuse, liver or renal failure with related metabolic disturbances, diabetes, respiratory pathology (including pneumonia and mechanical ventilation), leukemia, retroperitoneal tumors or history of pelvic radiation, and herpes zoster infection. Less commonly associated factors include chronic neurologic conditions, such as Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, and cerebrovascular accidents [2–5, 8, 11].
Medications that impair intestinal motility are often implicated in ACPO, including opiates, antihistamines, antipsychotics, tricyclic antidepressants, corticosteroids, and epidural anesthesia. In addition to Parkinson’s disease being a risk factor for ACPO, drugs used to treat the condition, such as dopamine agonists and anticholinergics, have been linked to ACPO (Table 8.2). Metabolic derangements are commonly present in ACPO patients and may be inciting or aggravating factors. Hypothyroidism, hyponatremia, hypocalcemia, hypokalemia, hypomagnesemia, and elevated urea nitrogen have all been described in association with ACPO (Table 8.1) [ 2–5, 8, 11].
Table 8.2
Medications associated with ACPO
Opiates |
Anticholinergics |
Histamine-2 blockers |
Calcium-channel blockers |
Tricyclic antidepressants |
Phenothiazines (chlorpromazine, prochlorperazine) |
Steroids |
Epidural anesthesia |
Antiparkinsonian drugs (dopamine agonists, anticholinergics) |
Clonidine |
Benzodiazepines |
Mechanical Colonic Obstruction
The specific etiology of MCO is usually more definitive (Table 8.3). The most common cause is colorectal cancer, accounting for 33–60 % of mechanical obstructions [1, 12–14], with three-quarters of these cancers being adenocarcinomas [4]. Overall, 10–30 % of colorectal cancer patients will develop obstruction [1, 4, 15]. Volvulus causes about 10–15 % of obstructions and chronic diverticular disease (abscess and stricture) accounts for 10 % [15]. In addition to primary colorectal cancers, metastatic tumors to the abdomen, including ovarian and uterine cancers, can lead to extrinsic compression of the colonic lumen [14]. Benign strictures due to ischemia, diverticular disease, diverticulitis and inflammatory bowel disease (secondary to acute inflammation or chronic strictures), nonsteroidal anti-inflammatory agents (NSAIDs), and high-dose pancreatic enzymes can cause MCO [12, 15–19]. Intussusception, adhesions, hernia, fecal impaction, and endometriosis are less frequent causes [12, 15, 20]. Very rarely, infectious sources, including Actinomyces, Taenia saginata, botulism, and Salmonella, have been reported to cause mechanical colonic obstruction [15].
Table 8.3
Etiologies of mechanical colonic obstruction
Etiology | Percent of colon obstructiona |
|---|---|
Primary colorectal cancer | 53–60 |
Volvulus | 15–17 |
Sigmoid | 76 |
Cecal | 22 |
Diverticular disease | 10 |
Extrinsic tumor compression | 6 |
Other | 9 |
Ischemic stricture | |
Anastomotic stricture | |
Inflammatory bowel disease | |
Intussusception | |
Fecal impaction | |
Adhesion | |
Infection |
Pathophysiology
Acute Colonic Pseudo-obstruction
The exact pathophysiology of ACPO has not been fully elucidated. In the gastrointestinal (GI) tract, parasympathetic innervation stimulates motility while sympathetic innervation inhibits peristalsis. Sir Ogilvie hypothesized that destruction of sympathetic ganglia caused relative parasympathetic overdrive leading to bowel spasm and clinical signs of obstruction [3, 8, 21]. In recent years, the successful treatment of ACPO with acetylcholinesterase inhibitors has substantially modified this original theory. Acetylcholinesterase inhibitors prevent the breakdown of the enteric neurotransmitter acetylcholine, leaving more stimulatory neurotransmitter available at the synapse. This enhances blood flow and smooth muscle contraction, stimulating bowel motility [8, 21]. Thus, the success of acetylcholinesterase inhibitors in treatment of ACPO may imply that decreased parasympathetic innervation (rather than increased activity as Ogilvie first hypothesized) is the main factor resulting in ACPO. In a case series of chronic colonic pseudo-obstruction, biopsies showed reduced number of myenteric (parasympathetic and sympathetic inputs) and submucosal (parasympathetic) ganglion cells in 4 of 6 patients.
Rather than decreased parasympathetic input to the bowel, an alternative hypothesis is that ACPO results from increased sympathetic drive due to tonic hyperactivity of inhibitory neurons. Animal models of postoperative ileus show sympathetic overactivity and leukocyte migration into the lamina propria [3]. Taken as a whole, these findings support the presence of autonomic dysfunction and imbalance of sympathetic (antimotility) and parasympathetic (promotility) inputs to the large bowel in ACPO. In addition, the colocolic reflex may also play a role in persistence of ACPO. In this case, distension of the distal colon sends inhibitory signals to the proximal GI tract, further inhibiting motility [2]. Once distension has progressed, it may be more difficult to resolve due to this negative feedback inhibition.
Mechanical Colonic Obstruction
MCO is, by definition, mechanical or anatomic in nature, whether internal or external to the bowel lumen. Compromise of blood flow leading to ischemia can occur due to increased intraluminal pressure, twisting of the mesentery (as in volvulus), or direct extrinsic compression of the vasculature [15]. Local inflammation and edema can cause or contribute to mechanical obstruction, as in endometriosis, inflammatory bowel disease, diverticulitis, or diverticular abscess [12, 15].
Mechanisms of Injury
Patients with ACPO and MCO are at risk for ischemia and/or perforation as wall tension increases. With increasing wall tension, venous congestion occurs and results in impaired blood flow. As intraluminal pressures continue to rise above diastolic blood pressure, arterial flow slows. Ischemia results when pressures exceed systolic blood pressure and ischemic bowel tissue is predisposed to perforation. Luminal stasis can cause bacterial overgrowth and translocation through the gut lumen, which may lead to peritonitis in the absence of perforation [15]. Laplace’s law states that wall tension is proportional to the intraluminal pressure and radius of the bowel [4, 15]. Therefore, the location at highest risk of perforation in ACPO is usually the cecum due to its larger diameter. However, there is an imperfect association with increased diameter and perforation and other factors, including the rate and duration of colonic dilation, are important as well [2]. In MCO, the presence of a closed-loop obstruction due to volvulus or obstruction with a competent/closed ileocecal valve is more likely to result in perforation due to increased intraluminal pressure [12].
Diagnosis and Evaluation
Radiographic imaging is . critical in the diagnosis and management of either type of colonic obstruction. In ACPO, plain abdominal X-ray typically reveals massive gas-filled dilation of colon without air-fluid levels and little or no small bowel dilation (Figs. 8.1 and 8.2). Stool and gas can be seen distal to the dilated segment since a mechanical obstruction is not present [22]. Careful attention should be paid to the amount of stool in the rectal vault to exclude distal stool impaction, which would be managed differently than ACPO. The cecum and right colon are usually the sites showing dilation of the largest diameter, averaging 10–16 cm on radiographs and conferring the highest risk of perforation due to Laplace’s law [5, 23]. In MCO, plain abdominal films reveal dilation proximal to the obstruction with air-fluid levels in the colon and small bowel. Distal to the obstruction, the colon is decompressed and devoid of stool and air [12]. Cecal volvulus typically displays a markedly distended loop of large bowel extending from the right lower quadrant to the epigastrium or left upper abdomen (Fig. 8.3). Sigmoid volvulus can present with an inverted-U or a coffee bean shape on X-ray due to massive dilation (Fig. 8.4) [24]. Upright abdominal and chest films are more useful than supine films in determining if free air due to perforation is present. If bowel ischemia is present, plain films may reveal thumbprinting due to mucosal edema and submucosal hemorrhage [5].



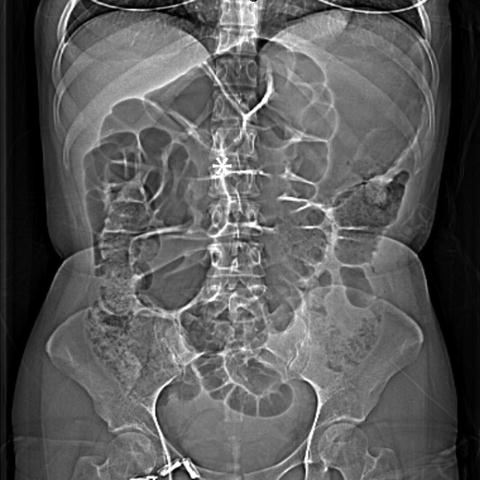

Fig. 8.1
Acute colonic pseudo-obstruction . Abdominal X-ray reveals diffusely dilated loops of small and large bowel

Fig. 8.2
Acute colonic pseudo-obstruction. Abdominal X-ray reveals diffuse gaseous distension of small bowel loops and colon causing diaphragm elevation bilaterally

Fig. 8.3
Cecal volvulus . CT topogram with * indicating marked dilation of the cecum

Fig. 8.4
Sigmoid volvulus . Abdominal X-ray with * indicates classic coffee bean appearance
Unfortunately, plain radiographs have poor sensitivity in diagnosing colonic obstruction. In a study of 120 patients, the sensitivity was only 33 % while the specificity was 100 %; subsequent CT imaging increased the sensitivity to 67 % [25]. In another series of 140 cases, plain abdominal X-ray alone had an 84 % sensitivity and 72 % specificity for colonic obstruction [15]. Plain abdominal imaging may also not be reliable in differentiating between ACPO and MCO. In another series, 30 % of patients diagnosed with MCO on plain X-ray actually had ACPO, whereas 20 % of those diagnosed with ACPO had mechanical obstruction [15].
CT with oral or rectal contrast is advised in all suspected cases to differentiate ACPO from mechanical obstruction and to assess for evidence of complications (Fig. 8.5). Contrast CT studies have the added ability to characterize bowel mucosa for signs of ischemia or perforation [3]. Water-soluble contrast enema is preferred over barium enema due to the risk of barium impaction at the site of obstruction and barium peritonitis if perforation is present [4]. CT findings that are characteristic of ACPO include preserved haustral markings and luminal dilation in the absence of an obstructive lesion [23]. If mechanical colonic obstruction is present, the source is very likely to be seen on these studies (Fig. 8.6). Volvulus can be diagnosed by the presence of a bird’s beak pattern on contrast studies. On CT imaging, sigmoid volvulus is characterized by limbs of the twisted loop converging toward a fulcrum point, which appears as a “whirl sign” when the view plane is orthogonal to the rotation axis of the loop. In most cases, the whirl sign is found in the left lower abdomen with a craniocaudal axis (Fig. 8.7). The rectum and the upstream colon are usually flat, whereas the twisted loop is highly distended and located in the anterior part of the abdomen. Cecal volvulus is the torsion of a mobile cecum around its own mesentery, which often results in a closed-loop obstruction; twisted terminal ileum, distended cecum, and twisted ascending colon are seen. Cecal volvulus may occur by three mechanisms: type 1 develops from clockwise axial torsion or twisting of the cecum around its mesentery; type 2 loop volvulus develops from counterclockwise axial torsion of the cecum around its mesentery; and type 3 or cecal bascule involves upward folding of the cecum as opposed to axial twisting [24]. In most cases of cecal volvulus, the whirl sign is found in the right part of the abdomen with a lateral or an anteroposterior axis (Fig. 8.8). Pneumatosis or gas in mesenteric veins in concert with bowel wall thickening strongly suggests that bowel infarction has occurred [5, 24].





Fig. 8.5
Sigmoid volvulus . CT topogram with * indicates classic coffee bean appearance

Fig. 8.6
Malignant sigmoid colon obstruction . CT with arrow indicating abrupt transition from dilated to decompressed sigmoid colon in the setting of cancer involving the proximal sigmoid colon

Fig. 8.7
Sigmoid volvulus . CT with swirling mesentery or “whirl sign” in the lower abdomen in the setting of a sigmoid volvulus

Fig. 8.8
Cecal volvulus . CT with arrow indicating swirling of the mesentery or “whirl sign” within the right lower quadrant of the abdomen
Management
Initial management of . ACPO and MCO is conservative unless there is significant concern for present or impending complications. Endoscopic interventions are central to the management of both MCO and ACPO and are detailed in a separate chapter. ACPO can often be managed conservatively, with reported success rates ranging widely from 20 to 92 % [2–4, 8]. Cases unresponsive to conservative measures after 24–48 h, symptom duration more than 3–4 days, and colonic diameter more than 10–12 cm warrant further treatment [8]. MCO can also be managed conservatively for a short time interval while preparing for more definitive endoscopic or surgical therapy. Close monitoring with serial abdominal examination and plain abdominal radiographs obtained every 12–24 h should be performed to monitor for peritoneal signs suggestive of ischemia or impending perforation while conservative measures are being instituted [2].
Conservative Measures
Initial conservative management of ACPO consists of noting by mouth, intravenous fluids, placement of a nasogastric tube to intermittent suction for proximal decompression, and rectal tube placement to gravity drainage. Metabolic and/or electrolyte imbalances should be corrected and any underlying associated condition(s) treated. All medications that can worsen GI motility should be discontinued whenever possible [2, 3, 8].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








