Fig. 15.1
Pathophysiology of nocturnal polyuria
Glomerular Filtration
The glomerulus receives its blood supply from an afferent arteriole of the renal circulation and drains into an efferent arteriole. The resistance of these arterioles is autoregulated and determines the fraction of blood plasma that is filtered through the glomerular capillaries into the Bowman’s capsule, which empties the filtrate into the proximal tubule.
An increase in intravascular volume and blood pressure (e.g., in patients with cardiac failure, hypertension, obesity, reabsorption of peripheral edema, polydipsia) can lead to an increase in glomerular filtration, which also affects osmotic and water diuresis.
In healthy controls, there is a circadian rhythm with a decrease (15–30 %) in glomerular filtration during the night. A subgroup of patients with NP shows an increase in nighttime glomerular filtration. This is called glomerular hyperfiltration.
Osmotic Diuresis
Endocrinological, neurological, renal, and cardiovascular stimuli are responsible for the reabsorption of the majority (95–99 %) of the filtered osmoles together with water. This process mainly takes place in the proximal tubule but also in the loop of Henlé and the distal tubule of the kidney. Natriuresis is the most important type of osmotic diuresis in the pathophysiology of NP. It is regulated by salt dietary intake and several hormones, including the atrial natriuretic peptide (ANP) and the renin-angiotensin-aldosterone system (RAAS). Other types of osmotic diuresis are glucosuria in patients with diabetes mellitus and excretion of urea due to a high-protein diet or hepatic diseases.
An increase in intravascular volume and blood pressure (e.g., in patients with cardiac failure, hypertension, obesity, reabsorption of peripheral edema, polydipsia) not only stimulates glomerular filtration but also rises the secretion of ANP and inhibits the RAAS, both leading to an increase in natriuresis.
In healthy controls, there is a circadian rhythm with a decrease in nocturnal natriuresis. Some patients with NP, especially older persons, have an absent or even inversed circadian rhythm in natriuresis.
Water Diuresis
In order to decrease water excretion and increase urine osmolality, different endocrinological, neurological, renal, and cardiovascular stimuli are responsible for the reabsorption of free water in the collecting duct of the kidney.
The most important regulator is the antidiuretic hormone (ADH), vasopressin. In response to hyposmolality or an increase in intravascular volume and blood pressure (e.g., in patients with cardiac failure, hypertension, obesity, reabsorption of peripheral edema, polydipsia), the secretion of ADH is inhibited and water diuresis is stimulated.
In healthy controls, there is a circadian rhythm with an increase in ADH during the night, leading to a decrease in water diuresis. A subgroup of patients with NP lacks this circadian rhythm and shows an increased nocturnal water diuresis.
24 h Polyuria
In 24 h polyuria the same mechanisms as in NP play a role. However, in this case, a disturbance in circadian rhythm does not only occur at night but also during daytime.
Glomerular hyperfiltration as underlying mechanism for 24 h polyuria can occur due to polydipsia or cardiac failure. For a detailed description, see section “Glomerular filtration”.
Osmotic diuresis in 24 h polyuria is usually caused by glucosuria. Normally all glucose is reabsorbed by the kidneys; however, in untreated or poorly treated diabetes mellitus, the elevated blood glucose levels result in excretion of glucose in urine which leads to excessive water loss.
Diabetes insipidus is characterized by increased water diuresis over 24 h, regardless of fluid intake. Central diabetes insipidus is caused by a deficiency of ADH, whereas nephrogenic diabetes insipidus is caused by an insensitivity of the kidneys to ADH.
Reduced Functional Bladder Capacity
The pathophysiology of an RFBC depends on the cause. Overactive bladder syndrome is caused by disturbances in the nerves, smooth muscle, and urothelium. Prostate enlargement and bladder, prostate, or urethral cancer resulting in lower urinary tract obstruction can lead to bladder decompensation with increased mass, decreased compliance, and cholinergic denervation. As bladder contractions occur in a response to cholinergic stimuli, any cholinergic medication, for example, beta-blockers and cholinesterase inhibitors, can increase voiding frequency and reduce bladder capacity.
Sleep Disorders
The pathophysiological mechanism of sleep apnea leading to nocturia is based on the hypoxemia due to airway obstruction stimulating ANP release, leading to natriuresis and nocturnal polyuria.
Impact
Nocturia is a major cause of sleep fragmentation and associated with a reduction in quality of life, impairment of daily activities and productivity at work, additional comorbidities (falls, traffic accidents, cardiovascular diseases, metabolic syndrome), and mortality. Although many experts consider nocturia to be clinically significant only when patients void at least twice during the night, the need to get up once to void is often related to disruption of the deep sleep (slow wave sleep) within the first 3 h of sleep, which is considered to have the most impact on quality of life. The first hours of undisturbed sleep are the best parameter to evaluate the impact of Nocturia.
Diagnostic Evaluation
A complete diagnostic evaluation is invaluable to initiate an adapted and individualized treatment. All patients with bothersome nocturia need to complete a frequency volume chart and a questionnaire on sleep quality and lower urinary tract symptoms to diagnose the underlying cause(s) of nocturia: NP, 24 h polyuria, RFBC, and sleep disorders. Patients with NP or 24 h polyuria should have an additional evaluation with a renal function profile to evaluate abnormalities in glomerular filtration, osmotic diuresis, and water diuresis.
Frequency Volume Chart (FVC)
Patients have to record voided volumes, volume and type of fluid intake, and the time of going to bed and getting up in the morning during 3 days. In case of urinary incontinence, the frequency of urinary leaks has to be reported together with (an indication of) the quantity and the circumstances (e.g., coughing, sneezing, handwashing) (Fig. 15.2).
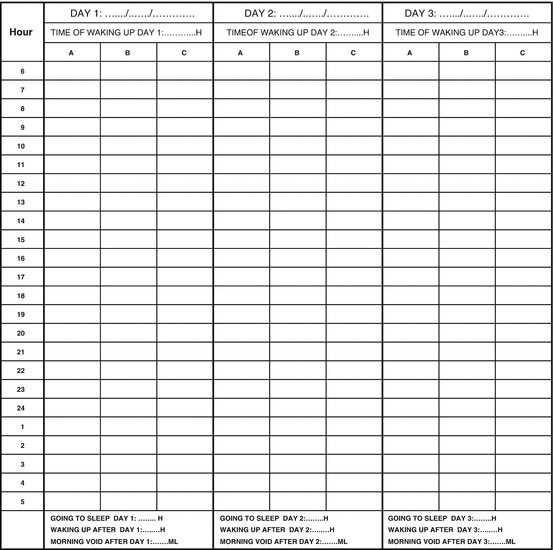

Fig. 15.2
Frequency volume chart. Column A: Write down the voided volumes of each micturition during 3 × 24 h (in ml). Do not forget to note the volume of the first morning void of the next day, the time of going to bed with the intention to sleep and the time of waking up in the morning. Column B: Measure the involuntary urine loss: weight of wet diaper – weight of dry diaper. If that is not possible, give an indication of the estimated urine loss: N1 = some drops of urine; N2 = leakage that requires a new diaper. Describe the situation of the urine leakage (e.g. during coughing, laughing, following urgency, etc.). Column C: Note the amount (in ml) and type of fluid intake (e.g. water, alcohol, coffee, soft drinks, etc.)
A lot of information can be deducted from an FVC (Table 15.1). When considering calculations with volumes, the first morning void of day 1 is excluded but the first morning void of day 2 is included. When considering calculation with frequencies, the first morning void of day 1 is included and the first morning void of day 2 is excluded.
Table 15.1
Information deducted from an FVC
Unit | |
|---|---|
Diurnal voiding frequency Daytime voiding frequency Nighttime voiding frequency | Number |
Diurnal urine production Nocturnal urine production (NUP) | ml |
Diurnal diuresis rate Nocturnal diuresis rate | ml/min |
Functional bladder capacity (FBC) = maximal voided volume in 24 h Mean FBC daytime = mean of voided volumes during daytime Mean FBC nighttime = mean of voided volumes during nighttime | ml |
Mean hours of sleep | h |
Diurnal fluid intake | ml |
Frequency of diurnal incontinence episodes Frequency of nocturnal incontinence episodes | Number |
Mean volume of incontinence | g |
Nocturia index (NI) = NUP/FBC → Nocturia if >1 | / |
NP33 definition: NP index (NPi) = NUP/24 h diuresis → NP if >33 % | % |
NUP90 definition = NUP/h of sleep → NP if >90 ml/h | ml/h |
Nocturnal bladder capacity index (NBCI) = actual – predicted number of voids (= NI-1) → RFBC if >1.3 | / |
Nocturnal Polyuria
Various definitions for NP can be used when analyzing an FVC (e.g., NPi33 or NUP90). In order to adapt treatment according to the underlying cause, we suggest to perform a renal function in all patients with NP.
24 h Polyuria
In patients with a 24 h diuresis exceeding 40 ml/kg body weight, the most common disorders should be considered by evaluating:
Fluid intake (polydipsia)
Glucose or HbA1c and osmotic diuresis on RFP (diabetes mellitus)
Water diuresis on RFP (diabetes insipidus)
Further diagnosis and treatment have to be organized by an internal medicine practitioner.
Reduced Functional Bladder Capacity
Because there are no cutoff values for a normal bladder capacity, the diagnosis of an RFBC is based on the NBCI (Table 15.1). Further urological investigation with uroflowmetry, ultrasonography, and urodynamics is recommended to explore the cause of the RFBC (e.g., outlet obstruction, hypocontractility of the bladder, overactive bladder syndrome).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








