As populations age, the prevalence of neurologic disease in the community continues to increase, and consultations relating to gastrointestinal motility problems in the patient afflicted with a neurologic disorder become ever more common.
Although in theory several gastrointestinal functions could be disturbed in neurologic disorders in relation either to a given disease process or to its therapy, this article focuses on the gastrointestinal function that has received the greatest attention in this context, namely, gastrointestinal motility.
Gut Motor Function and its Relevance to Neurologic Disease
Given its essential role in digestion, absorption, secretion, and excretion, the gastrointestinal tract and its associated organs play an essential role in homeostasis. The various physiologic processes of the gastrointestinal tract serve these functions; thus, motility propels food, chyme, and stool and promotes mixing to increase contact time and thereby digestion. Gut muscle and nerve are integrated into a “mini-brain” and are adapted to subserve these homeostatic functions. Throughout most of the gastrointestinal tract, gut smooth muscle is arranged in two layers, an outer longitudinal layer and an inner circular layer. However, at the beginning and end of the gut, striated muscle is found in the oropharynx, upper esophageal sphincter (UES), proximal part of the esophagus, external anal sphincter, and pelvic floor muscles. In these locations, somatic innervation plays a crucial role in the regulation of swallowing and defecation; these functions are, not surprisingly, particularly prone to disruption, and in neurologic disease and dysphagia, constipation and fecal incontinence are prominent issues.
Throughout the remainder of the gut, several levels of control are evident. Myogenic regulation of motility refers to intrinsic properties of gut muscle cells and their interactions with one another. The biochemistry and molecular biology of enteric smooth muscle has much in common with its striated counterpart, thus explaining the high frequency of gut involvement in muscular dystrophies.
The next layer of control is provided by the enteric nervous system (ENS), which is now recognized as a distinct and independent division of the autonomic nervous system. The ENS may represent the most important level of neuronal control of motility. It is capable of generating and modulating many functions within the gastrointestinal tract without input from the more traditional divisions of the autonomic system and central nervous system (CNS). Through variations in neuronal morphology and in the electrophysiologic properties of individual neurons, as well as through the presence of a wide variety of neurotransmitters and neuromodulatory peptides, the ENS demonstrates striking plasticity. Of relevance to any discussion of the gut in CNS disorders, it is now recognized that the ENS and CNS share many similarities, both morphologic and functional. Thus the basic organization of the ENS (neurons, ganglia, glia, an ENS-blood barrier), as well as the ultrastructure of its components, are similar to that of the CNS, and almost all neurotransmitters identified within the CNS are also found in enteric neurons. The concept of ENS involvement in neurologic disease should not, therefore, come as a great surprise.
Although the ENS is primarily responsible for the generation and modulation of most motor activities within the gut, input from autonomic nerves and the CNS also modulates motor activity. Autonomic input is now recognized to be exerted primarily though the modulation of ENS activity rather than through a direct input to effector cells in the gut, be they smooth muscle or epithelial secretory cells. Given the prevalence of autonomic dysfunction in a number of neurologic syndromes, as well as the existence of a number of primary and secondary disorders of autonomic function, disturbed autonomic modulation of gut motor function may be an important contributory factor to symptomatology in some scenarios.
It is now evident that the gut has important sensory functions. Although usually subconscious, gut sensation may be relayed to and perceived within the CNS. Sensory input is also fundamental to several reflex events in the gut, such as the viscerovisceral reflexes that coordinate function along the gut. The role of sensory dysfunction in the mediation of common symptoms, such as abdominal pain and nausea, in the patient with CNS disease with gastrointestinal manifestations has not been extensively investigated, however.
The Pathogenesis of Gastrointestinal Dysfunction in Neurologic Disease
Whereas a whole range of disease processes affecting central, peripheral, and autonomic nervous systems may affect gut motor function, the two predominant neurologic disorders encountered in gastrointestinal practice are cerebrovascular disease and parkinsonism.
Cerebrovascular Disease
Because of the aforementioned location of the swallowing center in the brain stem, it should come as no surprise that severe dysphagia can occur as a result of brainstem infarcts, especially if bilateral. Dysphagia following cortical strokes is a well-recognized and common complication, occurring in up to 50% of ischemic strokes, and its occurrence is associated with increased mortality and impaired functional outcome. Poststroke dysphagia is multifactorial and is largely attributable to oropharyngeal dysfunction. Abnormalities include incomplete lip closure, poor tongue movement, increased pooling, weak laryngeal elevation, and an increased frequency of airway penetration. Elegant imaging studies have provided considerable insights into the pathophysiology of dysphagia and its recovery in hemispheric strokes. These studies have emphasized the importance of cortical as well as brain stem control in the regulation of the swallowing mechanism. In humans, one hemisphere usually demonstrates dominance in the control of swallowing. At the cortical level, ischemic events that involve projections from the precentral gyrus to the internal capsule are most likely to be complicated by dysphagia. Brain stem strokes are especially likely to result in dysphagia, which may be slower to recover from and is more likely to result in aspiration. Given the role of the dorsal motor nucleus of the vagus in the medulla in the control of the smooth muscle esophagus and lower esophageal function, peristaltic dysfunction and gastroesophageal reflux would be expected in the stroke patient; however, there have been few studies of these aspects of esophageal function in the aftermath of acute stroke.
The good news is that, thanks to neuronal plasticity within the CNS, dysphagia resolves spontaneously in most cortical stroke patients within 2 weeks of the ischemic insult. Recovery seems to represent assumption by the unaffected hemisphere of the cortical control of swallowing rather than recovery of function in the damaged area; swallowing improvement may therefore occur independent of any recovery of limb function. This aspect of recovery is important and suggests that one should not be rushed into interventions in the interim but rather maintain close observation for risks for and the occurrence of aspiration.
Although little studied, there is indirect evidence to suggest that gastric emptying may be delayed following an acute stroke, which may have implications for feeding and drug administration. Stroke is also associated with constipation and anorectal dysfunction including incontinence, and instances of intestinal pseudo-obstruction have been reported. In contrast to swallowing function, gastric, small intestinal, colonic, and anorectal motor or sensory function have been little studied in the context of stroke, and therefore the pathophysiology of complications involving these parts of the gastrointestinal tract is less clearly understood. Whereas immobility may play some role, it is likely that cortical regulation of colonic and anorectal physiology is more important.
Parkinson Disease
Idiopathic Parkinson disease (PD) causes widespread and sometimes severe derangement of gastrointestinal motility. There are two basic contributors to gastrointestinal dysfunction in PD. First, striatal muscle dysfunction in the oropharynx, proximal esophagus, and anal canal is based on the same neurologic abnormalities that cause the cardinal manifestations of this disorder. The second component, dysfunction in the smooth muscle parts of the gastrointestinal tract, is less well understood but may reflect pathology in the autonomic and/or ENS systems. Indeed, neuropathologic changes reminiscent of CNS Parkinson features, such as dopamine depletion and the presence of Lewy neuritis, have been demonstrated in the myenteric and submucosal plexuses.
The pathogenesis of dysphagia in PD was studied in detail by Ali and colleagues in a detailed videofluoromanometric study of the swallowing process. The most prominent abnormalities, disturbed tongue movement and reduced amplitude of pharyngeal peristalsis, conspired to impair upper sphincter opening and thereby retard bolus passage into the esophagus. Cricopharyngeal bars and hypopharyngeal diverticula have also been described in PD. Involvement of the dorsal motor nucleus of the vagus and of central noradrenergic neurons, as well as the ENS of the esophagus, may contribute to the occurrence of esophageal dysphagia in PD.
With regard to the stomach, small intestine, and colon, similar hypotheses have been advanced to explain the high prevalence of symptoms related to these organs in PD, but in these regions the role of autonomic and ENS pathologic abnormalities looms large. The contribution of autonomic dysfunction is illustrated by the higher prevalence of gastrointestinal symptoms as well as postural instability among patients with a PD variant, multiple system atrophy, in which autonomic dysfunction is especially common. For some of these symptoms, such as nausea, the contribution of antiparkinsonian medications and dopaminergics in particular must be remembered.
Delayed gastric emptying has been well-documented in PD, and delayed emptying of solids has been linked in some studies to the severity of motor impairment. Here again, the impact of levodopa must be accounted for. The association between gastric emptying delay and the presence of levodopa response fluctuations, coupled with irregular patterns of drug absorption and the documentation of improved symptom control with intrajejunal or transdermal administration of antiparkinsonian drugs, underlines the potential clinical relevance of gastric emptying delay: by retarding drug delivery and absorption, gastroparesis could induce or further exacerbate response fluctuations. Several factors, however, limit the interpretation of reports of delayed gastric emptying and its association with upper gastrointestinal symptoms in PD. These factors include variations in patient population studied (eg, age, gender, disease severity, study location), the definition of gastroparesis, and the methodology used to assess gastric emptying rate (meal, test technique, study protocol, and manner of interpretation). Variations between studies in these parameters make it difficult to attempt real comparisons or draw firm conclusions. Nevertheless, delayed gastric emptying may occur in as many as 70% to 100% of PD patients attending specialist neurology clinics; the prevalence of symptomatic gastroparesis in PD, however, remains unknown. Indeed, there has been a lack of a consistent correlation between gastric emptying rate and upper gastrointestinal symptoms in PD. Although it is reasonable to assume that gastroparesis contributes to the weight loss that has been well-documented in PD, it is unclear whether nutrient delivery is affected by delayed gastric emptying, and a relationship between delayed gastric emptying and weight loss is yet to be demonstrated. Electrogastrography has also been used to study gastric motor activity in PD, but correlations with symptoms have been poor. Parenthetically, Helicobacter pylori and Helicobacter heilmannii infection have been implicated not only in contributing to gastrointestinal symptoms and weight loss in PD, but also in systemic proinflammatory cytokine activation and even in the pathogenesis of PD itself.
Orocecal and colonic transit times are significantly prolonged in patients with PD, and small intestinal bacterial overgrowth, presumed to be consequent to impaired small intestinal motility, has been documented.
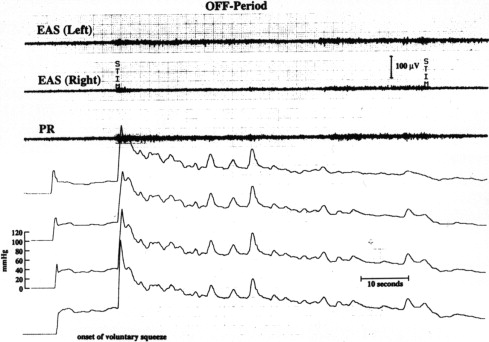
Constipation, a common and at times dominant symptom in PD, is probably multifactorial, with delayed colonic transit, anorectal dysfunction, drug therapy, and reduced physical activity all contributing to the problem. Difficulty with the act of defecation may be an especially distressing symptom for affected patients; this symptom seems to be associated with PD severity, and its pathophysiology is based on the involvement of the anal sphincter and pelvic floor musculature by the PD process ( Fig. 1 ). Accordingly, responses have been documented for apomorphine injection. Megacolon, at times requiring surgical intervention and even resulting in perforation and fatal outcome, has been well-documented in PD ( Fig. 2 ).
Motor Neuron Disease (Amyotrophic Lateral Sclerosis)
Motor neuron disease (amyotrophic lateral sclerosis, or ALS) features the progressive degeneration of motor neurons in the brain, brainstem, and spinal cord. When, as often happens, the cranial nerve nuclei become involved, swallowing is disrupted and difficulties ensue, and severe and potentially fatal aspiration-related events can occur. Unfortunately, the natural history is typically one of inexorable progression, and issues regarding alternative routes of feeding and fluid administration inevitably arise. Colonic function is also affected, and delayed transit in the right and left colon have been described ; it has been suggested that involvement of the autonomic nervous system might explain these findings. Fortunately, the pelvic floor muscles and Onuf nucleus motor neurons are relatively spared in ALS and, although involvement of the external anal sphincter has been documented by some but not others, fecal incontinence is infrequent.
Multiple Sclerosis
Among patients with long-standing (>10 years’ duration) multiple sclerosis (MS) in Finland, mortality rates from pneumonia and gastrointestinal disorders were four-fold more common than in the general population.
Dysphagia, previously thought to be rare, has in more recent studies been reported in up to 30% to 40% of MS patients and, although all phases of swallowing can be impaired, oropharyngeal dysphagia is the most common manifestation, and abnormalities in pharyngeal and cricopharyngeal function have been described. Dysphagia prevalence correlates with disease duration and extent ; it is reasonable to assume that aspiration may be a significant contributor to the previously mentioned excess deaths from pneumonia. When studied, dysphagia has, not surprisingly, been linked to evidence of brainstem involvement.
Delayed gastric emptying, isolated cases of gastroparesis, and even one instance of gastric perforation have been reported in MS, and their cause has been attributed to autonomic dysfunction of central origin. In one small series, parallel improvements in neurologic and gastric emptying function were documented in response to corticosteroid therapy.
In patients with MS, constipation is reported among 29% to 43% and fecal incontinence in over 50%, and these conditions exert a deleterious impact on quality of life. Bowel involvement is less common than bladder involvement and its pathophysiology less well-understood. Incontinence in MS has been attributed to abnormal rectosigmoid compliance and rectoanal reflexes, reduced rectal sensation, and loss of voluntary control of the external anal sphincter musculature, and constipation has been attributed to slow colon transit and paradoxical contraction of the puborectalis muscle.
Autonomic and Peripheral Neuropathies
Given its ever-increasing worldwide prevalence, diabetic autonomic neuropathy is by far the most common autonomic neuropathy encountered in clinical practice. However, involvement of other pathologic processes, especially those involving the ENS and the interstitial cells of Cajal, must be noted. Gastrointestinal involvement in diabetes can affect any or all parts of the gastrointestinal tract with delayed esophageal transit, gastroparesis, intestinal pseudo-obstruction, constipation, and incontinence being well-documented, and delayed gastric emptying reported in 30% to 50% of outpatients with longstanding type 1 or type 2 diabetes. Hyperglycemia delays gastric emptying in normal subjects and in diabetes; for patients with diabetes, this phenomenon must be accounted for in the performance and interpretation of gastric emptying studies and considered in patient management.
Gastrointestinal involvement occurs to a variable extent in the many types of autonomic peripheral neuropathies. Upper gastrointestinal symptoms (early satiety, nausea, and vomiting) as well as constipation are common in autoimmune autonomic neuropathies. Paralytic ileus is a common feature of the rare acute autonomic and sensory neuropathy.
Bulbar and oculomotor nerves are less commonly affected in the most common form of Guillain-Barré syndrome (GBS) seen in Europe and North America (approximately 85% of cases), which is the sensory-motor form referred to as acute inflammatory demyelinating polyradiculoneuropathy. However, bulbar and oculomotor nerve involvement is a common feature of other, less common, subtypes such as Miller Fisher syndrome. Bulbar involvement can lead to oropharyngeal dysphagia and respiratory difficulties. Autonomic dysfunction is commonly detectable in GBS but is usually of minor clinical importance. Delayed gastric emptying, gastroparesis, constipation, diarrhea, and fecal incontinence have all been described in GBS. The gastrointestinal tract has a role in the pathogenesis of GBS; 15% to 40% of cases of GBS in the west have followed infection with Campylobacter jejuni .
Muscle Disease
Myopathy and muscular dystrophy
Nasal aspiration is a prominent feature of myopathic involvement of the oropharyngeal musculature. Weakness of the tongue and pharyngeal and laryngeal musculature may result in ineffective bolus transfer, retention of components of the bolus in the valleculae or hypopharynx, and/or aspiration. Whereas a large number of myopathic disorders may involve the swallowing mechanism, oropharyngeal dysphagia is a characteristic feature of oculopharyngeal dystrophy. This form of muscular dystrophy features an autosomal dominant inheritance and presents with ptosis, oropharyngeal dysphagia, and proximal limb weakness. Whereas gastrointestinal involvement has been described in a number of muscular dystrophies, it has been most extensively documented in myotonic dystrophy and Duchenne muscular dystrophy. Gastrointestinal symptoms in myotonic dystrophy include dysphagia, aspiration, early satiety, nausea, vomiting, epigastric pain, constipation, difficult defecation, abdominal pain, pseudo-obstruction, and fecal incontinence. Of these, abdominal pain, dysphagia, vomiting, diarrhea, coughing while eating, and fecal incontinence were the most common in one survey ; up to 25% of patients consider gastrointestinal involvement as the most disabling feature of their disease. Functional correlates include impaired upper and lower esophageal sphincter pressures, esophageal peristalsis, and even aperistalsis leading to esophageal dilation ; delayed gastric emptying and gastroparesis ; abnormal small intestinal motility leading to pseudo-obstruction ; and impaired anal sphincter function. Gastric volvulus and megacolon have been described. In Duchenne dystrophy, esophageal symptoms such as dysphagia and aspiration are not common, although esophageal manometric abnormalities have been described. In contrast, gastric motor dysfunction is an early feature, resulting in hypomotility and gastroparesis, which can be profound and result in acute gastroparesis and gastric dilatation. Although when measured, orocecal transit time was found to be normal, instances of intestinal pseudo-obstruction have been reported.
Myasthenia gravis
Because the muscles controlled by the cranial nerves are commonly involved in myasthenia, dysphagia is prevalent. Severity of dysphagia is variable, ranging from fatigable swallowing difficulty evident only after a considerable duration of effort to constant difficulty, a poor prognostic sign. Two cases of intestinal obstruction have been described as a paraneoplastic manifestation of thymomas associated with myasthenia.
Alzheimer disease and other dementias
Pilot data indicate that swallowing duration is significantly delayed in Alzheimer disease, the delay being located principally at the pharyngeal level. There are no published data on the influence of Alzheimer disease and other dementias on gastric or small intestinal motility. Constipation is a well-described and common problem among patients with dementia. Despite this fact, there are no published studies of gastrointestinal motility in dementia. One large-scale retrospective survey of the medical records of 4 million patients discharged from United States veterans hospitals has shown that Alzheimer disease was associated with significantly increased risk of constipation, megacolon, volvulus, and intestinal impaction compared with patients without neurologic or psychiatric disease.
The Pathogenesis of Gastrointestinal Dysfunction in Neurologic Disease
Whereas a whole range of disease processes affecting central, peripheral, and autonomic nervous systems may affect gut motor function, the two predominant neurologic disorders encountered in gastrointestinal practice are cerebrovascular disease and parkinsonism.
Cerebrovascular Disease
Because of the aforementioned location of the swallowing center in the brain stem, it should come as no surprise that severe dysphagia can occur as a result of brainstem infarcts, especially if bilateral. Dysphagia following cortical strokes is a well-recognized and common complication, occurring in up to 50% of ischemic strokes, and its occurrence is associated with increased mortality and impaired functional outcome. Poststroke dysphagia is multifactorial and is largely attributable to oropharyngeal dysfunction. Abnormalities include incomplete lip closure, poor tongue movement, increased pooling, weak laryngeal elevation, and an increased frequency of airway penetration. Elegant imaging studies have provided considerable insights into the pathophysiology of dysphagia and its recovery in hemispheric strokes. These studies have emphasized the importance of cortical as well as brain stem control in the regulation of the swallowing mechanism. In humans, one hemisphere usually demonstrates dominance in the control of swallowing. At the cortical level, ischemic events that involve projections from the precentral gyrus to the internal capsule are most likely to be complicated by dysphagia. Brain stem strokes are especially likely to result in dysphagia, which may be slower to recover from and is more likely to result in aspiration. Given the role of the dorsal motor nucleus of the vagus in the medulla in the control of the smooth muscle esophagus and lower esophageal function, peristaltic dysfunction and gastroesophageal reflux would be expected in the stroke patient; however, there have been few studies of these aspects of esophageal function in the aftermath of acute stroke.
The good news is that, thanks to neuronal plasticity within the CNS, dysphagia resolves spontaneously in most cortical stroke patients within 2 weeks of the ischemic insult. Recovery seems to represent assumption by the unaffected hemisphere of the cortical control of swallowing rather than recovery of function in the damaged area; swallowing improvement may therefore occur independent of any recovery of limb function. This aspect of recovery is important and suggests that one should not be rushed into interventions in the interim but rather maintain close observation for risks for and the occurrence of aspiration.
Although little studied, there is indirect evidence to suggest that gastric emptying may be delayed following an acute stroke, which may have implications for feeding and drug administration. Stroke is also associated with constipation and anorectal dysfunction including incontinence, and instances of intestinal pseudo-obstruction have been reported. In contrast to swallowing function, gastric, small intestinal, colonic, and anorectal motor or sensory function have been little studied in the context of stroke, and therefore the pathophysiology of complications involving these parts of the gastrointestinal tract is less clearly understood. Whereas immobility may play some role, it is likely that cortical regulation of colonic and anorectal physiology is more important.
Parkinson Disease
Idiopathic Parkinson disease (PD) causes widespread and sometimes severe derangement of gastrointestinal motility. There are two basic contributors to gastrointestinal dysfunction in PD. First, striatal muscle dysfunction in the oropharynx, proximal esophagus, and anal canal is based on the same neurologic abnormalities that cause the cardinal manifestations of this disorder. The second component, dysfunction in the smooth muscle parts of the gastrointestinal tract, is less well understood but may reflect pathology in the autonomic and/or ENS systems. Indeed, neuropathologic changes reminiscent of CNS Parkinson features, such as dopamine depletion and the presence of Lewy neuritis, have been demonstrated in the myenteric and submucosal plexuses.
The pathogenesis of dysphagia in PD was studied in detail by Ali and colleagues in a detailed videofluoromanometric study of the swallowing process. The most prominent abnormalities, disturbed tongue movement and reduced amplitude of pharyngeal peristalsis, conspired to impair upper sphincter opening and thereby retard bolus passage into the esophagus. Cricopharyngeal bars and hypopharyngeal diverticula have also been described in PD. Involvement of the dorsal motor nucleus of the vagus and of central noradrenergic neurons, as well as the ENS of the esophagus, may contribute to the occurrence of esophageal dysphagia in PD.
With regard to the stomach, small intestine, and colon, similar hypotheses have been advanced to explain the high prevalence of symptoms related to these organs in PD, but in these regions the role of autonomic and ENS pathologic abnormalities looms large. The contribution of autonomic dysfunction is illustrated by the higher prevalence of gastrointestinal symptoms as well as postural instability among patients with a PD variant, multiple system atrophy, in which autonomic dysfunction is especially common. For some of these symptoms, such as nausea, the contribution of antiparkinsonian medications and dopaminergics in particular must be remembered.
Delayed gastric emptying has been well-documented in PD, and delayed emptying of solids has been linked in some studies to the severity of motor impairment. Here again, the impact of levodopa must be accounted for. The association between gastric emptying delay and the presence of levodopa response fluctuations, coupled with irregular patterns of drug absorption and the documentation of improved symptom control with intrajejunal or transdermal administration of antiparkinsonian drugs, underlines the potential clinical relevance of gastric emptying delay: by retarding drug delivery and absorption, gastroparesis could induce or further exacerbate response fluctuations. Several factors, however, limit the interpretation of reports of delayed gastric emptying and its association with upper gastrointestinal symptoms in PD. These factors include variations in patient population studied (eg, age, gender, disease severity, study location), the definition of gastroparesis, and the methodology used to assess gastric emptying rate (meal, test technique, study protocol, and manner of interpretation). Variations between studies in these parameters make it difficult to attempt real comparisons or draw firm conclusions. Nevertheless, delayed gastric emptying may occur in as many as 70% to 100% of PD patients attending specialist neurology clinics; the prevalence of symptomatic gastroparesis in PD, however, remains unknown. Indeed, there has been a lack of a consistent correlation between gastric emptying rate and upper gastrointestinal symptoms in PD. Although it is reasonable to assume that gastroparesis contributes to the weight loss that has been well-documented in PD, it is unclear whether nutrient delivery is affected by delayed gastric emptying, and a relationship between delayed gastric emptying and weight loss is yet to be demonstrated. Electrogastrography has also been used to study gastric motor activity in PD, but correlations with symptoms have been poor. Parenthetically, Helicobacter pylori and Helicobacter heilmannii infection have been implicated not only in contributing to gastrointestinal symptoms and weight loss in PD, but also in systemic proinflammatory cytokine activation and even in the pathogenesis of PD itself.
Orocecal and colonic transit times are significantly prolonged in patients with PD, and small intestinal bacterial overgrowth, presumed to be consequent to impaired small intestinal motility, has been documented.
Constipation, a common and at times dominant symptom in PD, is probably multifactorial, with delayed colonic transit, anorectal dysfunction, drug therapy, and reduced physical activity all contributing to the problem. Difficulty with the act of defecation may be an especially distressing symptom for affected patients; this symptom seems to be associated with PD severity, and its pathophysiology is based on the involvement of the anal sphincter and pelvic floor musculature by the PD process ( Fig. 1 ). Accordingly, responses have been documented for apomorphine injection. Megacolon, at times requiring surgical intervention and even resulting in perforation and fatal outcome, has been well-documented in PD ( Fig. 2 ).

Motor Neuron Disease (Amyotrophic Lateral Sclerosis)
Motor neuron disease (amyotrophic lateral sclerosis, or ALS) features the progressive degeneration of motor neurons in the brain, brainstem, and spinal cord. When, as often happens, the cranial nerve nuclei become involved, swallowing is disrupted and difficulties ensue, and severe and potentially fatal aspiration-related events can occur. Unfortunately, the natural history is typically one of inexorable progression, and issues regarding alternative routes of feeding and fluid administration inevitably arise. Colonic function is also affected, and delayed transit in the right and left colon have been described ; it has been suggested that involvement of the autonomic nervous system might explain these findings. Fortunately, the pelvic floor muscles and Onuf nucleus motor neurons are relatively spared in ALS and, although involvement of the external anal sphincter has been documented by some but not others, fecal incontinence is infrequent.
Multiple Sclerosis
Among patients with long-standing (>10 years’ duration) multiple sclerosis (MS) in Finland, mortality rates from pneumonia and gastrointestinal disorders were four-fold more common than in the general population.
Dysphagia, previously thought to be rare, has in more recent studies been reported in up to 30% to 40% of MS patients and, although all phases of swallowing can be impaired, oropharyngeal dysphagia is the most common manifestation, and abnormalities in pharyngeal and cricopharyngeal function have been described. Dysphagia prevalence correlates with disease duration and extent ; it is reasonable to assume that aspiration may be a significant contributor to the previously mentioned excess deaths from pneumonia. When studied, dysphagia has, not surprisingly, been linked to evidence of brainstem involvement.
Delayed gastric emptying, isolated cases of gastroparesis, and even one instance of gastric perforation have been reported in MS, and their cause has been attributed to autonomic dysfunction of central origin. In one small series, parallel improvements in neurologic and gastric emptying function were documented in response to corticosteroid therapy.
In patients with MS, constipation is reported among 29% to 43% and fecal incontinence in over 50%, and these conditions exert a deleterious impact on quality of life. Bowel involvement is less common than bladder involvement and its pathophysiology less well-understood. Incontinence in MS has been attributed to abnormal rectosigmoid compliance and rectoanal reflexes, reduced rectal sensation, and loss of voluntary control of the external anal sphincter musculature, and constipation has been attributed to slow colon transit and paradoxical contraction of the puborectalis muscle.
Autonomic and Peripheral Neuropathies
Given its ever-increasing worldwide prevalence, diabetic autonomic neuropathy is by far the most common autonomic neuropathy encountered in clinical practice. However, involvement of other pathologic processes, especially those involving the ENS and the interstitial cells of Cajal, must be noted. Gastrointestinal involvement in diabetes can affect any or all parts of the gastrointestinal tract with delayed esophageal transit, gastroparesis, intestinal pseudo-obstruction, constipation, and incontinence being well-documented, and delayed gastric emptying reported in 30% to 50% of outpatients with longstanding type 1 or type 2 diabetes. Hyperglycemia delays gastric emptying in normal subjects and in diabetes; for patients with diabetes, this phenomenon must be accounted for in the performance and interpretation of gastric emptying studies and considered in patient management.
Gastrointestinal involvement occurs to a variable extent in the many types of autonomic peripheral neuropathies. Upper gastrointestinal symptoms (early satiety, nausea, and vomiting) as well as constipation are common in autoimmune autonomic neuropathies. Paralytic ileus is a common feature of the rare acute autonomic and sensory neuropathy.
Bulbar and oculomotor nerves are less commonly affected in the most common form of Guillain-Barré syndrome (GBS) seen in Europe and North America (approximately 85% of cases), which is the sensory-motor form referred to as acute inflammatory demyelinating polyradiculoneuropathy. However, bulbar and oculomotor nerve involvement is a common feature of other, less common, subtypes such as Miller Fisher syndrome. Bulbar involvement can lead to oropharyngeal dysphagia and respiratory difficulties. Autonomic dysfunction is commonly detectable in GBS but is usually of minor clinical importance. Delayed gastric emptying, gastroparesis, constipation, diarrhea, and fecal incontinence have all been described in GBS. The gastrointestinal tract has a role in the pathogenesis of GBS; 15% to 40% of cases of GBS in the west have followed infection with Campylobacter jejuni .
Muscle Disease
Myopathy and muscular dystrophy
Nasal aspiration is a prominent feature of myopathic involvement of the oropharyngeal musculature. Weakness of the tongue and pharyngeal and laryngeal musculature may result in ineffective bolus transfer, retention of components of the bolus in the valleculae or hypopharynx, and/or aspiration. Whereas a large number of myopathic disorders may involve the swallowing mechanism, oropharyngeal dysphagia is a characteristic feature of oculopharyngeal dystrophy. This form of muscular dystrophy features an autosomal dominant inheritance and presents with ptosis, oropharyngeal dysphagia, and proximal limb weakness. Whereas gastrointestinal involvement has been described in a number of muscular dystrophies, it has been most extensively documented in myotonic dystrophy and Duchenne muscular dystrophy. Gastrointestinal symptoms in myotonic dystrophy include dysphagia, aspiration, early satiety, nausea, vomiting, epigastric pain, constipation, difficult defecation, abdominal pain, pseudo-obstruction, and fecal incontinence. Of these, abdominal pain, dysphagia, vomiting, diarrhea, coughing while eating, and fecal incontinence were the most common in one survey ; up to 25% of patients consider gastrointestinal involvement as the most disabling feature of their disease. Functional correlates include impaired upper and lower esophageal sphincter pressures, esophageal peristalsis, and even aperistalsis leading to esophageal dilation ; delayed gastric emptying and gastroparesis ; abnormal small intestinal motility leading to pseudo-obstruction ; and impaired anal sphincter function. Gastric volvulus and megacolon have been described. In Duchenne dystrophy, esophageal symptoms such as dysphagia and aspiration are not common, although esophageal manometric abnormalities have been described. In contrast, gastric motor dysfunction is an early feature, resulting in hypomotility and gastroparesis, which can be profound and result in acute gastroparesis and gastric dilatation. Although when measured, orocecal transit time was found to be normal, instances of intestinal pseudo-obstruction have been reported.
Myasthenia gravis
Because the muscles controlled by the cranial nerves are commonly involved in myasthenia, dysphagia is prevalent. Severity of dysphagia is variable, ranging from fatigable swallowing difficulty evident only after a considerable duration of effort to constant difficulty, a poor prognostic sign. Two cases of intestinal obstruction have been described as a paraneoplastic manifestation of thymomas associated with myasthenia.
Alzheimer disease and other dementias
Pilot data indicate that swallowing duration is significantly delayed in Alzheimer disease, the delay being located principally at the pharyngeal level. There are no published data on the influence of Alzheimer disease and other dementias on gastric or small intestinal motility. Constipation is a well-described and common problem among patients with dementia. Despite this fact, there are no published studies of gastrointestinal motility in dementia. One large-scale retrospective survey of the medical records of 4 million patients discharged from United States veterans hospitals has shown that Alzheimer disease was associated with significantly increased risk of constipation, megacolon, volvulus, and intestinal impaction compared with patients without neurologic or psychiatric disease.
Issues in Clinical Practice
Assessment
Oropharyngeal dysphagia
The evaluation of oropharyngeal dysphagia is firmly based on two modalities: the bedside clinical assessment and dynamic imaging of the swallowing mechanism, both preferably performed in collaboration with a speech and language therapist with expertise in the assessment and management of dysphagia. The imaging modality most commonly used and most extensively documented in the literature is videofluoroscopy, which, when performed by an experienced clinician, can evaluate the various components of the oropharyngeal phase of the swallow, define dysfunction, and predict therapeutic response. For example, the observation of impaired laryngeal closure predicts tracheal, and soft palate dysfunction, nasopharyngeal aspiration. Impaired UES opening leads to dysphagia, postswallow aspiration, and, in the case of cricopharyngeal bars, diverticulum formation. Tongue dysfunction with attendant impaired bolus propulsion leads to a sluggish, misdirected bolus. Finally, an impaired pharyngeal contraction results in postswallow residue in the valleculae and pyriform sinuses with the risk of postswallow aspiration. A key issue for the clinician is the detection of aspiration and/or the determination of risk for aspiration. Unfortunately, such traditional clinical signs as the gag reflex have not stood the test of time. Even when performed by a skilled evaluator and according to a standard and detailed protocol, the bedside assessment of swallowing has its limitations. In one prospective study among patients with acute stroke, sensitivity was only 47%, whereas specificity was 86% for the presence of aspiration. Among the various symptoms and signs evaluated, a weak voluntary cough and the presence of any alteration in conscious level were the most predictive, the combination having a sensitivity of 75% and specificity of 91%. Furthermore, a prior study from the same group found that the routine use of videofluoroscopy added little to the bedside assessment in the detection of aspiration. Indeed, it has been pointed out repeatedly that videofluoroscopy was never designed as a test for aspiration. The bottom line: current commonly used methods for the prediction of aspiration have their limitations and should be interpreted with caution. It is evident, however, that clinical and radiologic evaluation by a multidisciplinary team continues to be the best approach.
The esophageal phase of swallowing is primarily a function of the smooth muscle esophagus and is regulated centrally via vagal afferents and peripherally by the intrinsic properties of esophageal smooth muscle. The assessment of the esophageal phase of swallowing is dealt with in detail by Dr Kahrilas elsewhere in this issue.
Gastroparesis
Modalities used to diagnose gastroparesis include scintigraphy (still the gold standard) where the time taken to empty a solid radiolabeled test meal is measured. Optimum results are obtained if scintigraphy is performed according to a standardized protocol and scanning is extended to at least 4 hours postprandially. Regional gastric emptying can be used to assess fundic and antral function. Dual-labeled scintigraphy can offer insights into the differential handling by the stomach of liquids and solids. Based on its ability to identify transit into the duodenum by a sudden and profound change in pH, the wireless motility capsule is able to estimate the rate of gastric emptying and provide estimates of gastric and colonic motor function in the absence of radiation exposure, although availability remains limited and the costprohibitive for many. Other modalities under evaluation for use in the diagnosis and research of gastroparesis include the octanoic acid breath test, functional magnetic resonance imaging (MRI), and both 2-dimensional and 3-dimensional ultrasonography.
Chronic intestinal pseudo-obstruction
Although chronic intestinal pseudo-obstruction (CIP) may involve any part of the gastrointestinal tract and may result in symptoms related to that organ (eg, gastroesophageal reflux disease, dysphagia, achalasia, gastroparesis, constipation, megacolon), symptoms referable to small intestinal obstruction classically dominate the clinical picture. The typical history of the patient with CIP is repeated admissions with symptoms, signs, and radiologic evidence of “obstruction” with no convincing cause for obstruction being found. Unfortunately, these patients have usually been subjected to a number of fruitless laparotomies before the diagnosis is even entertained. Attention to the clinical context should prompt suspicion of CIP. CIP has been described in association with diabetic neuropathy, myotonic dystrophy, dermatomyositis, and amyloidosis, and iatrogenic cases have complicated therapy with antiparkinsonian medications, phenothiazines, and tricyclic antidepressants. Details of the clinical features, diagnostic approach to, and management of CIP are provided by Dr De Giorgio and colleagues elsewhere in this issue.
Colonic inertia and megacolon
Colonic motor dysfunction is common in neurologic disorders and may manifest as slow transit constipation or acute or chronic megacolon.
Acute megacolon
Acute colonic pseudo-obstruction, or Ogilvie syndrome, is defined as an acute dilatation of the colon without evidence of mechanical obstruction distal to the dilated segment. Progressive abdominal distention is the clinical hallmark of this condition. Lower abdominal pain is present in 60% to 80% of patients, and nausea and vomiting is present in 50%. It is important to realize that although the vast majority of patients with Ogilvie syndrome are completely constipated, megacolon can develop in individuals who continue to pass both stool and flatus. Only 40% of patients will have hypoactive or absent bowel sounds. The overall risk of perforation in Ogilvie syndrome is low, in the region of 3%, but mortality following perforation in this context may be as high as 50%. Cecal diameter is valuable in predicting risk of perforation; a diameter in excess of 9 cm is abnormal, and when greater than 12 cm indicates a significant risk of perforation.
Chronic megacolon
Chronic megacolon has been described in PD, MS, motor neuron disease, Alzheimer disease, and autonomic neuropathies and in relation to spinal cord injury, among others. In many instances chronic megacolon may be asymptomatic and discovered only on clinical or radiologic examination; depending on the level of consciousness and cognitive function, the patient may be aware of abdominal distension and discomfort. Although the risk of perforation with chronic megacolon is apparently low, instances have been reported in association with neurologic disease, and fatal outcomes have occurred.
Constipation
Two related aspects of colonic function are amenable to clinical testing: transit and contractile activity. Of these two, colon transit is by far the most widely used, and its popularity owes much to the validation of a simple test of transit: the radioopaque marker study. Using ingested radioopaque markers and following their movement through the colon by timed abdominal radiographs, an accurate and reproducible assessment of overall colonic transit can be obtained. More accurate and dynamic assessments of colon transit, including the determination of transit within segments of various segments of the colon, can be obtained from radioisotopic approaches, although these methodologies have been largely confined to a few centers and to clinical research protocols. Smart Pill technology has also been used to measure colon transit and in an initial study in constipation, a good correlation was noted between transit measurements obtained by this technique and the traditional radioopaque marker method.
In contrast to these relatively simple techniques, colonic manometry presents formidable challenges, foremost being positioning the catheter assembly in the first place and ensuring that it retains its position throughout the period of study. Furthermore, patterns of colonic motility are poorly defined and subject to tremendous variation between normal individuals, not to mention in disease states. The American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of colonic motility concluded that “in children with chronic constipation, colonic phasic pressure measurements can identify patterns suggestive of neuropathy and predict success of antegrade enemas via cecostomy.” The society’s stand regarding colonic manometry in adults was more circumspect; it stated that manometry “may be used to document severe motor dysfunction (colonic inertia) prior to colectomy.” There are no data on the utility of this approach in the patient with neurologic disease.
Anorectal sphincter and pelvic floor dysfunction
Two clinical problems that may coexist may be addressed in the assessment of anorectal and pelvic floor muscle function: fecal incontinence and difficult defecation (anismus, pelvic floor dyssynergia). Digital rectal examination, performed by a skilled clinician, has been shown to be of value and, in the appropriate clinical context, endoscopic and/or radiologic studies may be required to uncover other abnormalities such as inflammatory bowel disease or a rectal neoplasm. In the evaluation of the patient with fecal incontinence, both endoanal ultrasound and endoanal MRI are widely used to define anatomic (usually obstetric or postsurgical) defects in the internal and external anal sphincters, with ultrasound being the preferred modality for the former and MRI for the latter. Static images of the anorectal angle can be obtained during defecography (whether performed using fluoroscopy or MRI), which can also follow the transit of feces (or, more usually, a simulated stool) through the rectum and anal canal using standard contrast imaging, scintigraphy, or MRI. The first two modalities involve radiation exposure and the use of a customized “throne” on which the patient sits and performs various maneuvers following the insertion of a material to simulate the consistency of feces into the rectum. In this manner the behavior of the pelvic floor musculature can be recorded as the patient attempts to retain or expel stool. MRI offers many advantages over barium defecography but for a truly physiologic test requires a dedicated “open” system, a facility that is available at only a few highly specialized centers. Anorectal manometry has been used for decades to assess the integrity of the internal and external sphincters and is a well-established technique for the identification of Hirschsprung disease and the definition of poor sphincter tone in patients with incontinence. In the latter context, the clinician can go on to use manometry as the basis for biofeedback approaches to improving sphincter function. A variety of manometric assemblies have been used: multiple balloon, perfused catheter, solid-state, and high-resolution. In the most commonly used approach, a perfused catheter assembly incorporating multiple radially arrayed (to allow for sphincter asymmetry) closely-spaced side holes (typically 0.5 to 1 cm apart) with an inflatable balloon at its tip is placed in the rectum and anal canal so that the sensors straddle the sphincter, previously identified by a series of slow pull-through maneuvers. Most recently the technique of high-resolution manometry, now widely used in studies of the esophagus, has been used in the anorectum and has been shown to correlate well with conventional water perfused manometry yet provide greater anatomic detail.
Management
Oropharyngeal dysphagia
In cases of less severe impairment in which aspiration is not an issue and the patient is willing and able to cooperate, a number of maneuvers combined with dietary changes can be taught to compensate for impaired laryngeal (chin tuck and “supraglottic swallow”) or nasopharyngeal closure, UES opening (Mendelsohn maneuver, which helps to raise the larynx and facilitate UES opening), and impaired pharyngeal clearance.
There have been isolated reports of cricopharyngeal myotomy and botulinum toxin injection into the cricopharyngeus in patients with PD and MS, respectively, who seemed to have oropharyngeal dysphagia related to holdup at the cricopharyngues muscle. Given the predilection of such patients to aspiration, these are not approaches to embarked upon lightly.
As for the assessment of oropharyngeal dysphagia in the neurologic patient, management must be guided by a team approach, being mindful of the severity of the dysphagia, nutritional status of the patient, nature of the underlying illness, general health status, and above all of the wishes and well-being of the patient.
To PEG or not to PEG
Despite initial enthusiasm for the insertion of percutaneous endoscopic gastrostomy (PEG) tubes in patients with Alzheimer disease and other dementias, it is now clear that PEG does not prolong life or relieve suffering for these patients but is accompanied by significant risk, including local infection and aspiration. Less than 40% of older patients in whom a PEG tube is placed survive beyond 1 year after the procedure, and survival was even lower among those with malignancy or advanced age. Furthermore, the likelihood of aspiration may be enhanced and not, as commonly believed, prevented by PEG feeding. Nevertheless, gastroenterologists continue to come under pressure from families and nursing homes. (In some instances these patients will not be accepted without a PEG tube in situ.) It is also evident that the caloric requirements of these patients may have been overestimated and that oral feeding, despite its difficulty, may not only be adequate but more appropriate in many instances. It should come as no surprise, therefore, that a recent Cochrane systematic review concluded that there was “insufficient evidence to suggest that enteral tube feeding (including by PEG) was beneficial in patients with advanced dementia.”
As already mentioned, dysphagia is common in acute stroke but is often transient and, if persistent, a predictor of a poor prognosis in terms of survival and neurologic recovery. Here again, the role of PEG feeding has undergone a significant reappraisal with the publication of the FOOD trial, which showed that early PEG feeding (as compared with nasogastric tube feeding) was associated with an increased risk of death or poor outcome.
In considering PEG placement in patients with oropharyngeal dysphagia related to chronic neurologic diseases such as MS or ALS and where dysphagia is of such severity as to make oral intake impossible and/or where aspiration is a real threat, a number of factors must be considered. If aspiration is deemed significant and especially if recovery is unlikely or progression inevitable, alternative routes of nutrition must be considered if ethically justified. It must be emphasized that PEG placement offers no significant protection against aspiration pneumonia ; indeed, aspiration pneumonia is the most common cause of death in PEG-fed patients. A number of factors contribute including the aspiration of colonized oral secretions and/or gastric contents. The latter will, of course, be aggravated by the presence of gastroparesis. If aspiration is not a significant risk but alternative routes of nutrition are required, PEG placement should be considered, bearing in mind the very particular clinical context that presents itself to the gastroenterologist. A systematic review by the American Academy of Neurology concluded that although enteral feeding via a PEG tube “probably” prolonged survival in ALS, there was no evidence regarding PEG impact on quality of life, and there were insufficient data to support or refute specific timing of PEG insertion. The organization did suggest that an ALS patient would be exposed to less risk if a PEG tube was placed while forced vital capacity was above 50% of predicted. Gastrostomy tubes may also be placed radiologically (radiologically guided gastrostomy; RIG) A comparative study among ALS patients could detect no differences in terms of complications or survival. RIG may be more feasible and safe than PEG among ALS patients with significant ventilatory compromise, however.
Gastroparesis
In the patient with symptomatic gastroparesis, dehydration and electrolyte abnormalities should be corrected by oral or intravenous routes, as appropriate. Gastric decompression by nasogastric suction remains an important component of management in the acute stage. Malnutrition develops in many patients who have chronic established gastroparesis as a result of inadequate oral intake and vomiting. Therefore, attention to diet and nutrition remains of paramount importance. Low roughage low-fat diets are recommended. When oral intake fails, jejunostomy feeding may be considered. Percutaneous or, preferably, surgical placement of a combined gastrostomy-jejunostomy tube simultaneously decompresses the stomach and permits enteral nutrition.
Gastroparesis may be complicated by the development of phytobezoars. When symptomatic, these may be approached endoscopically or surgically, but enzyme dissolution and Coca-Cola lavage are less invasive alternatives. By infusing Coca-Cola in a volume of 3 L over 12 hours, in one study complete resolution of all 5 cases of bezoar was recorded.
Although the real clinical impact of chronic hyperglycemia on motility is unclear, it seems reasonable to advise that diabetic control should be optimized in patients with diabetic gastroenteropathy. In diabetes, interactions between blood sugar level and gastric emptying rate are critical to nutrient delivery, and thus to postprandial glycemic control.
Few effective pharmacologic agents remain available to the clinician for the management of gastroparesis. Although many new agents have been evaluated, few have proven either sufficiently effective or adequately safe to merit approval for human use. Progress in this area has also been hampered by our poor understanding of the basic pathophysiology of these disorders.
In terms of pharmacologic therapy, metoclopramide or domperidone (where available), and erythromycin have the best evidence for efficacy in gastroparesis. Whereas erythromycin should probably be reserved for intravenous use in the acutely ill patient, metoclopramide remains the sole option for long-term oral therapy in the United States. This agent also has significant side effect issues, of which the prescribing clinician must be fully apprised.
Nausea may be a significant problem for many of these patients and may respond to concomitant administration of an antiemetic. In these circumstances, an antiemetic such as a phenothiazine derivative or a 5-HT 3 antagonist should be used and can often be used successfully as circumstances may require. In choosing a particular antiemetic, attention should be paid to the appropriateness of available formulations, duration of action, and cost.
Given the paucity of pharmacologic options, other approaches have been explored for the patient with intractable and disabling gastroparesis. The perendoscopic injection of botulinum toxin into the pyloric muscle ring has been shown to be effective, albeit in the short term, in the symptomatic relief of gastroparesis, in accelerating gastric emptying, and in suppressing isolated pyloric pressure waves.
In 1997, gastric stimulation was proposed as an alternative for individuals with intractable gastroparesis. Although results in uncontrolled trials have been promising in terms of symptom relief, nutritional status, diabetic control, and pancreatic function, the results of the only double-blind controlled trial to date were less impressive. Other studies have shown little impact on long-term outcome, and this procedure is not without complications. The precise mode of action of gastric stimulation remains uncertain and may be independent of an acceleration of gastric emptying.
In a small number of patients, usually those with long-standing, complicated type 1 diabetes, severely symptomatic and apparently intractable gastroparesis develops. Near-total gastrectomy with Roux-en-Y anastomosis has been performed for such patients, and good results have been reported, albeit in small series. Furthermore, although results in terms of gastric function may be good, many of these patients go on to develop renal failure and other complications of diabetes.
For the patient with PD and gastric emptying delay, it is critical to avoid metoclopramide in view of its central antidopaminergic effects; domperidone and mosapride, where available, may provide some potential therapeutic solutions. The main aim of pharmacologic therapy in correcting delayed gastric emptying in PD is primarily to reduce response fluctuations rather than deal with upper gastrointestinal symptoms. If pharmacologic approaches do not work, jejunal or transdermal delivery of L-dopa is an interesting option, but the former may be technically challenging. The role of gastric electrical stimulation has not been defined in this population.
Megacolon, acute and chronic
Whereas obstruction of any cause must be included in the differential diagnosis of megacolon, fecal impaction must be high on the list in the patient with neurologic disease and chronic constipation, especially if immobile and in the context of sensory impairment. Plain abdominal radiography may be the only essential investigation. Typically, there is dilatation of the cecum and ascending and transverse colon with less gaseous distention in the left colon. If obstruction needs to be ruled out, nowadays computerized abdominal tomography may be the best option, with barium enema and colonoscopy as alternatives. If colonoscopy is contemplated, the endoscopist needs to be mindful of the risk of a cecal perforation due to the closed loop phenomenon, if obstruction is complete.
The first step in the management of acute megacolon is to search for and, where possible, treat any underlying contributing factor such as medication (eg, baclofen), electrolyte imbalance, sepsis, and, of course, recent surgery. Many cases will resolve spontaneously as the associated primary disorder improves; patient positioning may also promote resolution. In resistant cases, therapy should begin with a pharmacologic approach.
Cholinergic agonists are effective: Ponec and colleagues administered neostigmine in a dose of 2 mg intravenously to 11 patients with acute colonic pseudo-obstruction and compared their outcome with that of 10 who received intravenous saline. Ten of the 11 patients who received neostigmine had prompt colonic decompression compared with none of the 10 patients who received placebo. The median time for response was 4 minutes. Two patients who had an initial response to neostigmine required colonoscopic decompression for recurrence of colonic distention; 1 eventually underwent subtotal colectomy. In contrast, 7 patients in the placebo group and the 1 patient in the neostigmine group without an initial response received open-label neostigmine; all achieved colonic decompression. Given the risk of spontaneous perforation with its associated high mortality, colonoscopy has for some time played an important role in the management of patients with megacolon and significant cecal distention. By definition the colon will not be prepared in these patients, and the procedure may therefore be technically difficult. An overall success rate in achieving a reduction in cecal diameter of approximately 70% has been reported, but the recurrence rate has been as high as 40%. Some have advocated the placement of a decompression tube at the time of colonoscopy and have reported that this reduces the recurrence rate. This technique involves the placement of a guidewire through the biopsy channel of the colonoscope with the subsequent insertion of the decompression tube over the guidewire, under fluoroscopic control, following withdrawal of the colonoscope. Colonoscopy should be reserved for those for whom conservative therapy fails, in view of its risks in Ogilvie syndrome.
Surgical intervention, and the placement of a tube cecostomy in particular, may become necessary in the patient with megacolon who seems at high risk of perforation and for whom pharmacologic and colonoscopic attempts at decompression have failed. Clearly, surgery will also be necessary in those who unfortunately progress to ischemia or perforation. In all of these situations surgery has been associated with high morbidity and mortality rates. Prevention of acute megacolon will be based on the avoidance, where possible, of those circumstances that may precipitate this event; particular attention should be paid to medications known to induce hypomotility.
Constipation and incontinence
Laxatives continue to be the backbone of constipation management despite an overwhelming volume of rigorous clinical trial data. Whereas a number of prokinetic agents, including cisapride and tagaserod, which had been shown to be effective in the management of constipation in the patient with neurologic disease, have been withdrawn because of adverse events, the prokinetic approach continues to attract interest. “Old” compounds, such as pyridostigmine given orally in the maximum tolerated oral dose (180–540 mg daily), have been shown to accelerate colon transit and improve symptoms among patients with autonomic dysfunction and constipation. A “new” agent, mosapride, has also shown efficacy in constipation in relation to PD, and prucalopride, although not yet tested in PD, MS, or ALS, offers promise.
Nonpharmacologic approaches such as magnetic stimulation and abdominal massage have been shown to be effective in treating constipation related to neurogenic bowel dysfunction, and sacral nerve stimulation has demonstrated long-term efficacy in limited studies in chronic constipation. There are also a number of studies documenting successful treatment of constipation in MS by biofeedback.
The management of constipation in the context of neurogenic bowel dysfunction must include mindfulness of the possibility of precipitating fecal incontinence and soilage. One approach to this issue is the technique of transanal irrigation. In this technique, water is instilled into the colon and rectum to instigate a regular and controlled evacuation of feces; this approach has been shown to be effective in spinal cord injury and seems an attractive option in chronic neurologic disease. Other approaches to the management of incontinence in this context include biofeedback and sacral nerve stimulation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




