LARGE BOWEL DIVERTICULAR DISEASE (DIVERTICULOSIS OF THE COLON)
This is a very common acquired disorder of the colon, most prevalent in the Western Hemisphere.
1 It is characterized by a markedly thickened muscularis propria, through which herniations of the mucosa and submucosa pass through the muscularis propria into the subserosal connective tissue. The diverticula are distributed predominantly in the sigmoid and descending colon, although they may be found throughout the colon.
Clinically, the condition may be symptomatic or asymptomatic. There is not a good correlation between symptoms and pathologic findings unless there are inflammatory complications such as diverticulitis, abscess formation, or perforation. Diverticula may be the site of polyps or tumor, both within it and at their opening, although these are rare.
Pathogenesis
The
prevalence of diverticulosis varies greatly in different geographic areas of the world. It is most common in the Western Hemisphere and rare in Africa, Asia, and many parts of South America. In the Western Hemisphere, diverticular disease has an overall frequency of 15% to 35% using barium studies and is primarily left sided
5,
6 and up to 50% to 70% by age 75. In the West, about 95% of patients have disease involving the sigmoid and left colon, but with age, they occur progressively more proximally. Right-sided diverticular disease is therefore found in older patients with pan-diverticular disease.
7In Southeast Asia, right sided diverticular disease is the most frequent form of diverticular disease and has a barium enema frequency of 8% to 22% and affects the right side of the colon in 70% to 98% of patients.
8,
9 In sub-Saharan Africa, diverticular disease is uncommon and affects mostly the right colon.
10,
11,
12Interestingly, the risk of diverticular disease in black Africans living in Western countries is higher than that in their indigenous counterparts.
4 The risk of diverticular disease is related to a patient’s age and ethnicity.
4 Men and women are equally affected.
4 In Western countries, diverticulosis is uncommon before the age of 40. Diverticular disease incidence increases progressively,
4 so that by age 90 about 50% of the population have it. Men and women are equally affected.
The
pathogenesis of diverticulosis is not well understood and is likely multifactorial. Evidence suggests dietary fiber may be protective as one of the major differences between the populations of areas of low and high incidence is diet. In the Western Hemisphere, diets tend to be highly refined and fiber deficient, compared with those of African countries, which have a diet high in vegetable fiber and low in refined carbohydrates.
13 The incidence of diverticula increases when people migrate from low-prevalence areas to Westernized communities, for example, among Japanese migrants to Hawaii.
2,
4 Also it has been found that the prevalence of diverticulosis in vegetarians is three times less than in nonvegetarians.
14 Even rats fed with low-fiber diet seem to develop diverticulosis more frequently compared to controls.
15,
16 The question is: How does a low-residue diet result in diverticulosis? For diverticula to develop, two requirements are necessary: (1) increased intraluminal pressure of the colon and (2) points of relative weakness in the bowel wall through which the bowel mucosa protrudes to form diverticula:
1. It has been postulated that people with high-fiber diets have increased stool bulk, which increases the tone of the bowel wall, resulting in faster intestinal transit with more frequent stools and less fecal stasis. In contrast, lack of dietary residue (i.e., fecal bulk) has been postulated to produce irregular and uncoordinated peristalsis, which converts the colon into a series of saccules sealed off from one another by a valvular mechanism produced by alternating and overlapping semicircular arcs of thickened circular muscle. This is thought to result in a markedly increased intraluminal pressure, hypertrophy of the muscularis propria, and consequent diverticular outpouchings.
17 Another interesting hypothesis put forward for increased intraluminal pressure is related to the posture during defecation. It has been suggested that the natural position for defecation for human is squatting, where the rectum and sigmoid are more aligned in a straight line requiring less intraluminal pressure for the passage of stools from sigmoid to rectum. In contrast, in the sitting position, the rectosigmoid angle is close to 90 degrees requiring higher intraluminal pressure for defecation. This would also explain the lower rates of diverticulosis in parts of the world where defecation in sitting position is still the preferred mode, while diverticulosis is far more common in Western societies or
in populations in developing countries who have been increasingly adopting Western lifestyle.
18,
19,
20 Muscular thickening of the bowel wall usually accompanies diverticulosis, but it is still unclear whether this is a secondary phenomenon resulting from a need for increased intraluminal pressure or is a primary pathology.
2. The points of diminished resistance of the bowel wall occur at those sites where the nutrient arteries pass through the muscularis propria into the sub-mucosa, between the mesenteric and antimesenteric tenia. These areas are covered by connective tissue. It has been suggested that the collagen in these areas gradually loses its flexibility and tensile strength with age, resulting in weakening of the bowel wall and a predisposition to diverticula formation. This mechanism may help to explain the presence of diverticula in those cases of left-sided diverticulosis in which increased luminal pressure, reduced fecal weight, and prolonged intestinal transit are not found.
21 What is less clear is the role that veins play as these accompany the arterioles or whether the outpouchings are really along the tracts created by the veins. And if so, does this create local congestion? Which shows how little we know about this disease.
The importance of an intact connective tissue in maintaining the structural integrity of the colon is further attested to by the following observations:
a. Cases of diverticulosis occurring primarily in the cecum and right colon with luminal pressures that are lower than normal and do not show muscular thickening
b. The finding of diverticulosis in young patients with connective tissue disorders, such as
Marfan’s disease
22,
23 and
Ehlers-Danlos syndrome24
c. Acromegaly is also associated with an increased prevalence of diverticular disease.
25
However, the notion that interplay between muscle fibers, nerves, interstitial cells of Cajal (ICC), neurotransmitters, and possibly inflammatory cells plays a part in the development of diverticula is increasingly recognized. In one study, ICC and glial cells were decreased in colonic diverticular disease, whereas enteric neurons appear to be normally represented.
26 Mast cells have also shown to be increased,
27 but how these all fit together is not entirely clear and many of the histologic changes in the neuromuscular apparatus reported are likely secondary in nature.
Diverticulitis has been attributed to trapped fecaliths in diverticula.
28 Since these have at most a muscularis mucosae, impacted feces cannot be ejected so become hard and they cannot pass back into the lumen. Subsequently, edema and inflammation may further narrow the diverticula necks and impede the outflow of feces from diverticula.
28 Mucosal inflammation can readily extend to the submucosa of the diverticula, which is directly subjacent to either the peritoneal cavity or the retroperitoneum where further extension of the inflammation admixed with fecal contents may occur, and can result in fistula tracts.
Clinical Features
Diverticular disease increases with age and is found in approximately one-third to one-half of the patients over 60. Men and women are equally affected.
29,
30 Though presumed to be a rare entity, diverticulitis in patients younger than 40 years old has gradually risen.
31,
32 Uncomplicated diverticular disease (diverticulosis) is often asymptomatic
33 but may be associated with crampy abdominal pain and diarrhea alternating with constipation.
33 In this setting, symptoms may be attributed to diverticula, but this is usually difficult to prove.
33,
34,
35Diverticulosis may be present for months or years before complications ensue. Complications in diverticulosis occur in only about 20% to 25% of patients,
36 and only a small minority develop severe or life-threatening complications.
2Diverticulitis or its complications may be confined to a single attack with permanent remission or may consist of repeated episodes over many years. The symptoms and signs of diverticulitis are left lower abdominal pain, fever and, commonly, a palpable, tender, rope-like mass. Acute diverticulitis almost invariably results from perforations that are often quite small at the tip of the diverticulum. This area usually becomes walled off, with local abscess formation, fever, and abdominal tenderness. Generalized peritonitis is very uncommon.
37 Sometimes adjacent structures, such as the bladder, intestinal loops, vagina, anterior abdominal wall, and, rarely, adjacent vascular structures, become adherent to the inflamed colon, resulting in fistula formation. In 5% to 10% of cases, resolution of diverticulitis results in severe scar formation with large bowel obstruction, which may mimic carcinoma clinically.
38Hemorrhage is an uncommon complication of diverticular disease and is not usually associated with acute diverticulitis clinically,
38 although necrosis of arteriolar walls must occur, which presumably results from inflammation. Interestingly, often arterioles adjacent to the diverticula have marked luminal obliteration resulting from reactive intimal proliferation, even in the absence of active inflammation. Vascular injection studies have shown a close relation between blood vessels and diverticula, as well as features of vascular ectasia.
39 It has been estimated that diverticular hemorrhage accounts
for between 20% and 48% of lower GI bleeding,
40,
41 but these figures are likely erroneous, since endoscopic experience has shown that many patients with GI hemorrhage previously thought to be due to diverticular disease on clinical and radiologic grounds are due to other patients such as vascular ectasia. Clinically, bleeding from diverticula is usually abrupt and painless but, in some patients may be massive and life threatening. The blood is usually bright red and arteriolar, being most overt if the hemorrhage occurs from disease in the sigmoid colon.
38 The site of origin of hemorrhage can be difficult to localize. Selective arteriography may be helpful, especially in those patients with persistent, life-threatening hemorrhage in whom surgery is contemplated.
42Some patients develop inflammation indistinguishable from IBD in the segment of colon with diverticula, also referred to as diverticular colitis or segmental colitis associated with diverticular disease (SCAD). Prolapse-type polyps may also develop at the orifices of diverticula. Its differential diagnosis is Crohn’s disease especially if the rectum is spared and ulcerative colitis when the disease extends into the rectum. Thus, demonstration of a normal rectal biopsy is intrinsic in the diagnosis, as is the presence of proximal lack of involvement.
Endoscopy
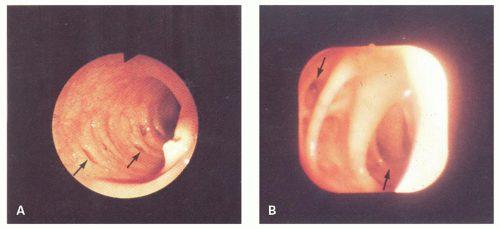
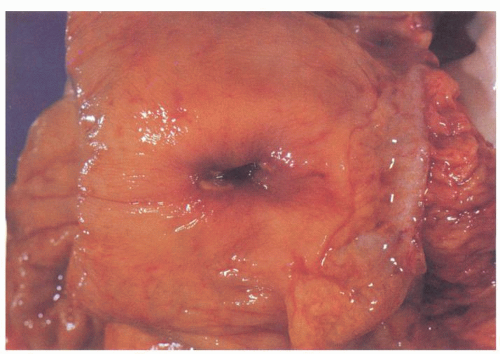
At colonoscopy, diverticula are characterized by 3- to 5-mm-wide orifices lying in shallow haustral pouches commonly separated by ridgelike elevations (
Fig. 6-1).
43 The mucosa around the mouths of the diverticula may be inflamed, with patchy erythema, and may sometimes be raised above adjacent mucosa mimicking polyps. Only rarely is blood or purulent material seen extruding from the diverticulum. In diverticular strictures, the lumen becomes quite distorted and narrowed, but overlying mucosa is intact, and the valvulae appear symmetrical and regular, in contrast to their appearance with carcinoma.
Imaging
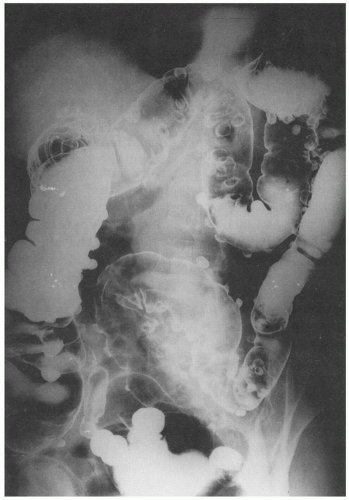
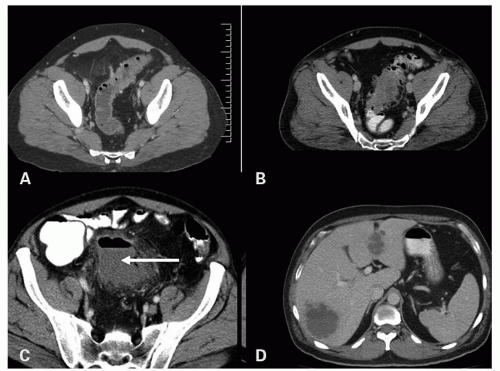
Imaging is important diagnostically, and ultrasound, barium enema, computed tomography (CT), and magnetic resonance imaging are all used. CT has largely replaced barium enema primarily because of its ability to identify extracolonic disease and its extent, and CT is also the procedure of choice in acute disease. If barium enema is used, the presence of extraluminal barium pockets, which persist on the postevacuation film (
Fig. 6-2), remains useful in some settings. Other findings include the demonstration of mucosal ridges and sacculations, mirroring the gross appearances. CT can also demonstrate diverticulosis, inflamed diverticula, and abscess both intra- and extramural, as well as less common complications such as hepatic abscesses (
Fig. 6-3).
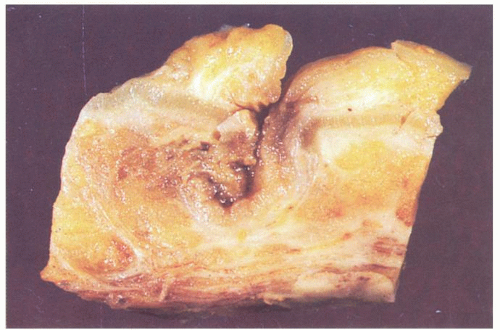
Gross Pathology
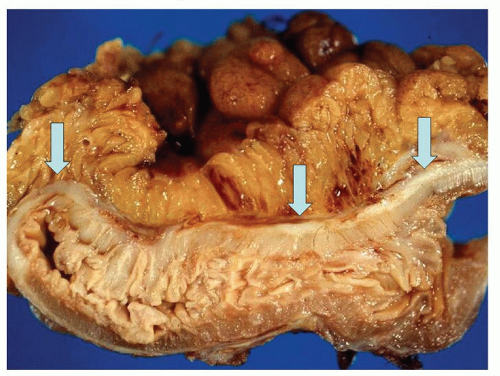
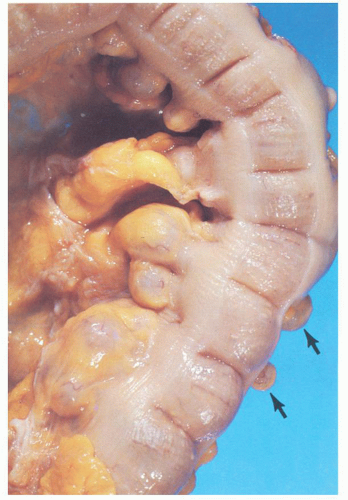
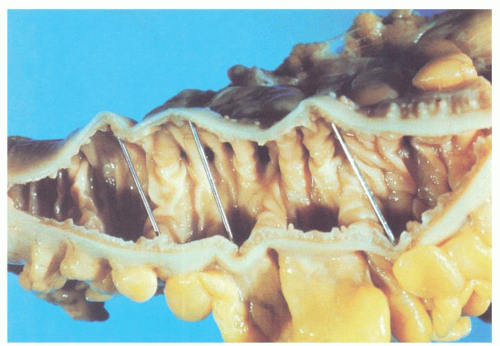
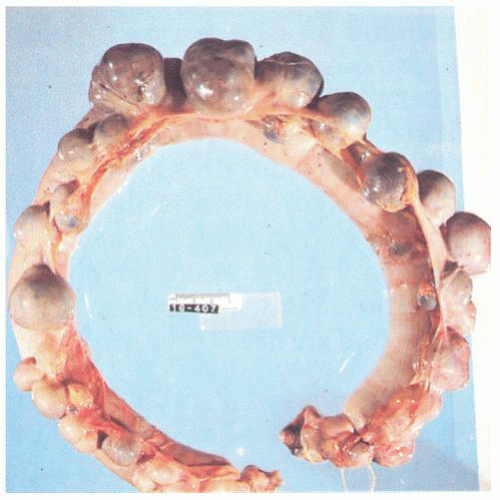
The vast majority of diverticula occur in the sigmoid colon, but they can extend proximally to the cecum and do so to some extent in about 15% of patients.
44 Externally, they typically appear in two rows on either side of the colon between the mesenteric and antimesenteric (lateral) teniae at points of entry of the nutrient arteries within the pericolic fat, especially the appendices epiploicae, and have a globular or fusiform configuration. They measure around 1 cm in diameter, although this may vary from a barely perceptible bump to sacs 2 cm or larger (
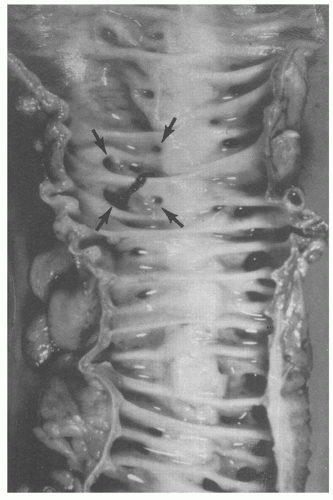
Fig. 6-4). Internally, there are one or more rows of diverticula that are visible to varying extent but are enhanced if the bowel is inflated with formalin overnight. When the disease is severe, there are invariably mucosal ridges longitudinally marking the position of the taeniae coli between which diverticula can be seen to be protruding but also numerous transverse ridges that appear either circumferential (
Fig. 6-5) or interdigitating (
Fig. 6-6). The ridges do not usually extend around the circumference of the bowel wall but tend to consist of overlapping semilunar arcs of thickened circular muscle confined to
the zone between the mesenteric and antimesenteric teniae.
44,
45 Between the ridges, the colon shows saccular dilatation, and this is where the orifices of the diverticula lie. The latter vary in size from 3 to 5 mm in diameter, may contain impacted fecaliths, and are connected to the diverticula by necks of varying lengths (
Figs. 6-4 and
6-5). Invariably, the muscularis propria is markedly thickened, often to over 5 mm when it appears shiny white to the point of looking almost cartilaginous; this is invariably a prominent feature of the disease (
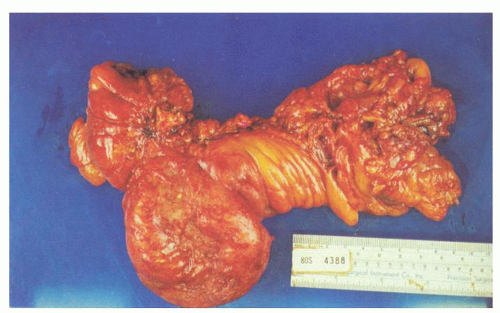
Fig. 6-7). The affected bowel is shortened (“myochosis”) with exaggerated mucosal folds, which appear almost redundant. Polyps either on the redundant mucosal folds or around ostia of the diverticula can form, which show many of the features of mucosal prolapse (see later).
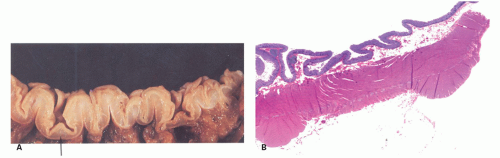
Prediverticular disease. In some patients, the muscle abnormality of diverticular disease is present in the absence of true diverticula, although outpouchings of what looks like excess mucosa and submucosa are present that initially resemble diverticula but which do not actually pass through the muscularis propria
(
Figs. 6-7 and
6-8). This is presumably because of persistent contraction of both circular and longitudinal muscle of the muscularis propria. Since radiologic follow-up of some patients with this type of thickening showed development of diverticula later (hardly a controlled trial), this does suggest this may be an early stage of the disease.
46,
47 The same changes may be seen in patients with typical diverticulosis in other areas of the colon, so it is generally believed that it represents the earliest phase of diverticular disease. It is also possible that the intraluminal pressure required for the mucosa and submucosa to pass through the potential spaces adjacent to the arterioles and veins never occurs in some of these patients.
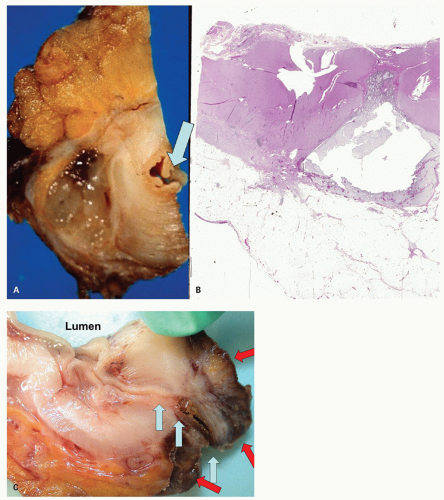
Handling of specimens. There are two ways in which the specimens can be handled on receipt from the operating room. The first is to insufflate the segment of colon with formalin, after first rinsing out the intestinal contents with saline, and fix it overnight. It can then be opened either longitudinally or transversely. This demonstrates virtually all diverticula so that those that are inflamed can be more readily identified. This method has the advantage of distending all the diverticula and making their examination easier. The second method is to open the colon immediately on receipt and then, after gross examination, to pin it out for fixation. One can insert a little finger in the lumen prior to making any cuts to avoid cutting through any unsuspected lesions such as large adenomas or a carcinoma. This method thus has the advantage of identifying any unexpected pathology that rarely may alter the immediate intraoperative management of the patient and also allows detection of abnormalities that are sometimes difficult to find in the fixed state, such as the points of origin of diverticular hemorrhage. It should be stressed that the mere finding of hemorrhage in a diverticulum does not prove that it originated from that site, since it may well have filled with blood from the lumen. To prove that blood has originated from a given diverticulum, it is necessary to demonstrate a
bleeding point histologically. This can be impractical if it requires examining every diverticulum, which are often numerous; however, examining most or all of them grossly is not too onerous. Many of the changes of diverticular disease may not be clearly visualized if the opened colon is examined in random transverse cuts unless these are done every few millimeters. In fixed specimens, abnormalities are also well demonstrated using serial longitudinal cuts parallel to the length of bowel wall through the rows of diverticula.
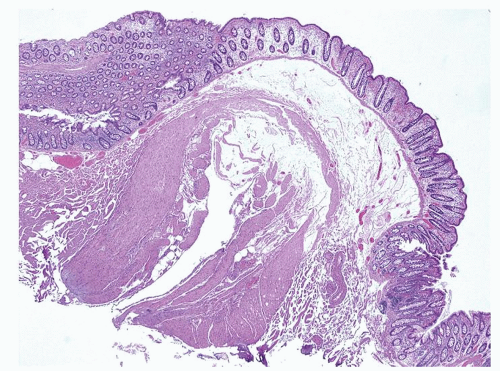
Histology
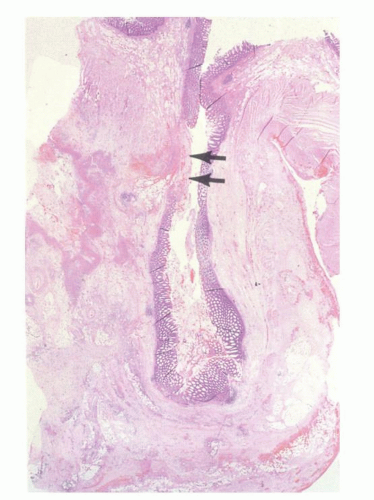
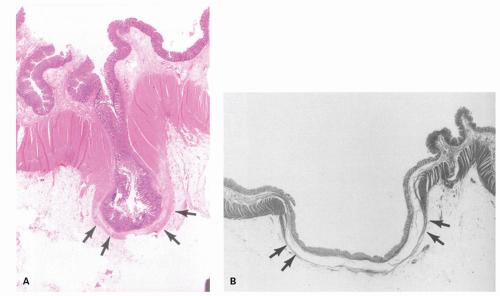
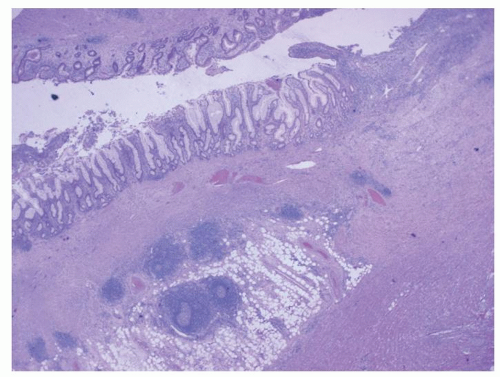
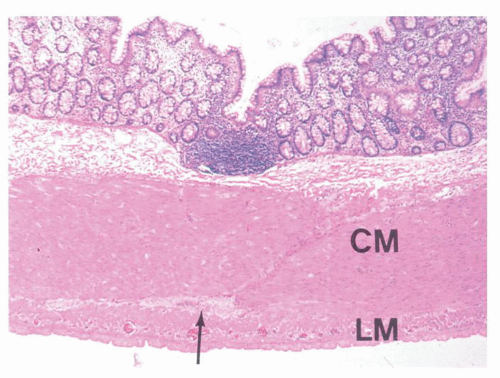
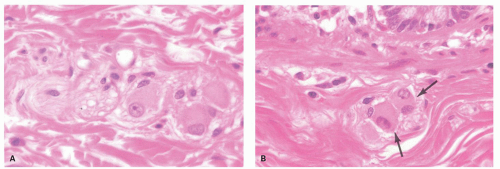
Diverticula consist of mucosa and muscularis mucosae and covered by pericolic adipose tissue, serosa, and, occasionally, a few fascicles of longitudinal muscle (
Fig. 6-9). The necks of the diverticula lie between the hypertrophied fascicles of circular muscle between the taeniae coli. The muscularis propria is often hugely thickened and the neural plexi similarly hypertrophied.
The inflammation in diverticulitis invariably has a chronic lymphoplasmacytic component and can mimic diversion disease, infection, and IBD. There can therefore be lymphoid aggregates often with germinal centers, similar to that seen with diversion colitis or bacterial overgrowth, which may spill into the pericolonic fat. Cryptitis and crypt abscesses can mimic infectious/self-limited colitis, while crypt architectural distortion, and rarely even granulomas can mimick IBD. Diverticular colitis can have all of the histologic features of IBD (see
Chapter 18).
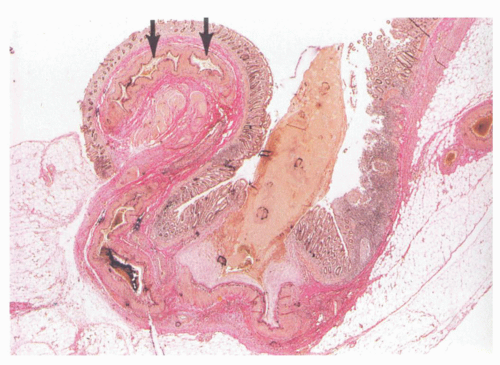
Usually, one or at most a few diverticula are affected, and these commonly contain fecaliths. Rarely, all diverticula are acutely inflamed. The inflammation usually involves the apical region of the diverticulum where it is frequently associated with a fecalith but may begin as a focus of acute inflammation and then an erosion and finally an ulcer. It can also start in the neck of the diverticulum (
Fig. 6-10). This results initially in microperforations and so may form peridiverticular inflammation and abscess (
Fig. 6-11). Sometimes, there is a foreign body giant cell reaction and occasionally genuine granulomas to the inspissated feces. The inflammation is often restricted to the diverticulum, and spread to the mucosa around the diverticular orifices and beyond (diverticular colitis) is uncommon (
Fig. 6-12).
In most instances, diverticulitis remains localized, being contained by the appendices epiploicae, pericolic fat, mesentery, or adjacent organs. In severe diverticulitis, inflammation may spread along the outside of the bowel wall, ensheathing it in fibrous tissue to produce a large mass around the colon. Occasionally, the abscess dissects along the serosa parallel to the outer aspect of the deep muscle layers. Consequently, inflammation from one diverticulum may spread widely up and down the bowel wall, ensheathing it with inflammatory tissue and producing a large mass around the colon.
17 However, inflammation rarely spreads within the bowel itself, and the luminal mucosa usually remains uninvolved (
Fig. 6-12), but its presence in the absence of diverticulitis represents diverticular colitis/SCAD segmental colitis associated with diverticular disease— (see
Chapter 18). Rarely, perforation occurs into the peritoneal cavity resulting in peritonitis, and occasionally, the perforation is unaccompanied by inflammation, but the ruptured diverticulum exudes mucin forming a peridiverticular mucin lake (
Fig. 6-13).
Resolution of the inflammation results in fibrosis leading to a thickened and stenotic colon with marked distortion of the lumen. A variable mononuclear infiltrate can be present, which may include numerous submucosal and serosal lymphoid aggregates, mimicking Crohn’s
disease
28 (see subsequent discussion). Involvement of adjacent structures such as the bladder wall, other intestinal loops, vagina, and the anterior abdominal wall is also rare and may result in fistula formation and massive hemorrhage if vessels are involved. Occasionally, rupture occurs and mucus is extravasated into the surrounding tissue (
Fig. 6-13). This is similar to nonneoplastic mucoceles of the appendix and carries no increased risk of pseudomyxoma. Whether “chronic diverticulitis” is a true clinicopathologic entity is debatable. However, when symptoms persist, one must consider the diagnosis of pericolic abscesses.
Since most resections in patients with diverticulosis are performed for diverticulitis or their complications, clinicians hope the pathologic examination can confirm the cause, while pathologists also want the same result with reasonable sampling not requiring submission of every diverticulum. Sometimes, this does requires going back to the specimen more than once for additional sections.
Defining diverticulitis. This becomes an issue when the question is whether the patient’s symptoms can be attributed to the inflammation present. In some ways this duplicates the same issue with appendicitis and mucosal inflammation. There are no established guidelines for minimal criteria. There is frequently more inflammation in and around the diverticula than the adjacent mucosa, but it is unclear if it is of any significance. Traditionally, the presence of neutrophils
and extension of the inflammatory infiltrate beyond the muscularis mucosae have been used as pathologic definitions for diverticulitis. If carefully examined, most diverticula even in asymptomatic individuals show at least some increase in lamina propria inflammation with lymphoid follicles, indicative of bacterial stasis; however, it is the presence of neutrophils in the inflammatory infiltrate that seems to correlate clinically with acute diverticulitis.
48
Hemorrhage Hemorrhage in diverticular disease is traditionally said to be associated with erosions of arterioles on the necks of the diverticula as it is often bright red. However, it may also be associated with vascular ectasia and/or erosion into submucosal vessels (
Fig. 6-14), but this is often difficult to demonstrate grossly and microscopically. It is not usually associated with symptoms associated with diverticulitis. As most arterioles are accompanied by at least one vein, it is unclear why the walls of veins are not just as susceptible as arterioles to involvement in the inflammatory process. Medications such as NSAIDs and ASA increase the risk of diverticular hemorrhage, (and perforation).
DIVERTICULAR DISEASE AND IBD
While there is no reason that these two diseases should not coexist,
66,
67 it should also be recognized that resolving diverticulitis can exhibit many of the features of Crohn’s disease. These include focal mucosal ulcerations and inflammation, transmural inflammation in the form of numerous submucosal and serosal lymphoid aggregates (
Fig. 6-16), and rarely even granulomas. Interpretation of these changes is clearly subjective. It has been shown that most of these patients do not represent Crohn’s disease unless they already have it.
68 In one review of 50 patients with Crohn’s-like features in diverticular disease, only 2 patients recurred with Crohn’s disease following resection.
69 Similarly, of the 77 cases with ulcerative colitis-like diverticular colitis, only 8 developed classical ulcerative colitis on follow-up.
69 A diagnosis of diverticulitis
and IBD should therefore
not be made unless there is other clinical evidence of IBD elsewhere. In some cases, even though the findings of diverticulitis are absolutely indistinguishable from IBD, it is wise to wait for the latter to reveal itself in due course of time and
not generate an extensive clinical workup except possibly for a full colonoscopy (if not performed recently) or start presumptive treatment. The finding of diverticula that are incidentally involved with contiguous inflammatory disease occurs but is surprisingly rare.
DIVERTICULOSIS OF THE DUODENUM AND SMALL INTESTINE
Small intestinal diverticula occur most frequently in the duodenum with fewer cases in the jejunum and ileum.
83 They are surprisingly common, may be single or multiple, and are usually asymptomatic. Most busy radiology departments will probably encounter one of these daily as an incidental finding in upper GI studies.
84
Pathogenesis
Small intestinal diverticula may be congenital or acquired. Though familial aggregation has been described in some cases,
85 for the most part, pathogenesis appears to be multifactorial. Herniation appears to occur where paired blood vessels penetrate the mesentery into the bowel wall. Diverticula are most readily identified in the duodenum, proximal jejunum, and distal ileum, where vasa recti are of greatest diameter.
86,
87 Diverticula are also frequently associated with intestinal motility disorders, such as progressive systemic sclerosis, visceral neuropathies, myopathies, and sometimes mitochondriopathies where abnormal contractions and fibrosis of the muscularis propria can result in the formation of diverticula.
88,
89 The pathology is that associated with motility disorders (vide supra).
Duodenal Diverticula (Single or Isolated)
These are by far the most common diverticula of the small bowel.
90 The number decreases distally with nearly 80% of the remaining in the jejunum and 15% in the ileum.
91 Duodenal diverticula can be either extraluminal or rarely intraluminal.
Extraluminal diverticula. The vast majority of duodenal diverticula are extraluminal. They usually occur in the medial portion of the second part of the duodenum within 2 cm of the papilla of Vater.
90 Most are asymptomatic and found incidentally on imaging (
Fig. 6-18). Thus, prophylactic removal is not necessary. Only rarely do they cause symptoms resulting from bleeding, perforation, obstruction, or malabsorption secondary to bacterial overgrowth.
92,
93,
94 In addition, there is an association between periampullary duodenal diverticula and choledocholithiasis that has been ascribed to papillary dysfunction.
95 In contrast to their frequent detection using imaging studies, they are rarely recognized at endoscopy because the orifice communicating with the duodenal lumen is typically a mere slit and is obscured by the mucosal folds. Duodenal diverticula consist of mucosal and submucosal outpouchings into the serosa and lack a muscle coat, appearances similar to those of left-sided colonic diverticular disease.
Intraluminal duodenal diverticulum (prolapsed diaphragm). This is an uncommon congenital anomaly characterized by an intraluminal mucosal pouch, usually located in the second portion of the duodenum adjacent to the papilla of Vater. The anomaly is thought to be part of the spectrum of failure of recanalization of the duodenum in embryonic life. Depending on its severity, lesions may range from atresia to stenosis, diaphragm formation, and intraluminal diverticulum.
96 There may be an association with other congenital defects, such as Down’s syndrome, cardiovascular lesions, intestinal malrotation, and omphalocele.
97,
98 In adults, intraluminal diverticula usually presents between the third and fifth decades with symptoms of partial obstruction. Rarely, pancreatitis and hemorrhage secondary to ulceration have been reported.
99,
100 Resection is the usual form of treatment for symptomatic diverticula, although therapeutic endoscopy with
incision or excision of the membrane may be indicated in selected patients.
101
Gross Appearance Endoscopically, these lesions may resemble polyps,96 up to 12 cm in length and 6 cm in width, usually attached to part of the bowel wall, and material such as stones and foreign bodies may become trapped in the pouch.
Histologic Examination This shows duodenal mucosa lining both sides of the pouch with submucosa but no muscle.
Jejunoileal Diverticula
Pathogenesis and clinical features. The most common location of diverticulosis is in the proximal jejunum; ileal diverticula are rare. Most cases are asymptomatic and found incidentally on imaging. Sometimes they cause symptoms due to bleeding, perforation, obstruction, or malabsorption secondary to stagnation of intestinal contents.
82,
102,
103,
104
This is a rare heterogeneous group of conditions with a reported incidence in the general population of <0.1%.
82 They may be congenital or acquired; the congenital ones are exceeding rare and are often associated with enteric duplications and defects in the spinal column
105 as well as motility disorders. Intestinal diverticula of the acquired type result from mucosal herniation through the muscle layer along the mesenteric border in relation to the perforating arteries. Concurrent colonic diverticula have been reported in up to 40% of cases, but this is approximately the prevalence in the general population.
91 Most cases of acquired small intestinal diverticulosis occur in older patients, and many are associated with intestinal pseudoobstructions, such as
familial visceral myopathy or neuropathy, mitochondriopathies, scleroderma, and Fabry’s disease,89,
106 or have local pathology indistinguishable from that of these disorders. Indeed, at any age, the battery of special stains carried out in intestinal pseudoobstruction should be carried out, or these changes are readily missed. If the muscle has disappeared, they are interpreted as “western type” rather than considering the possibility that the muscle has been lost from disease associated with motility disorders.
Gross appearance. Acquired small intestinal diverticula are usually multiple, wide-mouthed, paper-thin mucosal herniations situated along the mesenteric border of the bowel, measuring up to 4 cm in diameter (
Fig. 6-17). Rarely, obstructing phytobezoar may be lodged within the diverticula.
104 The “congenital” diverticula are usually solitary and present on the antimesenteric border (
Fig. 6-18).
84
Histology. Jejunoileal diverticula may be composed of mucosa and submucosa only or of all layers of the jejunal wall. In the congenital diverticula, there is protrusion of all coats of the intestinal wall, which is greatly thinned. The morphology of the acquired diverticula depends upon whether the primary lesion is neural or muscle related, so adjacent and remote muscle in the specimen always needs to be carefully sampled and examined for neuromuscular disorders.
Some diverticula have an appearance indistinguishable from hollow/familial visceral myopathy lesions, some have scleroderma-like changes, while mitochondriopathies such as familial mitochondrial neurogastrointestinal encephalopathy (MNGIE) also have them (see section on mitochondriopathies). However, in “congenital” diverticula, there is invariably a degree of atrophy of the muscularis propria that gives rise to diverticular outpouchings made up of remaining layers of the bowel wall. All are covered by serosa, so it is necessary to find where the muscle stops and then identify the associated lesion (see section on intestinal pseudoobstruction). The trap is that if the muscularis propria is completely fibrosed, the appearance may at first glance resemble an acquired diverticulum as only the muscularis mucosae is visible. Careful examination of a trichrome stain usually reveals the fibrosis that is all that remains of the thickened muscularis propria, but examination away from the diverticula invariably reveals focal or complete loss of muscularis propria indicative of the underlying disease. The usual panel of histochemical and immunostains is therefore desirable to demonstrate muscle layers, nerves, ICCs, fibrosis, etc., as discussed later.
Diverticulitis and its complications, such as perforation, hemorrhage, inflammation, and obstruction, may occur but are rare. Those cases secondary to pseudoobstruction will show histologic features of the underlying disorder, namely, either a myopathy or a neuropathy.
GASTROINTESTINAL MOTILITY DISORDERS
The Normal Motility Apparatus of the Gut
Normal intestinal motility depends on the coordinated activity and interplay of intestinal smooth muscle, the extrinsic and intrinsic nervous system and their neurotransmitters, the ICCs, and the GI hormones. Abnormalities in any of these result in abnormal
intestinal motility and potentially failure of sphincteric function. Luminal contents also modify motility either by using and modifying the intrinsic apparatus. The clinical manifestations depend on the location and extent of the abnormalities and may be localized, as is usually the case in Hirschsprung’s disease, or diffuse, as in visceral myopathy and degenerative neural diseases. Lesions in the esophagus can result in achalasia; in the stomach, they can result in gastroparesis, in the small intestine lead to pseudoobstruction, and in the colon lead to severe constipation and/or pseudoobstruction.
Musculature of the Gut
This is covered in detail in each chapter on the respective organ or site. To summarize here, there are two major muscle coats within the intestines, the muscularis mucosae and muscularis propria. Both have two layers, although that in the muscularis mucosae can be more difficult to see, but especially when hyper-trophied, both are readily visible and are oriented in the same directions as the muscularis propria. While in some conditions with myopathies similar changes occur in the muscularis mucosae however, these tend to be subtle and diagnostically unhelpful.
With the exception of the stomach, the external muscle coats of the GI tract are organized into two layers, an inner circular and an outer longitudinal coat (
Fig. 6-19). The circular layer is complete throughout the intestines. While the longitudinal muscle is complete in the small intestine, in the large intestine, it is focally condensed to form three taeniae coli, one on the mesenteric border, and two antimesenteric taeniae. They all fuse in the appendix proximally and splay out distally in the rectum again to form a complete muscularis propria.
All layers of the muscularis are richly innervated; indeed, all of the small “holes” seen on hematoxylin and eosin (H&E) sections are nerves. The ICCs are limited to the muscularis propria and myenteric plexus. This becomes important when assessing innervation.
The musculature of the upper third of the esophagus consists of striated muscle, and the remainder consists of smooth muscle, although there is a mixture of the two where they merge.
The musculature of the stomach is more complex. It consists of three layers, namely, an outer longitudinal coat, a middle circular coat, and an inner oblique coat. The outer longitudinal coat is continuous with the corresponding muscle of the esophagus and duodenum. It is relatively sparse anteriorly and posteriorly, except at the pylorus, where the musculature of the greater and lesser curvatures converges to form a circumferential sheet. The middle circular muscle coat is continuous with the esophagus and surrounds the whole stomach. However, at the pyloric sphincter, it bends on itself to form a muscular thickening and is not continuous with the circular muscle coat of the duodenum. The inner oblique coat courses from the cardia to the greater curvature, gradually merging with the circular muscle coat. Absence of this layer in the lesser curvature creates a depression, the so-called magenstrasse.
The cardiac, or lower esophageal sphincter, is poorly defined anatomically.
The pyloric sphincter consists of a thick band of muscle at the distal end of the stomach, which forms the gastroduodenal junction. The muscle bundle of the sphincter is composed of the circular muscle coat of the stomach, which is bent on itself and separated from the circular muscle coat of the duodenum by a thin band of fibroconnective tissue.
107 In cross section, smooth muscle fibers have a dense pink cytoplasm with round to irregular borders, depending on the state of contractility at the time of fixation. With autolysis, the cytoplasm may become swollen and stain poorly.
108 In H&E-stained sections, it may sometimes be difficult to distinguish poorly stained normal muscle fibers from atrophic fibers or collagen; thus, a trichrome stain is very useful for the interpretation of the intestinal musculature. The myenteric plexus lies between the longitudinal and circular muscle coats.
Demonstration of Smooth Muscle in Motility Disorders
Although demonstrable on H&E stains, fibrosis can be difficult to appreciate. Trichrome stains help considerably, enhancing not only fibrosis of the muscle, but whether it is occurring predominantly in the
circular, longitudinal, or both layers and also demonstrates the pattern that could be delicate interstitial or broad zones of fibrosis that frequently encases the muscularis propria on both internal and external aspects. It can also bring out additional layers of muscle that may be either in the submucosa or subserosa but can be subtle, especially the latter. Any marker for smooth muscle also enhances these changes.
ENTERIC NERVOUS SYSTEM
This system has two components, an extrinsic nerve supply and an intrinsic system. The intrinsic system is analogous to a microprocessor in close proximity to the effector system that initiates and programs the behavior of effector systems and automatically makes adjustments in behavior. The extrinsic system can be likened to a main computer that monitors sensory information from the gut and issues commands appropriate for the digestive tract.
109 The extrinsic nerves reach the gut via the vagus, mesenteric, and pelvic nerves and consist of sympathetic and parasympathetic nerve fibers, which mesh with the fibrillary network of the submucosal and myenteric plexuses and also interface with muscle fibers of the muscularis mucosae and muscularis propria.
108,
110,
111,
112,
113,
114,
115,
116 The sympathetic system originates from cells in the prevertebral ganglia and synapses directly with the enteric ganglion cells. It exerts an inhibitory effect on the motor activity of the entire gut, with the exception of the sphincters, and decreases blood flow during physical activity and enhances it after feeding. It does not appear to influence GI secretions. The parasympathetic cholinergic fibers consist of the vagal and pelvic outflow tracts and innervate primarily the esophagus, stomach, rectum, and anus.
3,
11 They control esophageal peristalsis, the esophageal sphincter, gastric emptying, and the internal sphincter of the anus.
The intrinsic (enteric) nervous system, in concert with ICC and gut hormones, is important in controlling intestinal motility and contributes to GI secretion. It is a complex but orderly system containing many chemically distinct types of nerves. Each type of nerve appears to have a characteristic cell shape and a precise projection pattern, sending processes in specific directions to specific targets.
12 Numerous different groups of chemically distinct peptides are found in the enteric nerves. These include substance P, vasoactive intestinal polypeptide (VIP), opioid peptides, somatostatin, cholecystokinin (CCK), neurotensin, and gastrin-releasing peptide.
12Histologically, the enteric nervous system consists of two nerve plexuses:
1. Auerbach’s myenteric plexus, situated between the circular and longitudinal muscle coats
2. The submucosal plexus, which has superficial, mid, and deep parts
117
i. Meissner’s submucosal plexus is the superficial component close to the muscularis mucosae (
Fig. 6-20).
ii. Henle’s plexus, which is the deep component and situated immediately superficial to the circular layer of the muscularis propria
iii. There is a less well-defined plexus between these two that tends to be in the superficial part of the muscularis mucosae and so, closer to the muscularis mucosae than the muscularis propria.
Microscopically, the plexuses consist of a mesh-work of neurons aggregated into ganglia, axons,
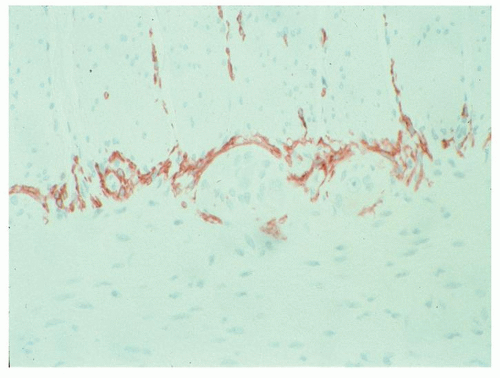
dendrites, and nerve fibers. The latter communicate between the ganglia, muscle fibers, and mucosa. In the myenteric plexus, there are also numerous ICC that extend into the muscularis propria (see subsequent section). By conventional histology in perpendicularly cut sections, only a tiny amount of the plexus is visualized because the primary plane of the plexus lies in a horizontal direction. Furthermore, neuronal processes and nerve fibers remain unstained by H&E. Neurons can be demonstrated using neural strains but also stain strongly with Bcl2 and mismatch repair gene proteins. A subpopulation of ganglion cells are immunoreactive with calretinin, and calretinin immunoreactive nerves are also present. In the mucosa, these are markedly enhanced in Hirschsprung’s disease.
Historically, the plexi were visualized in thick sections taken from between the muscle coats, cut parallel to the surface, and stained by a silver method
110 to produce a black stain in neurons, axons, dendrites, and nerve fibers (
Fig. 6-21). The intensity of staining varies from intense black for the so-called argyrophilic neurons to pale tan for argyrophobic neurons. Interpretation of these thick (up to 50
µm) silver-stained sections requires both specialized equipment and considerable experience with what is normal. However, there has been little correlation of silver studies using this method with either immunohistochemical or other histochemical techniques such that the interpretation of these stains in practice is fraught with many difficulties. However, there was much good work carried out that may therefore pass into obscurity.
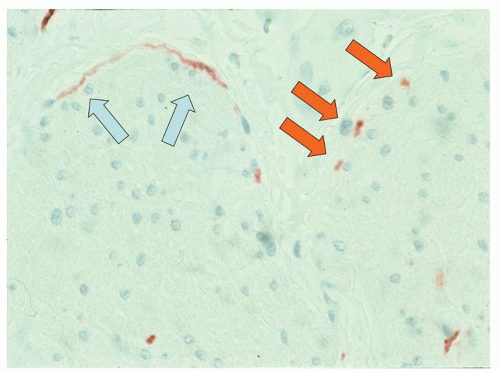
The second component of the neural plexi is the massive innervation of the muscle layers, and both components need to be examined. An immunohistochemical nerve stain is mandatory in evaluating motility problems and depends on what is working well in your lab. Protein gene product 9.5 (PGP9.5), glial fibrillary acidic protein (GFAP), and potentially CD56, neuron-specific enolase (NSE), etc., can all be used, while S100 that stains the supporting glia can usually be used as a substitute (
Fig. 6-22). Sometimes, they stain differently, so specialized centers may wish to do a specific neural stain as well as S100; however, there are few data on the correlations when these differ or
their significance. However, it is necessary to know what normal looks like in the part of the bowel being examined to interpret abnormal, so centers doing few cases should consider using normal controls if at all possible. Increasingly, the role of loss of nitric oxide synthase (NOS) producing nerves in motility disorders is being appreciated, and this may become a standard practice to evaluate this in motility disorders. Cal-retinin immunoreactive nerves are described in the previous section. There may be an argument for using this in motility disorders but it has to be regarded as experimental and controls are essential.
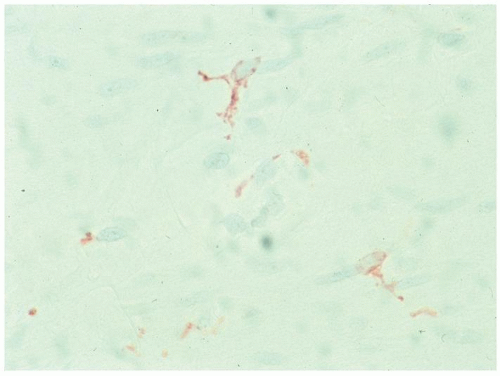
Interstitial Cells of Cajal
Interstitial cells of Cajal are thought to be the pacemaker cells of the gut initiating and maintaining its rhythmical contractions. They form from embryonic mesenchyme when cells differentiate into ICCs, likely as a result of sonic Hedgehog activation, and form a network that is found throughout the human GI tract and, when displayed appropriately, have numerous processes extending out akin to nerves. They are found
a) Primarily in and around the myenteric plexus (
Fig. 6-23).
b) Within the muscularis propria, especially in the circular muscle. When examining ICCs, both major components need to be considered even though their interpretation is still in its infancy (
Fig. 6-23).
c) Immediately beneath the internal margin of the circular margin, the deep submuscular plexus (
Fig. 6-24).
ICCs have processes similar to neurons that can sometimes be observed (
Fig. 6-25). To date, ICCs have not been identified in the mucosa or submucosa in man.
In patients with motility problems, most of the attention focuses on the myenteric plexus as that is where abnormalities are most easily recognized, but
loss of ICCs in this location is invariably accompanied by their loss within the muscle layers and loss of nerves. It is also unclear whether just one component of the ICC network can be affected in isolation. Many other aspects of ICC related to possible hypertrophy, their regulation, and development remain poorly understood.
ICCs are accompanied by a specialized fibroblast network of cells, that is CD34, and possibly PDGFRα, immunoreactive, but unreactive with CD117 or DOG1; they are thought to express small-conductance Ca(2+)-activated K(+) channels (SK3). ICCs are thought to be the cells of origin of GI stromal tumors, but it is unclear if the fibroblasts have any role in these tumors. A complex consisting of the cholinergic and nitric oxide (NO)-containing nerves with neural vesicle, ICCs, and the fibroblast-like cells form a network that interplays with adjacent smooth muscle cells, forming a cooperating mechanism that controls motility, and loss of any of the components can result in motility disorders. Further, loss of one component may result in degeneration of another part of the network, so that loss of nerves may result in loss of ICCs and muscle and fibrosis (denervation atrophy) and vice versa. It can therefore be difficult to determine the initial insult in motility disorders, as by the time they are examined several components may be abnormal.
The number and distribution of ICCS vary from organ to organ.
In the esophagus, ICCs are present within both muscle layers but appear not to be present in Auerbach’s plexus.
In the stomach, they are also present in the circular and longitudinal muscle of the corpus, but in the antrum, they are also present in Auerbach’s plexus but are more frequent in antrum and corpus than in the fundus.
In the human small intestine, they are numerous in both longitudinal and circular muscle and abundant in around the Auerbach’s plexus where they form almost a complete sling (
Fig. 6-23).
In the large bowel, their distribution is similar to small bowel, but ICCs are much fewer around the Auerbach’s plexus. In the appendix, they are distributed diffusely throughout both muscle layers (as are the ganglion cells).
Whenever the motility apparatus is being examined, it is worth running normal controls from the same organ for comparison. ICCs are immunoreactive with CD117 (Kit) and DOG1. Increasingly, we use DOG1 in motility disorders as the possibility of misinterpreting mast cell fragments as ICCs is abolished. Although there are (to date) no papers on using DOG1 in this manner, we have found that both antibodies stain ICC virtually identically other than there being no mast cell staining with DOG1. We do not use CD34 as there are too many cells other than ICC that stain.
MOTILITY DISORDERS
The etiology, pathogenesis, and pathology of motility disorders are something of a moving target, such that these are continually evolving and a long-lasting classification becomes difficult to achieve. Simplistically, it is easy to subdivide these into disorders of muscle, ICCs, nerves (intrinsic and extrinsic), and hormonal/neurotransmitter modulation, but there is considerable overlap. For example, some that are inflammatory disorders primarily target nerves but may also cause abnormalities of ICCs. Similarly, mitochondriopathies that are increasingly recognized to play a role in myopathies likely affect many other components of the motility apparatus as well.
The neurogenic disorders may be limited to the GI tract or may be part of a generalized neurologic illness. Similarly, damage to the intestinal musculature may be limited to the GI tract or may be part of a systemic illness, such as scleroderma, polymyositis, or myotonic dystrophy (
Table 6-1). Currently, the most useful classification is the London classification
118 (
Table 6-2), but the multiple components of this exemplify the difficulty in readily classifying many of them. These fall into the same basic groups making up its function, namely, disorders of muscle, nerve, and ICCs. What is less clear is how these relate, as disorders in one are often accompanied by disorders in the others, making it difficult to determine the primary cause. Most of these are rare, but some disorders, such as diverticular disease, are common and readily included within motility disorders, hence included in this chapter. Practically, while it is possible to follow the London
classification, it is easier to discuss the more traditional diseases by anatomical sites of involvement, and we have chosen to do it this way.