This article highlights current and emerging pharmacological treatments for gastroesophageal reflux disease (GERD), opportunities for improving medical treatment, the extent to which improvements may be achieved with current therapy, and where new therapies may be required. These issues are discussed in the context of current thinking on the pathogenesis of GERD and its various manifestations and on the pharmacologic basis of current treatments.
Gastroesophageal reflux disease (GERD) has come to be regarded as a simple condition that is easy to treat; proton pump inhibitors (PPIs) are generally considered the most effective medical treatment of GERD. Patients with mild or infrequent symptoms often do not require PPI therapy but those with more severe or frequent symptoms may benefit significantly from regular PPI therapy.
One of the major challenges in GERD management is the persistence of symptoms despite regular, once-daily therapy, considered by some as PPI failure. However, this is not necessarily a failure of acid suppression therapy. Current, first-generation PPIs have a short plasma half-life and cannot provide day-long acid suppression; thus, there are opportunities for improved outcomes with (1) more frequent dosing using current PPIs, (2) longer-acting PPIs or potassium-competitive acid blockers (P-CABs), (3) antireflux agents, (4) visceral pain modulators, or (5) mucosal protectants. In the short-term, improved outcomes can be achieved by fine-tuning treatment using currently available medications, based on their pharmacology and the patient’s disease manifestations; a careful consideration of the choice of drug, dose, and treatment regimen is crucial in the day-to-day management of GERD. For the future, an improved understanding of GERD and the pharmacology of available medications is essential for improving symptoms and quality of life.
Background
The prevalence of GERD is increasing worldwide and it has become the most prevalent gastrointestinal disorder. It is responsible for a substantial proportion of health care expenditure in the developed world. In the last 3 decades, the development of increasingly potent acid suppressants has revolutionized the medical management of GERD. Initially, histamine H 2 -receptor antagonists (H 2 -RAs) supplanted antacids for the healing of erosive esophagitis (EE); H 2 -RAs were, in turn, supplanted, to a large extent, by PPIs, which provided healing and symptom relief in a significantly greater proportion of patients than H 2 -RAs. Despite the success of acid suppression, GERD is not primarily a disorder of acid secretion and there have been many attempts to develop pharmacologic, endoscopic, and surgical treatments to increase basal lower esophageal sphincter (LES) pressure, reduce the frequency and duration of inappropriate transient LES relaxations (TLESRs), improve esophageal clearance, and accelerate gastric emptying. The attractions of targeting the pathophysiologic basis of GERD rather than acid suppression have not yielded new medications that produce healing and symptom relief comparable with those reported for PPIs.
Documented costs for managing GERD are attributable predominantly to the costs of pharmacotherapy, although surgical therapy is also associated with substantial costs, as are over-the-counter remedies. The costs of treating GERD and its complications have prompted close scrutiny of prescribing habits with the expectation that management guidelines and consequent constraints on pharmacotherapy will reduce expenditure. Reports that antireflux therapy may be provided without documentation of a diagnostic indication, in up to one-third of patients, suggest a significant opportunity to reduce the costs of GERD treatments. However, GERD is associated with an increased risk of esophageal adenocarcinoma and other complications and with substantial impairment of patients’ sleep, work productivity, and quality of life that warrant therapy. Furthermore, despite apparently optimal therapy, many patients continue to experience symptoms attributable to GERD, suggesting that there are still unmet needs.
The aim of this article to is to highlight current and emerging treatments for GERD, opportunities for improving medical treatment, the extent to which improvements may be achieved with current therapy, and where new therapies may be required. These issues are discussed in the context of current thinking on the pathogenesis of GERD and its various manifestations and on the pharmacologic basis of current treatments.
Pathophysiology of GERD
The Montreal definition of GERD as “a condition which develops when the reflux of stomach contents causes troublesome symptoms and/or complications”, is widely accepted; however, this definition does not specify how and why reflux occurs, which organs are affected by the reflux, or what symptoms and complications may be associated with the reflux of gastric contents.
Gastroesophageal reflux (GER) occurs in healthy individuals without any obvious sequelae; it is generally considered normal for the esophagus to be exposed to gastric acid (pH < 4) for up to 3% to 4% of the day (approximately 45–60 minutes a day). Similarly, TLESRs are normal events that occur in response to physiologic gastric distension. The duration of esophageal acid exposure, documented at one point, 5 cm above the LES, is often termed the reflux time; this implies that it is gastric acid or, more precisely, esophageal luminal fluid with a pH less than 4.0, that causes GERD. However, in an unknown proportion of patients, GERD symptoms seem to be associated with the presence of weakly acidic or alkaline conditions in the esophagus, suggesting that factors other than gastric acid and pepsin may be responsible for GERD.
Thus, excessive GER can occur for several reasons ( Box 1 ), which may be amenable to pharmacologic therapy; however, the range of causes is such that not all of these causes could be responsive to the same medication or to a single, standard treatment regimen. Depending on the causes of an individual’s GERD, it may be appropriate to target dietary factors, motility factors, concurrent therapy, meal-related factors, daytime or night-time acid secretion, structural factors (eg, hiatal hernia), or psychological factors.
Excessive GER
- •
Delayed gastric emptying with retention of gastric contents
Gastroparesis
Gastric outlet obstruction
Small bowel dysmotility (carbohydrate, gluten intolerance)
- •
Hiatus hernia
- •
Reduced basal LES pressure
- •
Excessive TLESRs
- •
Increased gastric acid secretion
Zollinger-Ellison syndrome
Rebound hypersecretion after withdrawal of acid suppression therapy
Helicobacter pylori- negative status
Increased duodenogastroesophageal reflux
Bile acids
Pancreatic secretions
Excessive esophageal exposure to noxious agents
- •
Impaired esophageal clearance
Disordered esophageal body motility
Impaired salivary secretion
- •
Hiatus hernia
- •
Luminal agents
Acidic foods
Alcohol
Medications (eg, bisphosphonates, antibiotics)
Excessive esophageal sensitivity
- •
Functional esophageal disease (Rome)
- •
Esophageal hyperalgesia
- •
Central hyperalgesia
Depression
Irritable bowel syndrome
- •
Concurrent inflammation
Eosinophilic esophagitis
Pathophysiology of GERD
The Montreal definition of GERD as “a condition which develops when the reflux of stomach contents causes troublesome symptoms and/or complications”, is widely accepted; however, this definition does not specify how and why reflux occurs, which organs are affected by the reflux, or what symptoms and complications may be associated with the reflux of gastric contents.
Gastroesophageal reflux (GER) occurs in healthy individuals without any obvious sequelae; it is generally considered normal for the esophagus to be exposed to gastric acid (pH < 4) for up to 3% to 4% of the day (approximately 45–60 minutes a day). Similarly, TLESRs are normal events that occur in response to physiologic gastric distension. The duration of esophageal acid exposure, documented at one point, 5 cm above the LES, is often termed the reflux time; this implies that it is gastric acid or, more precisely, esophageal luminal fluid with a pH less than 4.0, that causes GERD. However, in an unknown proportion of patients, GERD symptoms seem to be associated with the presence of weakly acidic or alkaline conditions in the esophagus, suggesting that factors other than gastric acid and pepsin may be responsible for GERD.
Thus, excessive GER can occur for several reasons ( Box 1 ), which may be amenable to pharmacologic therapy; however, the range of causes is such that not all of these causes could be responsive to the same medication or to a single, standard treatment regimen. Depending on the causes of an individual’s GERD, it may be appropriate to target dietary factors, motility factors, concurrent therapy, meal-related factors, daytime or night-time acid secretion, structural factors (eg, hiatal hernia), or psychological factors.
Excessive GER
- •
Delayed gastric emptying with retention of gastric contents
Gastroparesis
Gastric outlet obstruction
Small bowel dysmotility (carbohydrate, gluten intolerance)
- •
Hiatus hernia
- •
Reduced basal LES pressure
- •
Excessive TLESRs
- •
Increased gastric acid secretion
Zollinger-Ellison syndrome
Rebound hypersecretion after withdrawal of acid suppression therapy
Helicobacter pylori- negative status
Increased duodenogastroesophageal reflux
Bile acids
Pancreatic secretions
Excessive esophageal exposure to noxious agents
- •
Impaired esophageal clearance
Disordered esophageal body motility
Impaired salivary secretion
- •
Hiatus hernia
- •
Luminal agents
Acidic foods
Alcohol
Medications (eg, bisphosphonates, antibiotics)
Excessive esophageal sensitivity
- •
Functional esophageal disease (Rome)
- •
Esophageal hyperalgesia
- •
Central hyperalgesia
Depression
Irritable bowel syndrome
- •
Concurrent inflammation
Eosinophilic esophagitis
Manifestations of GERD
GERD is remarkable for the variety of possible causes and for its protean manifestations ( Box 2 ). Although it is generally accepted that heartburn and regurgitation are diagnostic of GERD, these typical symptoms may occur in the absence of GERD (so-called functional heartburn). Moreover, GERD may present with other atypical symptoms referable to the esophagus or to other organs. Although GER and GERD symptoms are common postprandially, recent studies highlight the association between GERD and sleep disturbances and the observation that sleep disturbances may improve with effective antireflux therapy. The diurnal patterns of GER that lead to typical symptoms may therefore be different from those associated with sleep disturbance or noncardiac chest pain; similarly, the timing and duration of reflux episodes documented in patients with nonerosive reflux disease (NERD) are different from those observed in patients with EE or Barrett esophagus. Data are limited with respect to the characteristics of reflux in patients with laryngopharyngitis, asthma, otitis media, or dental erosions attributed to GERD. However, it would not be surprising if, for example, the diurnal reflux patterns in patients with presumed reflux laryngopharyngitis differed from those in patients with EE. Thus, even if the causes of the reflux episodes were similar in patients with different manifestations of GER, one might expect differing therapeutic requirements for different presentations.
- •
Symptomatic: esophageal
Typical symptoms
Heartburn, regurgitation
Chest pain
Dysphagia
Atypical symptoms
Abdominal pain or burning
Nausea, vomiting
- •
Symptomatic: nonesophageal
Cough
Wheeze
Hoarseness
Shortness of breath
Sleep disturbance
Earache
Dysphagia/globus symptoms
Glossitis
Toothache
- •
Complications
Esophageal mucosal breaks (erosions/ulcers)
Esophageal stricture
Barrett esophagus
Esophageal adenocarcinoma
Reflux laryngopharyngitis
Bronchitis
Pneumonia
Otitis media
Dental erosions
Acid suppression therapy
For a variety of reasons, including patient adherence and commercial considerations, emphasis has been placed on developing antireflux therapies that need to be taken only once daily. Although this strategy is associated with greater adherence than multiple daily doses, it is appropriate only if the pharmacologic profile is consistent with therapeutic goals. Prompt acid neutralization by antacids is associated with rapid relief of reflux symptoms but the effect is short-lived with no prospect of antacids being used as a once-daily therapy. Histamine H 2 -RAs have a significantly longer duration of action, offset by the fact that their effect is not immediate. In the treatment of GERD, H 2 -RAs were evaluated twice daily; although H 2 -RAs are more effective than placebo for healing EE, their long-term effectiveness is compromised by tachyphylaxis and there is little, if any, benefit from using doses up to twice the standard healing dose. The short duration of effect and tachyphylaxis of H 2 -RAs limits their benefit when used once daily.
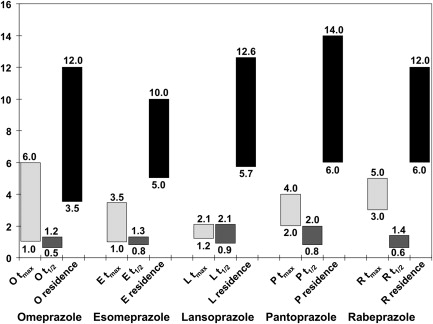
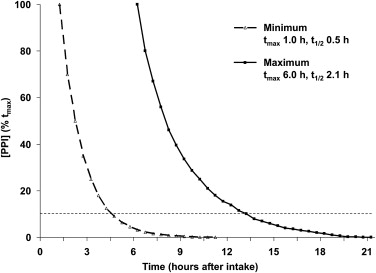
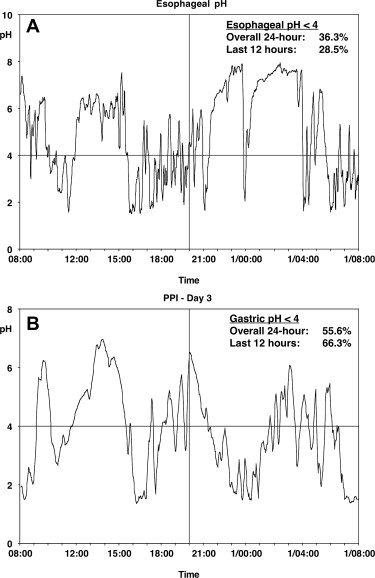
PPIs have proved significantly more effective than antacids or H 2 -RAs for healing and maintaining remission in EE and for maintaining symptom relief. Studies documented more prolonged acid suppression with PPIs than with H 2 -RAs and the degree of acid suppression, because the proportion of the 24-hour period during which gastric pH exceeded 4.0 correlated with the proportion of patients with healing of their EE over 8 weeks. Gastric pH studies confirmed that, although currently available PPIs have their C max (maximum concentration) at 90 to 120 minutes after administration, they produce more prolonged acid suppression (gastric pH ≥4.0) because they are covalently bound to the proton pumps; this suppression is overcome only when new proton pumps are inserted into the secretory membrane of the parietal cell canaliculus. As a result, current PPIs, taken once daily, can produce sufficient gastric acid suppression to achieve healing and symptom relief in many patients with GERD. Despite this situation, currently available PPIs have identifiable limitations related to their mechanism of action. Conventional PPIs do not have a rapid onset of action because they are prodrugs, administered in an enteric, acid-resistant formulation, to prevent premature inactivation by gastric acid; after dissolution of the enteric coating, the prodrug is absorbed in the small intestine. Because PPIs are weak bases, they are concentrated in the highly acidic, secretory canaliculus; activation of the prodrug to its sulphenamide form occurs only in the acidic secretory canaliculus of an actively secreting parietal cell, when the activated sulphenamide produces irreversible inhibition of those proton pumps (H+-K+ ATPase) that are actively inserted in the secretory canalicular membrane. These events occur only if circulating blood levels are high enough to allow sufficient PPI prodrug to concentrate in active parietal cells; however, because conventional PPIs have a t max (time of maximum concentration) of about 1 to 6 hours and a half-life of about 60 to 130 minutes, the PPI plasma residence time is less than 12 hours ( Fig. 1 ), such that plasma levels decrease below the therapeutic threshold within 4 to 10 hours of drug ingestion. Parietal cells that become active more than 4 to 10 hours after drug ingestion remain uninhibited because plasma PPI levels have fallen below the therapeutic threshold ( Fig. 2 ); as a result, gastric acidity steadily recovers over the 16 to 18 hours that remain until the next single daily dose. Because blocked proton pumps are not replaced for 3 to 4 days, PPIs have a progressively greater effect in reducing gastric acidity over the first 3 to 5 days of administration but, despite this, their effect diminishes over the 24-hour period between doses as new proton pumps are synthesized and acid secretion gradually increases. This situation may not be a problem for many patients with GERD whose symptoms occur predominantly during the daytime but, for other patients, the loss of PPI effect may permit nocturnal return of acid secretion ( Fig. 3 A ) and nocturnal acid reflux (see Fig. 3 B), leading to persistent night-time symptoms on once-daily PPI therapy. For some patients, the PPI effect is influenced by differences in PPI pharmacokinetics; variability in blood PPI levels, and hence in gastric acidity, and may be to the result of differences in absorption or differences in PPI metabolism related, for example, to CYP2C19 polymorphisms. As a result, 24-hour gastric acidity can vary by as much as 3 to 4 log units between individuals ( Fig. 4 ); furthermore, this interindividual variability does not seem to disappear after 3 days of once-daily oral dosing.
Pure PPI isomers, such as esomeprazole, dexlansoprazole, and TU-199 (an isomer of tenatoprazole), generally produce higher blood levels than the racemic mixtures, leading to an increase in gastric acid suppression; in early studies with esomeprazole, the increase in gastric acid suppression was attributed to an increased area under the curve (AUC) for the concentration-time curve but more recent studies with dexlansoprazole and other PPIs suggest that there is a threshold effect; that is, regardless of the peak concentration or AUC, acid suppression is achieved as long as blood levels exceed a threshold level. In clinical practice, threshold blood levels can be maintained by multiple daily oral dosing or by continuous infusion as in the management of upper gastrointestinal bleeding. Multiple daily dosing regimens raise concerns about patient adherence, and concerns remain that first-generation PPIs are slow to achieve an effective reduction in gastric acidity.
Recognition that current PPIs do not address all clinical needs has led to new antisecretory agents that offer more rapid onset and a more prolonged duration of action. Speed of onset has been addressed by an immediate-release (IR) omeprazole formulation that contains uncoated omeprazole powder (20 mg or 40 mg) plus sodium bicarbonate (1680 mg) ; the sodium bicarbonate neutralizes gastric acid, protecting the acid-labile omeprazole from degradation and, in addition, stimulating gastrin release by increasing gastric pH; the latter effect stimulates insertion of acid pumps, analogous to food-stimulated acid secretion, such that the parietal cells are activated and, hence, susceptible to omeprazole. The antisecretory effect of IR omeprazole is evident more quickly than that of classic delayed-release PPIs. Esomeprazole, the first pure isomer PPI, produces a greater AUC than racemic omeprazole, and the associated increase in acid suppression is associated with a small, but significant increase in healing rates for EE. Another approach to prolonged acid suppression is to extend the period during which blood levels of the PPI exceed the threshold level needed to achieve therapeutic levels of the PPI in the secretory canaliculus of the parietal cell. Dexlansoprazole, the R-isomer of lansoprazole, is metabolized more slowly than the S-isomer; it is now approved in the United States as a modified, dual-release formulation in which some of the R-isomer has a standard enteric coating and some a modified coating that releases the PPI at a higher pH; as a result, there is a second peak in blood levels and a more prolonged increase of dexlansoprazole levels above threshold levels. Clinically, dual-release dexlansoprazole (60 mg and 90 mg daily) healed EE after 8 weeks in 92.7% and 93.3% of patients, respectively, whereas standard lansoprazole, 30 mg daily, produced healing in 88.9% of patients ( P <.01). Although differences for healing, symptom relief, and maintenance of remission are comparable with the differences between esomeprazole 40 mg daily and omeprazole 20 mg daily, it is not clear how much of the effect is because of the change in formulation and how much to the absolute increase in dosage. Neither of the other isomeric PPIs, S-pantoprazole or dexrabeprazole, is available clinically and their benefit in clinical practice remains uncertain.
Other PPIs, still in development, include AGN 201,904-Z, ilaprazole (IY-81,149), and tenatoprazole (TU-199). AGN 201,904-Z, the sodium salt of an acid stable omeprazole prodrug, is designed to provide continued metered absorption throughout the gut and, thus, prolong the plasma PPI residence time; the prodrug is converted rapidly to omeprazole in the systemic circulation, producing faster and greater acid suppression than esomeprazole 40 mg daily in healthy, H pylori -negative male volunteers. Ilaprazole is a benzimidazole derivative that has a longer half-life and greater acid suppression than omeprazole. Racemic tenatoprazole, an imidazopyridine derivative, has a longer half-life (t ½ ∼ 8–9 hours) than first-generation benzimidazole-derived PPIs, with the result that it produces a significantly higher median 24-hour pH than esomeprazole, 40 mg daily, after 7 days’ administration. A crossover study comparing isomeric S-tenatoprazole sodium, 30 mg, 60 mg, and 90 mg daily, with esomeprazole, 40 mg daily, showed that the higher doses produced significantly greater and more prolonged dose-dependent 24-hour and nocturnal acid suppression than esomeprazole. A meta-analysis of individual subject data from 4 pharmacodynamic studies has shown, also, that S-tenatoprazole sodium, 60 mg daily, produces greater acid suppression than esomeprazole, 40 mg daily, and is comparable with esomeprazole, 40 mg twice daily, although there are no clinical trial data in patients.
In the last few years, another class of acid suppressants has been developed; the P-CABs also inhibit the H + -K + ATPase but, unlike PPIs, they target the potassium-binding region of the proton pump. P-CABs are lipophilic weak bases with a high pKa and are stable at low pH. They are absorbed rapidly and are concentrated, up to 100,000-fold, in the secretory canaliculus; the protonated form then binds ionically, but reversibly to the proton pump, producing rapid and profound acid suppression. Initial studies with P-CABs such as linaprazan (AZD-0865), revaprazan (YH1885), and soraprazan indicated that these compounds have a more rapid onset of action and the potential for greater acid suppression than conventional PPIs. Linaprazan (AZD-0865) given once daily was not superior to esomeprazole, 40 mg daily, in the treatment of EE and NERD, and the development program for this compound has been halted.
Although antacids and H 2 -RAs are less effective than PPIs, they still have a role in management of patients with GERD, particularly as over-the-counter medications. As prescription medications, H 2 -RAs alone are not, generally, effective for more severe grades of GERD; however, they are still recommended and used widely for patients with mild or infrequent symptoms and also, in combination with PPIs, for patients with persistent nocturnal symptoms. Initial enthusiasm for combined therapy with a PPI and an H 2 -RA was tempered by a diminution in benefit that was observed as tachyphylaxis to the H 2 -RA developed ; however, more recent data, and studies with combinations of a PPI and an H 2 -RA, suggest that there is still a role for combination acid suppression therapy in some patients with GERD.
Nonacid suppression therapy
Acid suppression therapy remains the mainstay of medical management for GERD, and PPIs provide the most effective acid suppression therapy. Despite this, PPIs are less effective for complete symptom relief and, furthermore, relapse of symptoms and complications such as erosions, strictures, and hemorrhage can occur, even with maintenance PPI therapy. There are good pharmacologic reasons why some patients might not respond to standard, once-daily therapy with a PPI and, although there is good reason to assume that increased acid suppression improves response rates, there are several other mechanisms that might underlie PPI failure in GERD, including the presence of esophagitis or other esophageal diseases, persistent weakly acidic reflux or duodenogastroesophageal reflux, delayed gastric emptying, esophageal hypersensitivity, concomitant functional bowel disorders, and psychological comorbidity. Identification of the individual underlying mechanisms for persistent symptoms, where possible, might be relevant for the management of refractory GERD. Several agents under development may have a role in selected refractory reflux patients. They include motility agents to accelerate gastric emptying and improve esophageal clearance, antireflux agents that reduce the frequency of TLESRs and thereby the number of acid and nonacid reflux episodes, visceral pain modulators to reduce visceral hypersensitivity, and mucosal protection agents. None of these agents, on its own, is likely to be as effective as a PPI in so many patients but there may be a role for these medications in combination with acid suppression therapy.
Motility agents
A significant factor in the pathophysiology of GERD is disordered gastroesophageal motility, including one or more of these abnormalities: delayed gastric emptying, reduced LES pressure, increased incidence of reflux during TLESRs, and ineffective esophageal clearance. Together with a hiatal hernia, disordered gastroesophageal motility may favor GER and/or determine the volume, proximal extent, and composition of the refluxates. Gastroesophageal hypomotility might be a cofactor involved in refractoriness to PPI treatment. Several prokinetic agents can stimulate gastrointestinal motility and many of these agents have been used, either alone or in combination with a PPI or H 2 -RA, for the treatment of GERD. Bethanechol, metoclopramide, domperidone, cisapride, and macrolides, such as erythromycin or ABT-229, have all been used in patients with GERD. Although these drugs are believed predominantly to impart their effect by enhancing esophageal motility, reflux clearance, basal LES pressure, reducing TLESRs, and by accelerating gastric emptying, many of these compounds are not highly selective and have off-target effects. This situation means that the mechanism of action responsible for their therapeutic effects often remains controversial, and undesirable side effects are often encountered. For example, cisapride, a 5-HT 4 agonist that was approved for use in GERD before subsequently being withdrawn for safety reasons, not only increased the rate of gastric emptying but also modified saliva secretion and bicarbonate content and gastric acid secretion, believed to occur via potassium channels, which also mediated the adverse cardiac effects of cisapride. Although cisapride was more effective than placebo in symptom relief and healing esophagitis, studies indicated that cisapride has little effect on esophageal motility and, notably, no effect on TLESRs after 4 weeks of oral (20 mg twice daily) administration. Tegaserod, another partially selective 5-HT 4 agonist, also showed some efficacy in small GERD trials. Tegaserod (1 and 4 mg/d) reduced postprandial reflux and TLESRs but did not alter LES tone. However, as with cisapride, it is uncertain whether the beneficial effects of tegaserod were because of the effect on motor function or other recently documented effects on salivary flow rate, salivary bicarbonate and epidermal growth factor secretion, and bicarbonate secretion from esophageal submucosal glands. Tegaserod has also been withdrawn from the market for safety issues.
Motilin is a peptide found in specific endocrine cells in the epithelia of the upper small intestine. Motilin receptor agonists can increase gastric emptying after ingestion of a meal; this action is mediated via the cholinergic system of the stomach. Much of this evidence is derived from the use of erythromycin, a macrolide antibiotic that also acts as a motilin receptor agonist. Erythromycin, given at low doses, which have no antibiotic effect, was the first motilin agonist used in several clinical situations, particularly in severe gastroparesis. Early studies found erythromycin to be of no value in the control of reflux. ABT-229, an erythromycin derivative devoid of antibiotic activity, accelerates gastric emptying and increases LES pressure in healthy persons; however, the therapeutic effect of ABT-229 in patients with GERD was limited in terms of modulation of both esophageal motility (LES pressure and TLESRs) and acid exposure. The development of ABT-229 has been discontinued because of a rapid onset of tachyphylaxis and worsening of symptoms in dyspeptic patients. In a recent study in patients after lung transplantation, another macrolide antibiotic, azithromycin, reduced the number of reflux events and total esophageal acid exposure and also reduced the proximal extent to which reflux regurgitated.
There are several limitations when using macrolides as prokinetics in GERD. The most consistent data supporting their role as prokinetics in reflux disease come from studies in which the drug was administered intravenously ; this would not be suitable for a broader GERD population. In addition, repeat administration of macrolides induces desensitization of the motilin receptor, reducing their efficacy over time. Macrolides also induce side effects, including nausea and abdominal cramping, which are believed to be caused by direct activation of motilin receptors on gastric smooth muscle, which occurs at higher doses and which makes macrolides difficult to tolerate for many patients. A more selective motilin agonist, which could be taken orally, would be highly desirable to test in patients with GERD with compromised gastroesophageal motor function.
Few studies have assessed the value of the current therapeutic approach to delayed gastric emptying in patients with GERD, who failed PPI therapy. However, it is likely that these patients also complain about other dyspeptic symptoms related to slow gastric motor activity. There are no data about the value of adding a promotility drug in patients who have failed PPI therapy, given once or twice per day. However, in patients with delayed gastric emptying and persistent GERD symptoms on PPI therapy, the use of a promotility agent remains an attractive option.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







