Erectile dysfunction is defined as the consistent or recurrent inability to attain or maintain penile erection sufficient for sexual performance. Self-reported erectile dysfunction has increased significantly as men seek effective therapy, such as oral phosphodiesterase 5 inhibitors (PDE5i). PDE5i are now the drugs of choice in the initial therapy of erectile dysfunction. This review compares the currently available PDE5i with the second-generation PDE5i, which are soon to be available.
Key points
- •
Neurologic and vascular contribution to erectile physiology
- •
The role of nitric oxide in smooth muscle relaxation
- •
First-line therapy for erectile dysfunction: phosphodiesterase 5 inhibitors
- •
Newer phosphodiesterase 5 inhibitors
- •
Safety; efficacy; and use of sildenafil, vardenafil, and tadalafil
- •
Future of oral therapy.
Erectile dysfunction (ED) is defined as the consistent or recurrent inability to attain or maintain penile erection sufficient for sexual performance. Self-reported ED has increased significantly as men seek effective therapy, such as oral phosphodiesterase inhibitors (PDE5i). It is estimated that 15 to 30 million men report sexual dysfunction. Although the search for the causes of ED has extended over centuries, it is only recently that we have gained an understanding of the neurovascular physiology of erection. As a result of these efforts therapeutic agents targeted at specific underlying pathology are now available. The dawn of effective pharmacologic treatment occurred in the 1980s with the introduction of vasoactive agents for self-injection. Intracavernosal therapy was the primary and most efficacious treatment at that time and remained so until the discovery of nitric oxide (NO) and its role in erection physiology in the next decade.
Twelve years ago the Food and Drug Administration (FDA) approved sildenafil as the first oral phosphodiesterase inhibitor, and there is a new algorithm of treatment that is centered on patients’ goals and motivations and evidence-based principles. More than 70% of erectile dysfunction can now be treated with oral medications. Oral pharmacotherapy is the first-line treatment without question for almost all types of erectile dysfunction according to the American Urological Association and European Urological Association guidelines and the World Health Organization-sponsored International Consultation on treatment for erectile dysfunction.
With the availability of effective oral medications, the point of care for men suffering from ED moved from urologists to primary care physicians; these health care providers now perform the majority of evaluation and management of men with erectile dysfunction. By 2002, the majority of sildenafil prescriptions were written by primary care physicians (69%) as compared with urologists (13%). With this transition, it is important for urologists to stress to the primary care community the need to be thoughtful in the treatment of erectile dysfunction. Recent evidence has demonstrated that ED may be a precursor to coronary artery disease and is associated with other chronic illnesses, such as diabetes mellitus, hyperlipidemia, obesity, hypertension, and depression; thus health care providers should discuss possible causes and all treatment options with patients. PDE5i are now the drugs of choice in the initial therapy of ED. This review compares the currently available PDE5i with the second-generation PDE5i, which are soon to be available.
Mechanism of erection
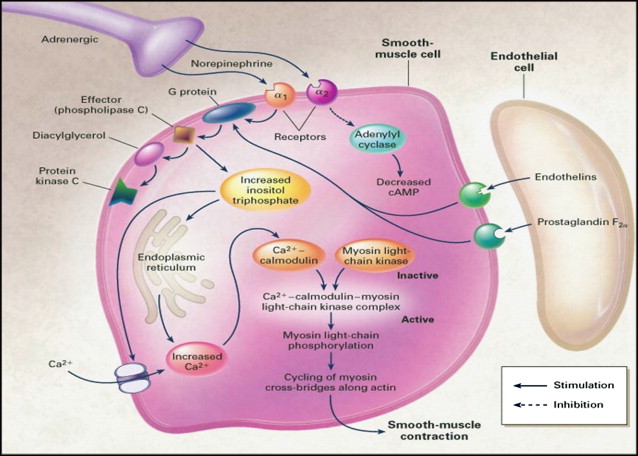
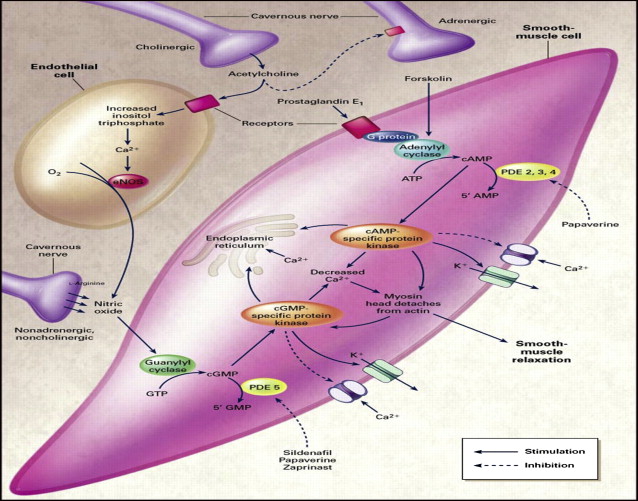
Erections are initiated, maintained, and terminated because of a complex interaction between the neural and vascular components. Both central and peripheral factors are responsible for successful erections. The main physiologic event is the release of nitric oxide both from the autonomic nerve endings and the endothelial cells in the corpora cavernosum. After release NO rapidly enters the smooth muscle cells leading to smooth muscle relaxation and tumescence followed by passive veno-occlusion as the subtunical venule plexus is compressed against the rigid tunica albuginea. Nitric oxide facilitates vasodilatation and relaxation by activating guanylate cyclase. This enzyme converts guanosine triphosphate to cyclic guanosine monophosphate (cGMP), which is directly responsible for smooth muscle relaxation by its effect on intracellular calcium levels. Hyperpolarization occurs at the cell membrane. There is a decrease in cytoplasmic calcium and the smooth muscle cell relaxes ( Fig. 1 ). Levels of cGMP in the smooth muscle cells of the penis are regulated by the enzyme phosphodiesterase type 5 (PDE-5). Detumescence occurs with sympathetic nerve firing. Adrenergic nerves release norepinephrine that binds to α1 or α2 receptors on the smooth muscle cell. This neurotransmitter is responsible for the activation of a G-protein and an influx of calcium into the smooth muscle cell. As PDE-5 continues to breakdown cGMP to guanosine monophosphate, the smooth muscle cells and endothelial cells contract. This condition is the chronic state of the flaccid penis ( Fig. 2 ).
Mechanism of erection
Erections are initiated, maintained, and terminated because of a complex interaction between the neural and vascular components. Both central and peripheral factors are responsible for successful erections. The main physiologic event is the release of nitric oxide both from the autonomic nerve endings and the endothelial cells in the corpora cavernosum. After release NO rapidly enters the smooth muscle cells leading to smooth muscle relaxation and tumescence followed by passive veno-occlusion as the subtunical venule plexus is compressed against the rigid tunica albuginea. Nitric oxide facilitates vasodilatation and relaxation by activating guanylate cyclase. This enzyme converts guanosine triphosphate to cyclic guanosine monophosphate (cGMP), which is directly responsible for smooth muscle relaxation by its effect on intracellular calcium levels. Hyperpolarization occurs at the cell membrane. There is a decrease in cytoplasmic calcium and the smooth muscle cell relaxes ( Fig. 1 ). Levels of cGMP in the smooth muscle cells of the penis are regulated by the enzyme phosphodiesterase type 5 (PDE-5). Detumescence occurs with sympathetic nerve firing. Adrenergic nerves release norepinephrine that binds to α1 or α2 receptors on the smooth muscle cell. This neurotransmitter is responsible for the activation of a G-protein and an influx of calcium into the smooth muscle cell. As PDE-5 continues to breakdown cGMP to guanosine monophosphate, the smooth muscle cells and endothelial cells contract. This condition is the chronic state of the flaccid penis ( Fig. 2 ).
Oral phosphodiesterase inhibitors
Phosphodiesterase is found in multiple tissues throughout the body and has been categorized into 11 families. Phosphodiesterase type 5 is the predominant subtype in corpora cavernosal tissue as well as vascular smooth muscle. Thus, drugs were targeted to inhibit this enzyme and increase the NO in corporal smooth muscle cells, which resulted in improved erectile function.
PDE5i were first marketed in 1998 with the introduction of sildenafil. This introduction was followed in 2003 by vardenafil and tadalafil. When choosing a PDE5i for patients with ED, considerations include the onset of action, efficacy, and duration of effect of the individual agent. Pharmacokinetic serum levels have defined the maximal plasma concentration, time to reach this plasma concentration (Tmax), and the plasma half-life (t½) for each agent and are included in the product label of the 3 current agents. How well these laboratory findings correlate to clinical results is sometimes difficult to determine and the measuring tool most commonly used to evaluate efficacy is the International Index of Erectile Function (IIEF) erectile function (EF) domain score.
Sildenafil Citrate
The first and most extensively investigated of these agents is sildenafil citrate. The registration trial for sildenafil, published in 1998, demonstrated a clinically and statistically significant improvement in IIEF erectile function domain scores for men suffering from ED for greater than 5 years. Sildenafil is absorbed in the small bowel after gastric emptying, with onset of action of sildenafil in approximately 20 minutes, a reported t½ of 3 to 5 hours, and duration of action as long as 12 hours. Sildenafil undergoes first pass metabolism in the liver by the P-450 enzymes CYP3A4 and CYP2C9 and metabolism within the gut wall. This metabolism allows only 38% to 41% of the drug available to elicit a drug effect. Further experience with sildenafil revealed that it should not be taken with high-fat meals because the delay in gastric emptying results in decreased absorption, reduced peak serum concentrations, and decreased efficacy.
Vardenafil
The FDA approved vardenafil in 2003 after a study of 805 men with ED demonstrated a clinically and statistically significant improvement in IIEF scores when compared with placebo. In this pivotal trial, 3 therapeutic doses were evaluated (5, 10, and 20 mg) and men were classified with mild, moderate, and severe ED based upon baseline IIEF EF domain scores. Approximately 40% of men with moderate or severe ED had improvement with the highest dose and more than 79% of men with mild ED had improvement. Vardenafil is quickly absorbed with a Tmax of 45 minutes and a reported t½ of 4 to 5 hours. Onset of action has been recorded as early as 10 minutes. Vardenafil is also metabolized by the liver; however, the degree of hepatic metabolism for vardenafil is less than that of sildenafil resulting in greater bioavailability of this agent. Just as with sildenafil, it is recommended that high-fat meals are avoided.
Tadalafil
Tadalafil was also approved in 2003 and is the most selective of the 3 PDE5i medications. Tadalafil has a completely different structure than the other 3 marketed PDE5i ( Fig. 3 ). The onset of action has been recorded at 20 minutes, whereas the Tmax of tadalafil is closer to 2 hours and the t½ is 17.5 hours with a clinical efficacy reported of 12 to 36 hours. The registration trial for tadalafil involved 1112 men with a mean duration of ED greater than 1 year. After ingestion more than 80% of the men taking tadalafil had improved erections with duration of efficacy up to 36 hours after administration of the drug. Because of slower uptake in the small bowel tadalafil does not seem to be as affected by high dietary fat intake, thus there are no dietary restrictions with the use of this agent.
Since the introduction of the last of the currently marketed PDE5i in 2003 several novel PDE5i that have been investigated, with the hope that different pharmacokinetic parameters may lead to a decrease in adverse effects while or increasing efficacy. To this date, most of the newer PDE5i have exhibited pharmacokinetic profiles similar to the older PDE5i, and although the newer agents show more promise in vitro, true clinical evidence is still lacking. At the time of this article, none of these have been approved in the United States.
Avanafil
Avanafil (TA-1790, Vivus, Inc) has a much shorter onset of action and half-life than currently approved PDE5i; after ingestion the Tmax has been measured at 35 minutes with a half-life of less than 1.5 hours. Preliminary trials examined doses of 50 mg, 100 mg, and 200 mg; all 3 doses significantly improved IIEF EF domain scores versus placebo. Phase I and II trials looking at safety and efficacy of the drug for ED have been completed and currently phase III trials are underway in the United States. The adverse event profile is similar to that of the other known PDE5i.
Udenafil
Udenafil is the only other PDE5i marketed and is available in South Korea and approved for distribution in the Russian Federation. The structure of udenafil is similar to that of sildenafil and vardenafil. The pharmacokinetic profile of udenafil demonstrates a Tmax of 60 minutes and a t½ of 11 to 13 hours; thus the duration of action of udenafil falls in between those of sildenafil/vardenafil and tadalafil. Animal studies showed improved erectile function with the use of udenafil versus placebo in diabetic rats and in rats with cavernosal nerve injury. In human studies, improvement in IIEF EF domain scores has been shown in both healthy men and men after prostatectomy ED. Other studies have looked at the effect of udenafil in a diabetic population with reports that improvement in IIEF scores from baseline were unaffected by initial glycosylated hemoglobin levels, which could be promising for this group of patients who can be refractory to oral medications.
Lodenafil
Lodenafil carbonate is a PDE5i developed in Brazil and has completed phase II and III trials that show safety and efficacy in treating ED. Lodenafil has a unique chemical structure; a carbonate bridge unites 2 molecules of lodenafil, after ingestion the carbonate bridge is broken freeing each molecule of lodenafil for biologic effect ( Fig. 4 ). Lodenafil has a Tmax of 80 minutes and a t½ of 2.4 hours.






