This article focuses on recent findings on the molecular mechanisms involved in esophageal columnar metaplasia. Signaling pathways and their downstream targets activate specific transcription factors leading to the expression of columnar and the more specific intestinal-type of genes, which gives rise to Barrett metaplasia. Several animal models have been generated to validate and study these distinct molecular pathways but also to identify the Barrett progenitor cell. Currently, the many aspects involved in the development of esophageal metaplasia that have been elucidated can serve to develop novel molecular therapies to improve treatment or prevent metaplasia. Nevertheless, several key events are still poorly understood and require further investigation.
Key points
- •
At present, the SHH-BMP4/pSMAD pathway, its antagonists, and downstream targets seem to be the important signaling pathways involved in the development of earliest stages of columnar metaplasia.
- •
SHH-BMP4/pSMAD signaling is crucial for the induction of columnar genes leading to the nonintestinal-type of columnar metaplasia.
- •
Expression of the intestine-specific genes as seen in the later stage of the intestinal-type of Barrett metaplasia seems to be mediated by a pSMAD-CDX2 interaction, Wnt, and Notch signaling.
- •
Current molecular findings provide important insight in the development of the columnar and intestinal-type metaplasia, which can be used for developing novel molecular therapies.
- •
With respect to the Barrett cell of origin, there is a need to better characterize the human Barrett progenitor cell to develop appropriate lineage tracing models.
Introduction
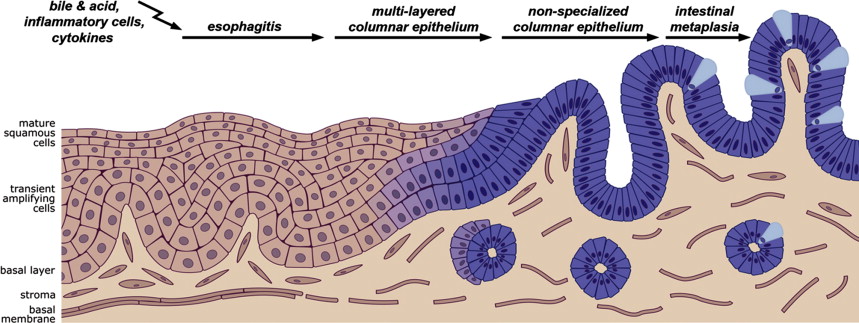
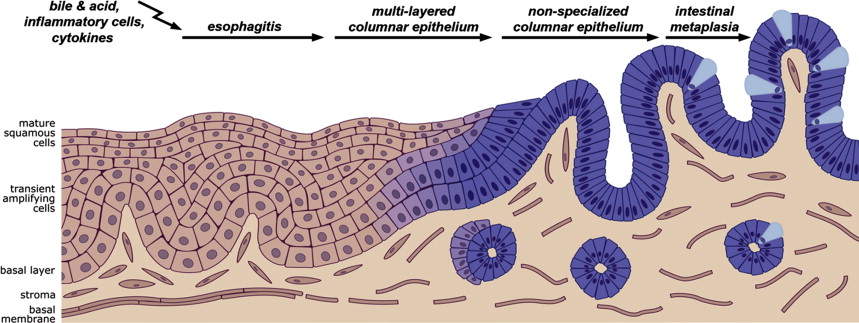
Metaplasia or the dedifferentiation of cells may occur in several organs of the gastrointestinal tract, such as pancreas, stomach, and the esophagus, but also in other locations, such as in lungs, bladder, and cervix. Epithelial metaplasia is caused by constant injury by internal or external factors that give rise to chronic inflammation. In general, metaplastic epithelia are mostly incompletely differentiated, and are often considered to be precancerous lesions. Metaplasia of the distal esophagus is the result of longstanding gastroesophageal reflux disease, in which bile and acid reflux cause chronic inflammation of the esophageal mucosa. In the subsequent process of healing, the epithelium attempts to adapt to its new environment, and hereto may undergo profound phenotypic changes leading to a different type of epithelium that is more resistant to its novel environment. In a surgical animal model, reflux of bile and acid induced the development of nonintestinal and later intestinal metaplasia, supporting the earlier observation in humans that indicated a nonintestinal-type of metaplasia may precede the development of the intestinal-type of metaplasia. Based on observations in human subjects and supported by animal studies and molecular data, the development of the intestinal-type of metaplasia is increasingly recognized as a stepwise process. Within this process, inflamed esophageal squamous mucosa is replaced by a transitional epithelium also referred to as multilayer epithelium followed by single layered nonintestinal-type columnar epithelium and finally by the specialized intestinal-type of columnar metaplasia. This novel concept indicates that the previously defined independent metaplastic phenotypes including the nonintestinal phenotypes, such as junctional/cardia or the gastric/fundic type, and specialized intestinal-type of metaplasia as can be observed in patients with Barrett esophagus are rather related than distinct entities ( Fig. 1 ).
Important progress recently has been made in understanding the underlying molecular mechanisms in the process of Barrett metaplasia. The current hypothesis is that the stepwise development of intestinal metaplasia is based on the upregulation of diverse signaling pathways involving SHH, WNTs, Notch, retinoic acid (RA), and bone morphogenetic protein (BMP), which normally are involved in development and homeostasis of the gut and other organs. Through renewed or upregulated activation, these signaling pathways drive the development of epithelial metaplasia by activating specific transcription factors leading to the expression of columnar and more specific intestinal target genes. Much of the current research has focused on the role of factors that regulate these pathways and their downstream targets.
Introduction
Metaplasia or the dedifferentiation of cells may occur in several organs of the gastrointestinal tract, such as pancreas, stomach, and the esophagus, but also in other locations, such as in lungs, bladder, and cervix. Epithelial metaplasia is caused by constant injury by internal or external factors that give rise to chronic inflammation. In general, metaplastic epithelia are mostly incompletely differentiated, and are often considered to be precancerous lesions. Metaplasia of the distal esophagus is the result of longstanding gastroesophageal reflux disease, in which bile and acid reflux cause chronic inflammation of the esophageal mucosa. In the subsequent process of healing, the epithelium attempts to adapt to its new environment, and hereto may undergo profound phenotypic changes leading to a different type of epithelium that is more resistant to its novel environment. In a surgical animal model, reflux of bile and acid induced the development of nonintestinal and later intestinal metaplasia, supporting the earlier observation in humans that indicated a nonintestinal-type of metaplasia may precede the development of the intestinal-type of metaplasia. Based on observations in human subjects and supported by animal studies and molecular data, the development of the intestinal-type of metaplasia is increasingly recognized as a stepwise process. Within this process, inflamed esophageal squamous mucosa is replaced by a transitional epithelium also referred to as multilayer epithelium followed by single layered nonintestinal-type columnar epithelium and finally by the specialized intestinal-type of columnar metaplasia. This novel concept indicates that the previously defined independent metaplastic phenotypes including the nonintestinal phenotypes, such as junctional/cardia or the gastric/fundic type, and specialized intestinal-type of metaplasia as can be observed in patients with Barrett esophagus are rather related than distinct entities ( Fig. 1 ).
Important progress recently has been made in understanding the underlying molecular mechanisms in the process of Barrett metaplasia. The current hypothesis is that the stepwise development of intestinal metaplasia is based on the upregulation of diverse signaling pathways involving SHH, WNTs, Notch, retinoic acid (RA), and bone morphogenetic protein (BMP), which normally are involved in development and homeostasis of the gut and other organs. Through renewed or upregulated activation, these signaling pathways drive the development of epithelial metaplasia by activating specific transcription factors leading to the expression of columnar and more specific intestinal target genes. Much of the current research has focused on the role of factors that regulate these pathways and their downstream targets.
Molecular pathways involved in the development of columnar and squamous epithelia
SHH and BMPs are highly influential morphogenes in endoderm development. Both proteins are secreted in the notochord of the esophagus during the early embryonic stages. SHH is a secretory protein. Its signaling is mediated by two membrane-bound receptors: patched (PTCH) and smoothened (SMO). Normally, PTCH inhibits the activation of SMO. On binding to SHH, PTCH is internalized and SMO is released. Active SMO mediates intracellular signaling via several molecules that subsequently cause translocation of transcription factors of the GLI family, which in turn regulates the expression of several genes including BMP4. BMP proteins are members of the transforming growth factor-β family. Once BMP proteins bind to their type II receptors, type I receptors (BMPR1A or BMPR1B) are recruited, and transduction of a signal by phosphorylation of the SMAD proteins is initiated giving rise to phosphorylated SMAD (pSMAD). The SMAD proteins can be classified into three subgroups based on their distinct functions. The receptor-regulated SMADS (RSMAD1, 2, 3, 5, and 8), on ligand stimulation, are directly phosphorylated by type I BMP receptors. Once activated, these SMADS associate with Smad4. The heteromeric complex translocates into the nucleus, where it mediates the response to specific ligands. Together with the RUNX cofactors, the complex induces transcription of target genes. Downstream targets are, for instance, ID1 and ID2. The RSMADS 1, 5, and 8 act in BMP pathways, whereas R-SMADS 2 and 3 respond to activin/transforming growth factor-β signaling. The inhibitory SMADS (ISMAD6 and SMAD7) prevent the activation of receptor-regulated SMADS or their heteromerization with SMAD4.
SHH-BMP cell signaling is essential for the development of organs and tissues and their function is highly conserved between species. Development of the esophagus has been most extensively studied in mouse, rat, and chicken embryos. The esophagus develops from the anterior foregut. SHH and BMP4 are critical for the development of the foregut and for the separation of trachea from the esophagus. In the foregut the expression of both molecules rapidly decreases at the time that the trachea and esophagus have separated. Importantly, the action of BMP4 on the development of the foregut seems to be closely regulated by Noggin, a natural antagonist of BMP proteins. It has been shown that ectopic expression of BMP4 in the esophageal foregut inhibits development of squamous epithelium and promotes columnar differentiation. Likewise knocking out Noggin is associated with esophageal defects and the presence of a columnar epithelial lining in embryos. In other transgenic mouse models it has been demonstrated that differentiation of the foregut epithelium toward stratified squamous epithelium is regulated by SHH and the transcription factors SOX2 and p63. Mice lacking SOX2 and p63 expression develop a columnar-type of epithelium in the esophagus. The RA pathway is another pathway that seems to be involved in the development of stratified esophageal epithelium. The effect of RA on cellular differentiation, however, depends on specific receptor activation. This involves the nuclear retinoid acid receptor-alpha and retinoid X receptor-gamma, which are highly expressed in squamous epithelium.
Molecular pathways involved in the development of columnar-type of metaplasia
The current concept of esophageal metaplasia is that chronic (inflammatory) injury caused by bile and acid reflux as seen in gastroesophageal reflux disease has led to the upregulation or renewed expression of the SHH-BMP4 signaling pathway, which changes the epithelial environment in favor of columnar cells or drives cells toward a columnar phenotype. In the microenvironment surrounding the epithelial cells these factors are mostly secreted by stromal cells. The stromal-epithelial interaction of SHH and BMP4 for epithelial remodeling is for instance demonstrated in xenopus. Thus, the cross-talk between epithelium and stroma plays a major role in deciding on cell fate and cell lineage commitment. In adult normal squamous epithelium the expression of SHH and BMP4 is low and confined to the basal layer. In Barrett biopsies SHH and its receptor PTCH are increased. Also BMP4 and its downstream target pSMAD are highly expressed, whereas the expression of Noggin, its natural inhibitor, is low. Of interest it that increased expression of BMP4 and activation of its pathway is indeed observed in the early stage of esophagitis caused by gastroesophageal reflux disease. In vitro studies showed that BMP4 induced a shift in the gene expression profile of squamous cells toward that of columnar cells, which also included an important shift of the cytokeratin (CK) expression pattern. The cytoskeleton of epithelia, mainly consisting of actin and CKs, highly determines the phenotype of cells. Different types of epithelia have different CK expression patterns. In Barrett esophagus, it is known that CKs specific for columnar cells, such as CK7 and CK8, are highly expressed, whereas normal squamous epithelium show high levels of, for instance, CK10/13 and CK14. In an in vivo transplant culture system using esophageal epithelium expressing SHH, BMP4 and SOX9 were found to be upregulated. SOX9 is a transcription factor of columnar-type of genes. In the organotypic model SOX9 was found to induce the expression of CK8 in squamous cells independent of BMP4. In intestinal cells, however, SOX9 was found to be a WNT target and found to repress CDX2 and MUC2 expression. Both, CDX2 and MUC2 are factors associated with a specific intestinal phenotype. In a transgenic mouse model of chronic inflammation, IL-1ß overexpression leads to columnar metaplasia in mice. Columnar metaplasia at the squamocolumnar junction developed after a relatively long period (∼12–15 months) of inflammation. Both, bile acids and carcinogens enhanced the development of metaplasia and dysplasia. Gene expression profiles of the metaplasia in these mice closely resemble gene expression profiles found in human Barrett and the associated cancer. In this model SHH and BMPs were found to be increased in the metaplastic epithelium. Early development of metaplastic columnar glands at the squamocolumnar junction was also demonstrated in a mouse model that overexpressed BMP4 in squamous cells. In these animals the metaplastic glands could be observed at 9 to 12 weeks. In a surgical mouse model in which bile and acid reflux is induced through an esophagojejunostomy, pSMAD and BMP4 were found to be upregulated in the inflamed esophagus and the metaplastic glands.
Molecular factors involved in intestinal differentiation
The intestinal-type of esophageal metaplasia has striking similarities with the specialized intestinal-type of epithelium that can be found in the small and large intestine. Intestinal metaplasia is, for instance, characterized by the presence of periodic acid–Schiff/alcian blue staining goblet cells. SHH and BMP4 are key players in the midgut and hindgut for the early transforming the primordial epithelium of the endoderm into a simple columnar epithelial lining. WNT and Notch signaling subsequently take part in the further differentiation of the intestinal mucosa into crypts and villi, and in the adult small and large intestine these factors are crucial in the homeostasis and crypt renewal. WNT ligands are secreted glycoproteins that bind to cell surface receptors including the transmembrane receptor Frizzled, and the low-density lipoprotein receptor–related proteins 5 and 6 (LRP5/6). WNT signaling may follow the WNT–β-catenin canonical pathway or less defined noncanonical pathways. WNT–β-catenin signaling is essential for growth and differentiation in many tissues, including the intestine. A key regulator of canonical WNT signaling is β-catenin. Activation of WNT signaling inhibits degradation of β-catenin, which in turn translocates into the nucleus. β-Catenin through interaction with TCF4/LEF transcription factors promotes transcription of genes involved in growth and proliferation and genes regulating the expression of intestinal-type of genes.
Notch signaling is also essential for differentiation, proliferation, and the homeostasis of intestinal epithelium. Activated Notch receptor results in release of the Notch intracellular domain, which can translocate into the nucleus, binds to the transcriptional repressor RBP-J kappa, and recruits coactivators to activate transcription of, for instance, the Hairy enhancer of split1 (HES1) and atonal homolog 1 (ATOH1) genes. Notch signaling has a critical role in controlling intestinal cell commitment. The repressor function of the Notch target HES1 and reciprocal function of ATOH1 are critical in the maintenance of stem cell populations and determine the differentiation direction of the intestinal cells.
CDX2 is an intestine-specific homeobox gene known to play a critical role in differentiation and maintenance of intestinal epithelial functions. CDX2 is a homologue of the Drosophila Caudal gene and belongs to the family of homeobox genes (HOM cluster in Drosophila). A common feature of these genes is the possession of a “homeobox” DNA binding motif coding for a consensus sequence of 60 to 63 amino acids that acts as a transcriptional regulator of “downstream” genes. Expression of several CDX genes may be regulated through the WNT signaling pathway. In turn CDX2 in cancer has been found to inhibit WNT signaling.
Molecular signals that drive intestinal metaplasia
Gene expression profiling of intestinal metaplasia showed that expression of several more specific intestinal-type of genes including CK20, Carbonic Anhydrase 1, VILLIN, Mucins (MUC2), sucrase-isomaltase, human defensin, fatty acid binding protein 6, gap junction protein beta 1, trefoil factors, and others is increased. Transcription of these genes is known to be mediated by the caudal-related homeobox gene CDX2 and CDX1. CDX2 is normally not expressed in the normal esophagus or stomach. Several studies demonstrated that nuclear CDX2 and CDX1 expression can be found in the intestinal-type of metaplasia. In vitro it was shown that exposure of a human esophageal squamous cell line to acid and bile upregulated CDX2 expression through promoter demethylation. These studies indicate that development of the specialized intestinal phenotype in esophageal metaplasia is mediated by expression of CDX2. To further investigate the role of CDX2 in the development of columnar metaplasia, a transgenic mouse that overexpressed CDX2 in the mouse esophagus under the cytokeratin 14 ( K14 ) promoter was generated. In this model CDX2 overexpression in squamous cells failed to induce a columnar metaplasia. Also, retroviral transduction of human squamous cells with CDX2 did not cause a columnar or intestinal phenotype. Therefore, these results suggest that CDX2 alone is insufficient to induce columnar metaplasia in squamous cells, unlike the metaplasia of the stomach, where overexpression of CDX2 under the H + /K + ATPase promoter clearly leads to intestinal metaplasia of the stomach. In a surgical mouse model it was shown that CDX2 and MUC2 expression are late events in columnar cells, which already have upregulated BMP4-pSMAD pathway. In vitro studies showed that pSMAD and CDX2 form a functional complex that target the Muc2 promoter. This indicates that CDX2 function for transcription of intestinal-type of genes depends on active BMP-pSMAD signaling. This could explain why transgenic overexpression of CDX2 in the stomach but not in the esophagus leads to intestinal metaplasia, because pSMAD is expressed in the stomach.
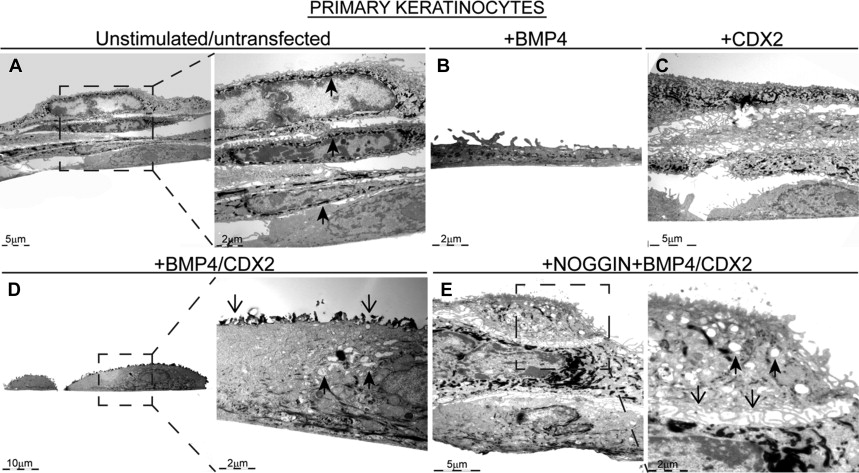
At the ultrastructural level, primary cultured squamous cells stimulated with an activated BMP4-pSMAD pathway and transfected with CDX2 show morphologic changes toward that of columnar cells. Most remarkable is that the cultured keratinocytes lose their multilayered appearance, reorganize into a monolayer, and develop such features as microvilli ( Fig. 2 ).