Neurogenic lower urinary tract dysfunction (NLUTD) affects many patients and requires close monitoring. Initial studies establishing patients at risk for upper tract disease revealed that high detrusor leak point pressures were predictive of upper tract disease. Urodynamics in patients with NLUTD have specific challenges. Initial studies in patients after an acute injury should be delayed until after the spinal shock phase. In children with spinal dysraphism, studies should be done early to established potential risk. The goals are maintaining low bladder pressures, decreasing risk of infection, and maintaining continence.
Key points
- •
Neurogenic lower urinary tract dysfunction (NLUTD) affects a large population of patients with variable bladder behaviors depending on extent of disease.
- •
Videourodynamics can be useful to evaluate outlet and upper tracts during filling and voiding.
- •
Monitoring blood pressure during urodynamics (UDS) for autonomic dysreflexia is especially important for patients with spinal cord injury (SCI).
- •
UDS in patients with NLUTD are challenging because of the inherent lack of sensation and lack of correlation of symptoms to upper tract disease.
- •
Patients with SCI undergo a period of spinal shock after injury usually lasting 4 to 6 weeks; initial study should be delayed until after bladder reflexes return.
Who: epidemiology of neurogenic lower urinary tract dysfunction
Neurogenic lower urinary tract dysfunction (NLUTD) (also referred to as neurogenic bladder [NGB]) is a condition in which neurologic disease manifests by alteration of bladder and sphincter activities through abnormal bladder innervation. NLUTD affects a large population of patients suffering from various conditions, including spinal cord injury (SCI), stroke, traumatic brain injury, brain tumor, meningomyelocele, cerebral palsy, multiple sclerosis, disk disease, Parkinson disease, and other diseases with long-term neurologic dysfunction, such as diabetes, pernicious anemia, and tabes dorsalis. Bladder behavior in each subset of patients is unique depending on extent and length of disease and may require close monitoring for symptomatic control and evaluation for potential upper tract deterioration.
Historical Perspective
Before the late 1970s, it was well recognized that patients with NGB developed bladder dysfunction and obstructive uropathy slowly in the first 5 years after injury, followed by a faster progression to eventual renal failure, hypertension, stone formation, incontinence, vesicoureteral reflux (VUR), autonomic dysreflexia (AD), and even death. The recognition that bladder storage pressure is related to upper tract damage was first published in 1978 by Light and colleagues, who reported upper tract deterioration in children with myelodysplasia. This was followed by the more familiar work of McGuire and colleagues, in 1981, who described more definitively that myelodysplastic children with elevated detrusor leak point pressure (DLPP) are at risk to develop upper tract disease. This landmark study evaluated 42 pediatric subjects with spinal dysraphism. These subjects underwent urodynamics (UDS) and 68% of subjects with a DLPP greater than 40 cm of water were found to have VUR and 81% had dilated upper tracts on excretory urography. In contrast, none of the subjects with a DLPP less than 40 cm of water had VUR and only 9% had dilation of upper tracts. Subsequently, in 1989, Ghoniem and colleagues described the relationship between high DLPP and poor bladder compliance leading to renal dysfunction, thus prompting the use of pharmacologic therapy in conjunction with intermittent catheterization or procedures, such as bladder neck incision, to decrease outlet resistance.
Who: epidemiology of neurogenic lower urinary tract dysfunction
Neurogenic lower urinary tract dysfunction (NLUTD) (also referred to as neurogenic bladder [NGB]) is a condition in which neurologic disease manifests by alteration of bladder and sphincter activities through abnormal bladder innervation. NLUTD affects a large population of patients suffering from various conditions, including spinal cord injury (SCI), stroke, traumatic brain injury, brain tumor, meningomyelocele, cerebral palsy, multiple sclerosis, disk disease, Parkinson disease, and other diseases with long-term neurologic dysfunction, such as diabetes, pernicious anemia, and tabes dorsalis. Bladder behavior in each subset of patients is unique depending on extent and length of disease and may require close monitoring for symptomatic control and evaluation for potential upper tract deterioration.
Historical Perspective
Before the late 1970s, it was well recognized that patients with NGB developed bladder dysfunction and obstructive uropathy slowly in the first 5 years after injury, followed by a faster progression to eventual renal failure, hypertension, stone formation, incontinence, vesicoureteral reflux (VUR), autonomic dysreflexia (AD), and even death. The recognition that bladder storage pressure is related to upper tract damage was first published in 1978 by Light and colleagues, who reported upper tract deterioration in children with myelodysplasia. This was followed by the more familiar work of McGuire and colleagues, in 1981, who described more definitively that myelodysplastic children with elevated detrusor leak point pressure (DLPP) are at risk to develop upper tract disease. This landmark study evaluated 42 pediatric subjects with spinal dysraphism. These subjects underwent urodynamics (UDS) and 68% of subjects with a DLPP greater than 40 cm of water were found to have VUR and 81% had dilated upper tracts on excretory urography. In contrast, none of the subjects with a DLPP less than 40 cm of water had VUR and only 9% had dilation of upper tracts. Subsequently, in 1989, Ghoniem and colleagues described the relationship between high DLPP and poor bladder compliance leading to renal dysfunction, thus prompting the use of pharmacologic therapy in conjunction with intermittent catheterization or procedures, such as bladder neck incision, to decrease outlet resistance.
How: performing UDS in a patient with NLUTD
Preparation for the Study
Many patients with NLUTD also have neurogenic bowel with a home bowel regimen. If the patent is not on a bowel regimen, bowel evacuation may be necessary before the study to allow for accurate rectal catheter pressure readings. If patients are already on a bowel program, rectal suppositories or enemas should be administered with enough time before the study to allow the medication to take effect and avoid bowel movements during the procedure.
The study can be performed in the supine, sitting, or standing positions, or during ambulation. Many patients with NLUTD have limitations in mobility, not allowing them to sit or stand at a commode. These patients do not usually void into a toilet and, therefore, it is acceptable to do the study in the supine position during the test. Patients should be comfortable regardless of position and care should be taken to avoid excess pressure on the limbs and to protect skin from breakdown. If patients volitionally void, the study should be performed in the position in which they usually void (standing or sitting) to allow for optimal pressure-flow measurement. If using fluoroscopy, it is ideal that the patient is positioned so that oblique images can be captured to adequately visualize the bladder neck. To perform the pressure-flow portion of the study, urine may be collected into a wide-bore drainpipe with length to reach the flowmeter. Multiple positions might be required, especially when expected results are not achieved in the supine position.
Filling Rate
Patients with NLUTD tend to be more sensitive to the speed of filling. A voiding diary is often helpful to determine if the filling rate should be decreased. A voiding diary that reveals low volumes and/or consistent leakage with or between each void or catheterization warrants lower filling rates. Generally, starting at a low rate of 10 mL/min or less is advised. If no increase in detrusor pressure is seen, the rate may be increased slowly. If the detrusor pressure continues to increase with filling, decreasing the filling rate or stopping the infusion can help determine if the increase in pressure is due to a detrusor contraction or impaired compliance. In a child with NLUTD, the rate can be calculated as 2% to 10% of the child’s age-related bladder capacity. Filling rates greater than 20% of estimated bladder capacity have been shown to artificially raise detrusor pressures.
Electromyography
Electromyography (EMG) during UDS is very useful in patients with NLUTD because it may confirm denervation of the pelvic floor musculature or identify discoordination of the external urethral sphincter. In patients with sensation, pad surface electrodes can be placed around the anus. The surface EMG is described as an indirect measure of external sphincter activity. Needle electrodes that more directly assess sphincter function are often used with patients with SCI who have no sensation.
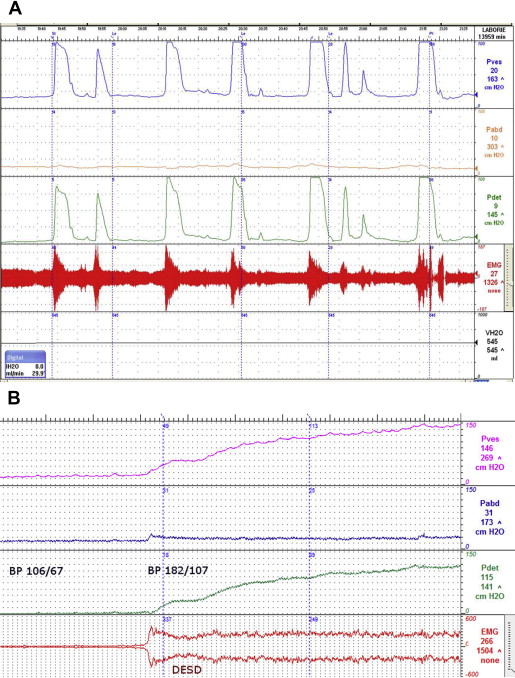
EMG is especially important in evaluation of patients with neurologic lesions suggestive of detrusor external sphincter dyssynergia (DESD) or other evidence of impaired bladder emptying. DESD is seen during the voiding phase of the UDS ( Fig. 1 ). During DESD, the external (voluntary) sphincter contracts (signified by increased EMG activity on UDS), which impairs the ability to empty the bladder by obstructing the outlet and may prevent a sustained bladder contraction, further impairing bladder emptying. EMG is also useful in monitoring patients who have undergone sphincterotomy (see later discussion).
Videourodynamics
Fluoroscopic imaging at the time of videourodynamics allows for the visual evaluation of the entire urinary tract during filling and voiding phases of the study. This imaging identifies anatomic and functional abnormalities of the urinary tract. Fluoroscopy may be performed on a radiograph table in the supine position, or in an radiograph-compatible UDS chair. Patients with NLUTD often have symptoms that are difficult to differentiate or they may be asymptomatic given their altered sensation. This is particularly challenging in patients with incontinence and incomplete bladder emptying, especially in aging men with benign prostatic enlargement or women with pelvic organ prolapse in whom other causes of dysfunction may be considered.
AD
In 1860, the first case of AD was described by Hilton as hot flushes in a patient with C5 SCI. Subsequent reports described a variety of symptoms, including hot flushes, sweating with bradycardia, and increase in blood pressure associated with a distended bladder. In 1947, Guttman and Whitteridge more fully described the autonomic response after distension of the abdominal viscera leading to effects on cardiovascular activity in subjects with SCI.
AD occurs in approximately 60% of cervical and 20% of thoracic SCI patients. The most common cause is bladder or rectal distension, either spontaneous or by instrumentation (ie, UDS). Other causes include plugged catheters, urinary tract stones, long bone fracture, decubitus ulcers, or even electroejaculation.
A classic sign of AD is an increase in blood pressure with bradycardia, although true bradycardia is seen in only approximately 10% of patients. In fact, tachycardia or no significant change in heart rate is more common in patients with AD. Other signs may include cardiac arrhythmias, changes in skin temperatures (vasodilation above the spinal cord lesion, vasoconstriction below the spinal cord lesion), or changes in mentation.
Common symptoms include sweating above the spinal cord lesion, pounding headache, hot flushes, piloerection, nasal congestion, dyspnea, and anxiety. Although we usually think of patients presenting with these classic symptoms, some patients may be entirely asymptomatic. A study by Linsenmeyer and colleagues demonstrated that 35 of 45 subjects with SCI above T6 were asymptomatic with a significant elevation of blood pressure. This emphasizes the importance of monitoring blood pressure during procedures in patients at risk for AD because significant changes in heart rate and blood pressure may be missed in an asymptomatic patient with possible devastating outcomes, including seizures, stroke, or even death. During UDS, it is generally recommended to obtain a baseline blood pressure and cycle the blood pressure during regular intervals throughout the study (see Fig. 1 B).
When AD is recognized, the first course of action should be removal of the stimulus. Usually this means ensuring that a patient’s catheter is draining correctly, checking for fecal impaction, or (if performing a urologic procedure) stopping and immediately emptying the bladder. It is also recommended to move the patient upright and remove any tight clothing or constrictive devices. If this does not alleviate symptoms and/or decrease blood pressure, the urologist can move to pharmacologic agents.
No particular pharmacologic agent is preferred for acute AD. Multiple drugs have been used, including nifedipine, nitrates, captopril, terazosin, prazosin, phenoxybenzamine, and prostaglandin E2. Nifedipine, a calcium channel blocker, has been the most popular pharmacologic agent for management of acute AD. The usual dose is 10 mg oral and the patient is asked to chew and swallow the medication for optimal absorption. The use of nifedipine is falling out of favor for patients without SCI secondary to adverse events seen in management of hypertensive emergencies, including stroke, heart attack, severe hypotension, and death. Nitrates have been used for acute AD but should be used with caution because of drug interaction, especially in patients who use phosphodiesterase 5 inhibitors. Topical nitrates are easy to use and can be removed quickly if necessary. Typically, they are placed on the shoulders or arms, above the level of injury. If a patient has a history of AD, consideration of prophylaxis given 30 minutes before a urologic procedure, including UDS, would be appropriate ( Fig. 2 ).
Patients with recurrent AD can be managed prophylactically. Terazosin, nightly, at 5 mg has been used without change in blood pressure or erectile function. Vaidyanathan and colleagues titrated terazosin from 1 to 10 mg daily in 18 subjects with resolution of AD in all subjects. One subject required discontinuation secondary to dizziness.
Although prophylaxis has been effective in prevention of AD, it is important to continue to monitor during any urologic procedure, including UDS. Other prophylactic medications include prazosin and phenoxybenzamine. Intravesical botulinum toxin and capsaicin have been demonstrated to decrease AD episodes but further studies are required. If conservative measures do not alleviate AD, sacral denervation has been described; however, studies are conflicting on its effectiveness for eliminating AD. If prophylaxis does not adequately manage AD, the study can be performed under general anesthesia with close monitoring.
Challenges of UDS in NLUTD
UDS in patients with NLUTD may present challenges not seen in neurologically intact patients. For example, many patients with NLUTD lack symptoms because of impaired or altered bladder sensation, or it may be difficult for the patient to define symptoms, such as timing of incontinence. Patients with NLUTD may have a difficult time describing whether leakage is associated with urinary urgency or stress maneuvers, such as transferring in and out of a wheelchair. We know that degree of symptoms does not necessarily correlate with findings on UDS in the neurologically intact patient, which also applies to patients with NLUTD. Importantly, the severity of symptoms does not always correlate with the magnitude of disease affecting the urinary tract. This is particularly crucial to remember in patients at risk of upper tract deterioration, such as children with spinal dysraphism. Dator and colleagues demonstrated a poor correlation between neurologic signs and symptoms and UDS assessment in 54 children with myelodysplasia. In addition, although we know that certain levels of injury in patients with SCI tend to have certain types of bladder dysfunction, the exact status of the both the bladder and sphincter behavior cannot be inferred solely from the neurologic evaluation. The importance of doing high-quality studies in subjects with NLUTD cannot be overstated because it is the only reliable indicator of the potential risk to upper tract deterioration and the optimal tool to guide appropriate lower urinary tract management.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




