Down Syndrome
Down syndrome (DS) is the most common chromosomal abnormality occurring in humans. In Europe, DS accounts for 8% of all registered cases of congenital anomalies. Throughout the world, the overall prevalence of DS is 10 per 10,000 live births, although in recent years this figure has been increasing. DS is characterized by several dysmorphic features, delayed psychomotor development, and low muscle tone in early infancy. DS is associated with dysfunctions that might affect almost every organ and system, including the gut. It has been reported that more than 77% of DS affected neonates have, or develop, associated gastrointestinal (GI) disorders. These conditions can be classified into mechanical and functional disorders and can be primary or secondary.
Next to the most common associated congenital malformations, such as tracheo–esophageal fistula, duodenal atresia/stenosis, and imperforate anus, the most frequently occurring GI disorders in DS are characterized by motor disturbances, particularly encountered in the esophagus and colon. DS affected infants have been reported to be at 100-fold increased risk for Hirschsprung disease (HSCRD). In addition, gastroesophageal reflux disease (GERD), achalasia, and unexplained chronic constipation often complicate the clinical course of DS children. According to a recent report by Van Trotsenburg and colleagues, GI disorders and feeding difficulties represent a very frequent cause of hospitalization (19%) in patients affected by DS. Unfortunately, the inherent difficulties of DS patients to express themselves could hamper clinical evaluation and delay the diagnosis, increasing the risk of related complications. The likelihood of the involvement of the enteric nervous system (ENS) in these associations, although not yet completely understood, is generally accepted.
Role of the Enteric Nervous System
The anomalous central nervous system (CNS) development, function, and intellectual impairment in DS has always been related to the genetic imbalance, resulting from the presence of an extra copy of chromosome 21. However, the underlying pathogenetic mechanisms are still unclear. Whether chromosome 21 trisomy determines a malfunction of dosage-sensitive genes or results in a more generalized alteration of homeostasis in a critical development period is still not clarified. In this context, it is not surprising to observe a high frequency of enteric nervous system alterations, as both embryonic brain and GI tract development are regulated by similar neural growth factors, under the control of the same genes. A link between brain and ENS development is well demonstrated by the high prevalence of cerebral dysgenesis in patients affected by neurocristopathies, such as HSCRD. The ENS develops from the colonization of neural crest cells, which migrate to populate the GI tract. This mechanism is regulated by a complex signaling system, which appears to be altered in DS. Decreased neuronal migration, as well as alterations of dendritic development, has been shown in an animal model of DS. At the same time, alterations in ENS structure and function have been demonstrated in DS patients. Nakazato and Landing showed a reduced number of neurons in esophageal plexus ganglia in DS patients. Hypothetically, this decrease, described in the esophagus, could occur throughout the entire gut. Further studies are needed to better clarify these fundamental pathogenetic mechanisms.
Esophageal Motor Dysfunction
In a recent prospective analysis, Zarate and colleagues reported that the most common functional GI symptoms described in DS patients are dysphagia for liquids and solids, vomiting, regurgitation, and heartburn. GERD remains one of the most frequent causes of esophageal symptomatology in DS. Previous studies described a high prevalence of severe GERD with the occurrence of serious complications, such as oropharyngeal aspiration and pneumonia, in 43% of DS patients. In one case report, GERD was associated with the development of pulmonary arterial hypertension in a 2-month-old DS-affected boy.
As is the case with all children with a neurologic impairment, many factors could contribute to the high prevalence of reflux and impaired esophageal clearance and, consequently, lead to the development and progression of GERD among DS patients. For example, abnormal swallowing, delayed gastric emptying, abnormal muscle tone, obesity, and constipation are commonly reported in this patient population. The severity of GERD may also result from poor self-protective mechanisms and delayed diagnosis caused by difficulties in obtaining an accurate history of symptoms. In addition, it seems that the severity of GERD correlates directly to the severity of neurologic impairment. For all these reasons it seems extremely important to investigate esophageal function in all patients with typical or such atypical GERD symptoms as food rejection, frequent vomiting, coughing, and failure to thrive. Early diagnosis is essential to prevent respiratory problems, growth retardation, and all other GERD complications. According to the latest European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN)–North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) guidelines on GERD, the evaluation of children with neurologic impairment, such as DS, should be based on a high index of suspicion and must not only confirm the diagnosis but also rule out alternative diagnoses. Contrast GI radiographic studies, upper GI endoscopy and biopsy, metabolic and drug toxicity screening, and pH/impedance studies may be required. Treatment should associate optimized antisecretory therapy to behavioral measures, including feeding and positional changes. Given the morbidity and high failure rates of antireflux surgery, patients whose symptoms are well controlled on medical therapy may not derive additional benefit from antireflux surgery. Despite optimized medical therapy, some DS patients affected by GERD need antireflux surgery. However, this should be considered only in highly selected cases, as antireflux surgery has been independently associated with poorer developmental outcome. In addition, it has been shown that preexisting esophageal dysmotility in DS patients could complicate the postoperative clinical course after corrective surgical procedures.
Next to GERD, esophageal motor disorders, such as achalasia, are not infrequent in DS patients. A number of instances of the association between achalasia and DS have been described in adult and pediatric DS populations ( Fig. 1 ). In a study conducted by Zarate and colleagues, the authors evaluated esophageal clearance in adult DS patients and controls, using scintigraphy. Patients with abnormal scintigraphic studies and suggestive symptoms proceeded to undergo radiologic and manometric examinations. Results clearly showed a significantly greater retention of both liquid and semisolid boluses in DS. Achalasia was diagnosed in two patients, providing an astonishingly high prevalence (2/58 patients enrolled) compared with the general population (prevalence 8/100,000). Further, another patient had total body aperistalsis and two had a nonspecific motor disorder. The same authors, in 1999, reported five cases of DS associated with achalasia. Two of the five reported cases were children. Why does DS carry a higher risk of achalasia?
Achalasia is a primary esophageal disorder of unknown etiology: infectious, autoimmune, and genetic factors have been implicated. Although the increased susceptibility of DS patients to infection, as well as their predilection to autoimmune disorders, could be the link between these disorders, genetic factors and associated alterations of ENS morphology and function probably play the most important role. HSCRD, another primary congenital disorder, is also more prevalent in DS. Both achalasia and HSCRD share some pathophysiologic mechanisms, such as a lack of nitric oxide synthase in the affected region, a reduced number of ganglion cells in affected regions, and a consequent failure of sphincter relaxation. Symptoms in pediatric patients with achalasia can be subtle and, in contrast to adults, dysphagia is not always present. Indeed, the main clinical features are respiratory symptoms and growth retardation. In any event, an early diagnosis of achalasia is extremely important in childhood and achalasia should always be included with GERD in the differential diagnosis of any esophageal or potentially esophageal-related symptoms in pediatric patients with DS.
Unexplained Chronic Constipation and HSCRD
Chronic constipation remains one of the most common symptoms experienced by both children and adults with DS : the reported prevalence in the literature varies from 19% to 56%. Further, these rates may represent an underestimate. All DS children with constipation must be considered to be potential candidates for HSCRD, because of the known association between these two entities. HSCRD remains the most common congenital dysganglionosis associated with DS. Reported incidence is approximately one in 200 to 300 DS patients, higher than the 0.15% to 0.17% expected incidence in the general population. Looking at it from another perspective, about 30% of HSCRD patients have a recognized chromosomal abnormality, syndrome, or additional congenital anomalies, the most frequent of which is DS. This well described association has led to the proposal that chromosome 21 may play a possible role in the pathogenesis of HSCRD. Nevertheless, although the presence of trisomy 21 seems to be associated with a higher risk for the disorder, it does not invariably lead to HSCRD. Several studies have investigated the role of chromosome 21 as a potential candidate area, capable of modifying the risk of HSCRD. Possible relationships between DS and major susceptibility HSCRD genes, such as RET and EDNRB , have also been examined. Arnold and colleagues showed that the RET enhancer polymorphism RET +9.7 correlates with HSCRD in DS. At the same time, a novel EDNRB variant has been identified in DS patients with HSCRD.
Although the precise pathogenesis of HSCRD in DS has yet to be clarified, HSCRD should always be considered in DS patients. Moore reported a prevalence of 3.2%, with a female preponderance and an 85% rate of other associated anomalies. The aganglionic segment was limited in extent to the rectosigmoid in 69% of cases. Early diagnosis is extremely important owing to the high reported mortality. DS patients show a significantly higher overall risk of preoperative and postoperative enterocolitis. These inferior outcomes could be explained by an impaired immunologic response. In addition, long-term outcome may also be inferior, as DS patients with HSCRD appear to have a less predictable prognosis. Thus, previous studies reported persisting soiling after the fourth year of life in 60% of patients.
Cerebral Palsy
Cerebral palsy refers to a group of chronic, nonprogressive disorders of movement, posture, and tone due to CNS damage before cerebral development is complete. The availability of neonatal intensive care units and high-technology diagnostic procedures has led to an increased survival rate for premature and term infants with neurologic impairment. The prevalence of cerebral palsy is estimated to be 2 per 1000 live births. The relative prevalence of the different types of cerebral palsy varies from series to series, with the spastic type considered to be the most frequent, while periventricular leukomalacia and cortical/cerebral atrophy represent the most typical neuropathologic substrates. GI motor dysfunctions, such as GERD, dysphagia, vomiting, and chronic constipation, have all been reported in children with different degrees of CNS damage. The degree of GI dysmotility seems to correlate with the degree of brain damage. The long-term survival of children with severe neurologic damage, such as cerebral palsy, has created a major challenge for medical care.
Esophageal Dysfunction
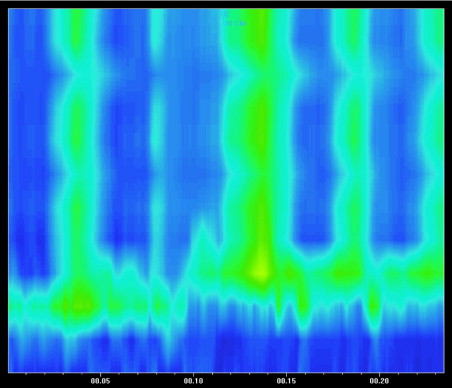
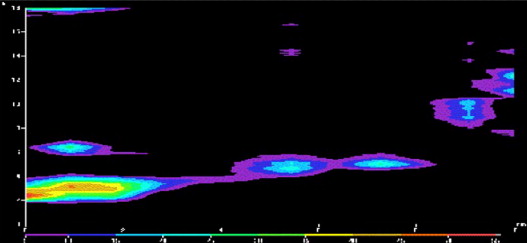
GERD is very common in patients with severe neurologic impairment. The incidence, in various studies, has been reported to be between 70% and 90%, depending in part, on the method of investigation, whether using esophageal pH studies or upper GI endoscopy. In 1999, our group clearly showed that neurologic patients affected by GERD had delayed gastric emptying and abnormal esophageal motility. This suggests that impaired GI motility is the main pathogenetic factor in the induction and progression of GERD, as well as of severe esophagitis ( Fig. 2 ). The main motor abnormalities consisted of significantly lower amplitude of both lower esophageal sphincter (LES) pressure and esophageal body contractions and an increased number of simultaneous waves, compared to control children ( Fig. 3 ). These findings, coupled with spasticity, prolonged adoption of supine position, scoliosis, seizures, and a reduction in the amount of swallowed saliva consequent upon drooling, increase the predisposition to the development of GERD and may be responsible for the high failure rate of both medical and surgical treatment approaches to this category of patients. Indeed, the optimal management approach to GERD in patients with brain damage is still controversial. According to the recent ESPGHAN–NASPGHAN guidelines on gastroesophageal reflux, antisecretory therapy should be optimized. Long-term treatment with proton pump inhibitors (PPIs) is often effective for symptom control and maintenance of remission. Baclofen is also recommended to control GERD symptoms and to reduce vomiting. Changes in feeding volume, consistency, and frequency, as well as positional changes, may be helpful. An alternative to this classic medical approach is represented by the use of an elemental diet. We reported a lower incidence of GERD in neurologically impaired children with refractory esophagitis treated with an amino-acid–based formula. However, conventional medical management is known to be less effective in neurologically impaired children and often vomiting tends to persist despite PPIs therapy. At the same time, surgical intervention is associated with a high operative risk and the benefit/risk ratio for antireflux surgery in these patients with persistent symptoms, despite optimized medical therapy, is not clear. The open Nissen fundoplication has been associated with several complications in neurologically impaired children. In addition, postoperative morbidity rates of up to 50%, reoperation rates up to 20%, and mortality up to 50% have been reported. More recently, the laparoscopic approach to the Nissen fundoplication has become the procedure of choice in the surgical management of GERD in general, and its results also appear superior among brain damaged children. Thus, Esposito and colleagues reported a 30% rate of postoperative complications and a 6% rate of reoperation.

Swallowing disorders represent another common problem in this patient group, an occurrence that may further exacerbate GERD and esophagitis. Our group reported an incidence of 85.7% of swallowing disorders in patients with cerebral palsy. Most patients showed dysfunction of the oral phase of swallowing, with abnormal formation of the food bolus due to either uncoordinated movements or contraction and rigidity of the tongue. Others, though demonstrating normal bolus formation, had huge defects in bolus propulsion toward the oropharynx, due to the lack of finely coordinated movements of the tongue against the palate. Swallowing disorders have significant implications for development, nutrition, respiratory health, and GI function in this group of patients. The development of dysphagia is associated with a progressive reduction in food intake and it represents the main pathogenetic factor for malnutrition. At the same time, swallowing disorders can cause recurrent episodes of pulmonary aspiration. Early diagnosis should be considered mandatory. A videofluoroscopic swallow study, being capable of simultaneously assessing pharyngeal motility and airways protection during the swallow, is considered the gold standard in children with neurologic impairment. Considering all of the problems related to oral feeding in these patients, a gastrostomy tube feeding is strongly recommended in neurologically impaired patients with dysphagia, undernutrition and associated respiratory diseases. The American Academy of Cerebral Palsy and Developmental Medicine considers gastrostomy feeding as a valuable alternative nutritional source in this group of children, capable of improving nutrition, ameliorating GERD and associated pulmonary problems.
Chronic Constipation
The prevalence of the chronic constipation varies from 25% to 75% among patients with cerebral palsy. It represents a common but often underdiagnosed condition in patients with neurologic impairment. Chronic constipation is the result of prolonged colonic transit, which is secondary to an underlying gut dysmotility. In a study conducted by our group in 1994, colonic transit time seemed to be delayed predominantly in the left colon and rectum. These findings differentiate this form of constipation from that observed in patients with functional fecal retention. In addition, we observed that, in contrast to neurologically normal children, none of the children with cerebral palsy presenting with chronic constipation reported fecal soiling. Disruption of the neural modulation of colonic motility may play a predominant role in the development of constipation in neurologic disease. This could be a possible explanation for the poor impact of prokinetic drugs on delayed colonic transit in children with brain damage. A low fiber and fluid intake, as well as the frequent delay in diagnosis, certainly contribute to the development and reinforcement of constipation in neurologically impaired children. Our group demonstrated the efficacy of the dietary fiber glucomannan in improving bowel frequency in children with severe brain damage, despite no measurable effects on delayed transit.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




