Mohs micrographic surgery (MMS) has been shown to reduce recurrence rates when used to excise many different mucocutaneous neoplasms, especially of the head and neck. The low recurrence rates are due to careful microscopic evaluation of the horizontal and vertical surgical margins. This article discusses the utility and limitations of MMS in controlling neoplasia of the male genitalia. Specific penoscrotal neoplasias discussed in this article include invasive and in situ squamous cell carcinoma, basal cell carcinoma, extramammary Paget disease, and granular cell tumor.
The inability to adequately assess margins of tumor excision specimens hampers the surgeon’s ability to obtain cure. False-negative histologic findings can result in leaving tumor behind and highlights the importance of careful and complete microscopic visualization of excision margins. Vertical sections through the center of an excision specimen (eg, bread loafing) are adequate for diagnostic purposes but often fall short of providing sufficient visualization of the margins of the excision specimen. It has been calculated that 0.1% or less of the margins is assessed using this approach (Ronald P. Rapini, MD, 2009, personal communication). Other methods of evaluating the surgical margins have been developed, but when applying the concept of surgical margin control to lesions of the skin and mucous membranes, perhaps the most effective is the Mohs surgery technique. Most commonly used for removal of skin cancers of the head and neck region, Mohs surgery also can be used in the eradication of skin cancers of the external genitalia. This article highlights the documented uses of Mohs surgery for treating malignancies of the genitalia and perigenital regions.
Mohs micrographic surgery
While using zinc chloride paste as a medical student involved in research at the University of Wisconsin in the 1930s, Frederic E. Mohs developed the technique that now bears his name. Mohs applied the paste directly to the surface of skin cancers and left it in place for 24 hours. While on the patient’s lesion, the paste acted as a fixative that preserved the histologic features of the cells and tissue architecture. When fixation was complete, he would dissect the fixed tissue sample off the patient and, using a microtome, slice thin sections from the undersurface of the fixed specimen. If tumor was found in these sections, then the process was repeated with application of more paste for another 24-hour fixation period. These horizontal sections cut from the undersurface of the fixed tissue and examination microscopically by the surgeon became the distinguishing features of the Mohs technique. They are the hallmark features of what is now referred to as Mohs micrographic surgery (MMS).
The original zinc chloride paste used by Mohs included 40 g of black granular antimony (stibnite), 10 g of Sanguinaria canadensis (blood root), and 34.5 mL of saturated zinc chloride solution. When compounded, it had a tarlike consistency and color ( Fig. 1 ). Tissue debulking with curettage or a saturated solution of dichloroacetic acid often was performed before applying the zinc chloride paste to aid paste penetration into the skin. The paste caused an intense localized inflammatory reaction where it was applied; therefore “chemosurgery,” as it was called, had significant morbidity associated with it. Drawbacks included severe discomfort for the patient and the fact that it often took several days to clear the tumor. In addition, patients had to wait several more days once the tumor margins were clear to allow the wound to mature before a repair of the defect could be considered.

In 1953, Mohs experimented with the use of fresh-frozen specimens to speed up the process for an educational film he was making on the removal of skin cancers of the eyelids. He noted that microscopic detail was preserved with this approach, and he continued to use this thereafter for removal of tumors in the periorbital area. In 1969, he reported on a series of tumors of the eyelid removed in this manner and noted a 5-year cure rate of 100%. In 1974 and later in 1976, Stegman and Tromovitch published a series of skin cancer removals from head and neck sites in which they used the fresh-frozen method to process tissue margins. The speed at which tissue could be processed allowed the surgeon to perform multiple stages in a matter of hours instead of days, and resultant defects could be repaired immediately following tumor clearance. Consequently, today it is rare to see the chemosurgery approach used by Mohs surgeons.
MMS is most successful when tumors grow in a contiguous fashion. For most mucocutaneous malignancies treated with MMS, visible and palpable tumor is debulked using a curette or scalpel. Then a 2 to 3 mm margin of tissue is excised from around and underneath the debulk defect. The excision most commonly is performed by beveling the incisions toward the center of the debulk defect during removal ( Fig. 2 ). These beveled incisions follow the contour of the debulk defect and usually approach 45°. Margin removal can be accomplished in a more completely horizontal fashion when removing from convex sites. Incisions that parallel the contour of the debulk defect usually result in a bowl- or pancake-shaped excision specimen that can facilitate manipulation of the entire surgical margin into a flat plane for laboratory processing. On occasion, a beveled incision is not possible when harvesting an MMS surgical margin. In these situations, one must provide relaxation cuts on the debulked side of the specimen to facilitate the flattening of the specimen.
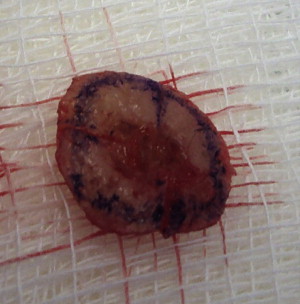
Orientation of the excised margin specimen relative to where it was removed is maintained by marking the margin specimen edge at two or more spots. These marked spots correspond to marks on the patient at the edge of the defect resulting from removal of the margin specimen. Also referred to as hash marks, these marks routinely are made at the 12, 3, 6 and 9 o’clock positions on both the margin specimen and the patient’s defect. The marks on the margin of the specimen are made as short shallow nicks using the scalpel. The patient is marked either with corresponding surgical nicks or an ink mark. The ink used on the margin specimen is applied in a unique fashion that is recorded on a map showing the margin specimen and its relation to the location of removal from the patient ( Figs. 3–5 ). Ink applied to the margin of the specimen can be visualized under microscopic examination, so that when residual tumor is visualized in microscopic sections from the margin specimen, its location is correlated relative to the inked hash marks and then documented on the map. The surgeon then returns to the patient and removes the involved areas with another 2 to 3 mm margin of tissue around and underneath the sites of tumor as noted on the map.
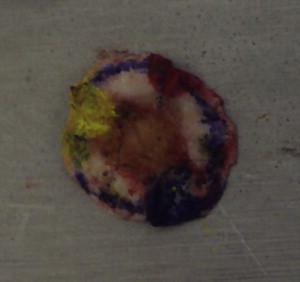
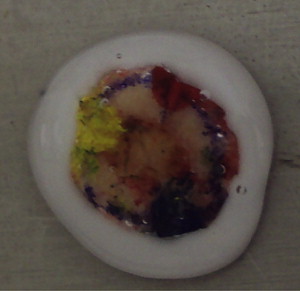

Hematoxylin and eosin or Toluene blue stains are used routinely for all frozen tissue Mohs specimens. Occasionally immunohistochemical stains are used to highlight tumor cells not easily seen on routine stains.
Standard excision for malignancy involving the male genitalia often involves extensive surgical margins resulting in defects that range from extensive loss of skin to total penectomy. Due to the tissue sparing capabilities of MMS many have attempted to apply the use of the technique to treat cutaneous malignancies of the male genitalia and perigenital regions.
Treatment of penile squamous cell carcinoma
Mohs and colleagues reported data on 35 patients with invasive squamous cell carcinoma (SCC) who were treated using the fixed-tissue (chemosurgery) technique over a 50-year period. Five-year follow-up data were available on 31 patients, which revealed a recurrence rate of 26%. Two of the 8 patients with recurrence had local recurrence due to extensive urethral involvement. The remaining six were reported to have occult lymph node metastasis at the time of MMS and died of SCC. In the first case series of MMS using the fixed-tissue technique for invasive penile SCC, Mohs and colleagues reported 0% recurrence in 5 years if the tumor size was less than 1 cm, 17% with tumors 1 to 2 cm, 25% if tumor size was between 2 and 3 cm, and 50% if tumor size was greater than 3 cm.
Brown and colleagues studied 20 patients with penile malignancies treated with Mohs surgery. Eleven patients had invasive SCC; seven had SCC in situ. One had verrucous carcinoma, and one had leiomyosarcoma. They reported a 29% recurrence rate over a mean follow-up period of 3 years. One of the 17 patients had only local recurrence, and four patients developed lymph node involvement, with one patient dying of metastatic disease.
Shindel and colleagues reported on 41 penile cancers in 33 patients treated with MMS. Twenty-five patients were identified, with overall average follow-up data of 58 months. Eight patients developed recurrence (32%), of whom seven were retreated with MMS. The eighth patient had a T3 SCC of the glans and was reported to be tumor-free for 4 years. Initially he was treated with a partial penectomy for recurrent distal T3 disease. Another recurrence 1 year later resulted in the patient receiving a total penectomy. One patient developed inguinal lymph node involvement, and one patient died of metastatic disease. Of the original 41 cases, 10 were invasive SCC; 25 were squamous cell carcinoma in situ. Four cases were verrucous carcinoma; one was epidermoid carcinoma, and one was basal cell carcinoma. The authors concluded their discussion by pointing out that despite a higher recurrence rate in their patients treated with MMS as compared with those receiving traditional surgery, recurrence was managed effectively in most by retreatment with MMS and did not have an adverse effect on overall survival rate or tumor progression. Their patients reported that they were satisfied with the resulting sexual and urinary function following MMS compared with the option of penectomy. The authors report they terminated five MMS procedures due to urethral involvement or size of defect during MMS with continued positive margins. They suggested that very large tumors may not be best treated by MMS, especially if they deeply involve the corpora, urethra, or urethral meatus.
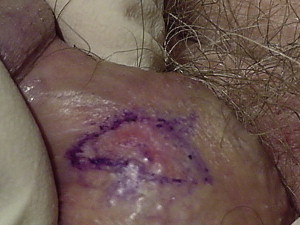
MMS may be most helpful in SCC in situ (SCCIS) of the penis ( Figs. 6–10 ). SCCIS of the penile shaft can be removed easily using MMS with the resultant defect allowed to heal by second intention for smaller defects or with primary closure or local skin flaps. SSCIS of the glans is more difficult because of the possibility of urethral involvement. Therefore for lesions located on the distal glans it is imperative that a careful cystoscopic examination be performed before formulation of the treatment plan. Rare reports of good outcomes using MMS for urethral SCCIS with or without distal urethrectomy have been described. Especially when used alone, MMS is not considered as effective when urethral involvement is present and should be discouraged.