Serum PSA
PSA <15 ng/ml PSA >15 ng/ml should be counselled with caution
Clinical stage
T1c–T2a
Pathology
Gleason score 3 + 3
Gleason score 3 + 4
Life expectancy
>10 years
Clinical stage
Any; except in case of HIFU: <40 mL
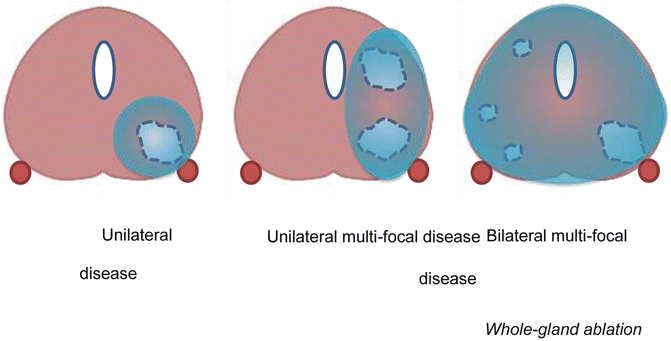
Fig. 10.1
Treatment scenarios
Table 10.2
Overview of techniques with indications, contra-indications, advantages and disadvantages
Indications | Contra-indications | Advantages | Disadvantages | |
|---|---|---|---|---|
Brachytherapy | Stage T1b to T2a | Recent TURP | Outcomes equal to radical approach | Chronic urinary morbidity in 20 % |
Widely available | ||||
Little interruption of daily life | ||||
High IPSS | ||||
Worse flowmetry | ||||
Prostate gland volume >50 mL | ||||
Previous pelvic irradiation | ||||
Cryotherapy | Stage T1 to T3 | Prostate gland volume >40 mL | Real time monitoring with TRUS and MRI | Cold sink effect |
High post-operatively erectile dysfunction (47–100 %) [66] | ||||
Short hospital stay (1–4 days) | ||||
Intraprostatic needles insertion required | ||||
High costs | ||||
Less side-effects than radical prostatectomy | ||||
Unfit for surgery | ||||
Life expectancy <10 years | ||||
HIFU | Stage T1-T2 | Anterior tumor or tumor located near apex or midline | No intraprostatic needles required | Heat sink effect |
Short hospital stay | Time-consuming (10 g prostate/h) | |||
High costs | ||||
Minimal rectal injury | ||||
Radiofrequency ablation | Clinically localized cancer; not further specified | Not described | RFA can be performed with IV sedation in an outpatient setting | Heat sink effect |
Few data about efficacy | ||||
Laser ablation therapy | Clinically localized cancer: not further specified | Not described | MRI-guidance possible | Heat sink effect |
Few data about efficacy | ||||
Erectile function preservation | ||||
Short hospital stay | ||||
Irreversible electroporation | Clinically localized cancer: not further specified | Not described | No heat sink issues | Intraprostatic electrodes required |
No data about efficacy | ||||
Real-time CT/US imaging | ||||
Nerves and vessel-sparing | ||||
Photodynamic therapy | Clinically localized cancer: not further specified | Not described | Photosensitizer possible selective for malign cells | Intraprostatic fibers |
Oxygen-dependency in hypoxic tumors | ||||
Technique only reviewed after failure of radio- or brachytherapy | ||||
Short hospital stay |
10.2 Thermal Ablations
Laser Ablation Therapy
Principles
Laser ablation therapy uses near infrared (NIR) light from a neodymium-yttrium-aluminum-garnet laser. It reaches the tissue of the prostate by laser fibers through a transperineal approach. The technique is based on the photo-thermal effect. This thermal action results from the absorption of NIR light by tissue chromophores, which is converted into heat in a very short time [10]. This effect depends on the intensity of light and the concentration of available tissue chromophores. Temperatures above 60 °C cause rapid coagulative necrosis in the targeted tissue followed by instant cell death. But also at lower hyperthermic temperatures (>42 °C) irreversible cell death is also achieved with prolongation of the procedure [11, 12]. Figure 10.2 shows the mechanisms of the thermal ablation techniques.
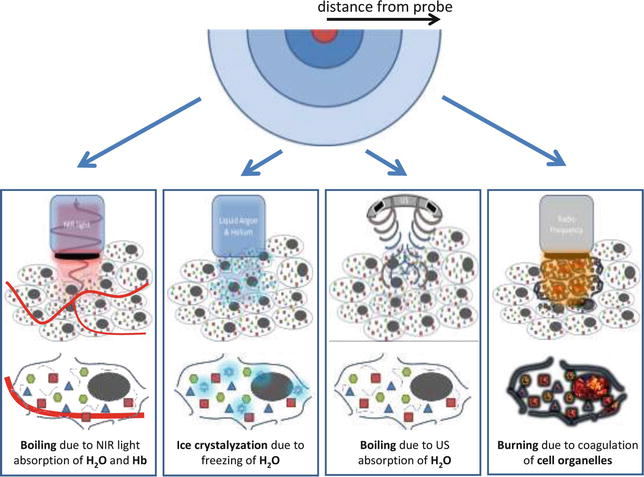

Fig. 10.2
Therapies based on hyper- and hypothermal ablation. From left to right: laser ablation therapy, cryotherapy, HIFU, radiofrequency ablation
Application and Outcome
Besides the destruction of cells by the photo-thermal effect, a reaction can be observed in the reduction of blood perfusion. It is therefore possible to observe the delineation between viable and nonviable tissue with contrast-enhanced ultrasonography (CEUS) [13]. Until now, all studies about laser ablation are phase I clinical trials or contain small cohorts with a maximum of 12 patients [14]. This study of Lindner et al. showed on biopsies after 6 months post-treatment 67 % was free of tumor in the targeted area and 50 % was free of disease. Side effects according to this technique included perineal discomfort, hematospermia, dysuria and fatigue [13–15]. Further research is needed to demonstrate the long-term effectiveness.
Cryotherapy
Principles
Cryotherapy induces cell death by freezing. In the past, urinary incontinence, urethral sloughing and recto-urethral fistula were common side effects and a mortality rate of 1.9 % was reported [16]. More recently, the technique has changed and improved using multiprobe-devices, guided by advanced imaging techniques. Cryotherapy is either used as primary treatment (partial or whole-gland) or as salvage treatment. It contains different mechanisms in destroying tumor tissue, including ‘Freeze rupture,’ a cellular response to freezing, which induces cell death known as necrosis and apoptosis [17]. Direct cell damage occurs when cell metabolism fails as a result of temperature drop. When temperature decreases until −20 °C, extracellular water crystallizes and causes a retraction of water out of the system. This results in a hyperosmotic extracellular environment followed by the extraction of water from the cells and end up in denaturation and electrolyte disturbances [18]. All parts of the freeze-thaw cycle can cause tissue damage. But the coldest tissue temperature is the main factor in generating cell death. It is also important that the cooling rate is as fast as possible. The optimal duration of freezing are unknown, but long lasting freezing increases tissue injury. Thawing rate is a prime destructive cause and it should be as slow as possible, thereby repetition of the freeze-thaw cycle is an important factor in effective therapy [19]. Furthermore, freezing until at least −40 °C is recommended since this causes intracellular ice crystal formation which is a severe threat to cell viability and nearly always lethal [20, 21]. Depending on the multifocality and extension of the tumor, the choice has to be made between whole or partial-gland cryoablation (See Fig. 10.2).
Application and Outcome
Cryotherapy is an option for low-, intermediate-, and high-risk patients [17]. The freezing is obtained by introducing transrectal ultrasound (TRUS) -guided needles using a transperineal approach. Limiting factor is the volume of the gland; the larger the prostate, the more difficult to achieve a uniformly cold temperature by pubic arch interference. Advantage is the ability of real-time visualization of the formed ice-ball by TRUS or Magnetic Resonance Imaging (MRI). Short-term complications are urinary retention because of gland swelling. Penile and scrotal swelling can occur, but are mostly self-limiting. Long-term morbidity differs between partial-gland and whole-gland treatment. A report from the National Cryo On-Line Database (COLD) Registry shows a high percentage of complete urinary continence (98.4 %) [22]. Erectile dysfunction ranged from 49 to 93 % at 1 year [23, 24]. Here for, cryoablation is considered as a treatment option in men who are not concerned with erectile function. Biochemical disease-free survival is diversely ranged along patients-cohorts. The 5-year biochemical disease-free survival rates for low-, intermediate-, and high-risk cases range from 65 to 92, 69 to 89, and 48 to 89 %, respectively [17, 24]. Another study (n = 60) shows biopsy proven recurrence found in up to 23 % of the patients after 15.2 months, mostly found in the untreated hemi-gland [25].
High-Intensity Focused Ultrasound
Principles
High-intensity focused ultrasound (HIFU) has seen several applications in tissue since the 1950s. Madersbacher et al. [26] stated in 1995 the value of HIFU in treating prostate cancer, leading to several clinical studies. HIFU uses focused ultrasound (US) to destroy tissue based on two principles; hyperthermia and cavitation. When the US beam is focused transrectally at a specific depth inside the prostate, the high-energy instigates heats above the denaturation-temperature of proteins inducing cell death. Besides, the US beam can interact with aqueous micro-bubbles in the sonicated area, leading to coagulative necrosis [27–29]. Two companies are providing HIFU devices: Ablatherm and Sonablate (See Fig. 10.2).
Application and Outcome
HIFU is used as both a primary treatment and as a salvage treatment. Best candidates for HIFU are patients with T1c-T3 tumors smaller than 40 mL that are not suitable for radical approach. Contra-indication is the absence or an inaccessible rectum since the technique applies a transrectal approach. Also, major calcifications larger than 1.0 cm have negative influence on treatment [30, 31]. The advantage of this procedure over other focal therapies is the ability to destroy cells over a distance from the US probe without being invasive. Most common complications of HIFU therapy are urinary retention (<1–20 %) caused by edematous prostate tissue, urinary tract infections (1.8–47.9 %) and incontinence (<1–34.3 %). Erectile dysfunction is reported in 20.0–81.6 %, which in less compared to other modalities. The incidence of recto-urethral fistulas (<2 %) has decreased with the improvement of devices and treatment procedures. Most complications are transient or treatable. Less common complications are urethral or bladder neck stenosis, urethral stricture, chronic perineal pain, infravesical obstruction, epididymitis and prostatitis [32, 33]. Cordeiro et al. [32] reviewed the outcome of 31 HIFU studies and stated that negative biopsy rates (mostly taken after 3–6 months) ranged from 35 to 95 %. Percentage of patients with a PSA nadir of 0.5 ng/mL ranged from 61 to 91 %. The 5-year biochemical disease-free rate (according to Phoenix criteria) was 72 %; 84 % for low-risk patients, 64 % for intermediate-risk patients and 45 % for high-risk patients [31].
Radiofrequency Ablation
Principles
This technique uses radiofrequency energy to ablate tissue. Through transperineal needles the monopolar electrodes can be inserted which are able to reach 50 W with a frequency of 460 kHz. It causes an irreversible destruction of tissue by hyperthermia of approximately 100 °C. Hyperthermia occurs by gradually raising the power. For 5 min this heat has to be maintained. This results in coagulative necrosis of the targeted tissue [34]. During this treatment, the urethra and rectum were cooled by cold saline. The procedure has been assessed as feasible, safe and reproducible in prostate cancer [35, 36] (see Fig. 10.2).
Application and Outcome
Radiofrequency ablation has been investigated in two different groups of patients. Firstly in patients with clinically localized prostate cancer; this showed no complications [35, 36]. Shariat et al.[37] treated patients after failed radiation and patients unfit for surgery. This study showed transient side effects as macrohematuria in 19 %, bladder spasms and dysuria in 9 %. At 12 months after RITA, 50 % of patients with sufficient follow-up had no residual cancer on repeat systematic 12-core biopsy cores and 67 % were cancer-free in biopsy cores sampled from the RITA-treated areas. No long-term outcomes have been reported in literature.
10.3 Non-thermal Ablations
Irreversible Electroporation
Principles
Bio-electrics is an interesting new area of medicine combining pulsed high-voltage engineering and cell biology [38–40]. Pulsating current alters the transmembrane potential of biological cells. If the duration of the applied electrical pulses is below the charging time of the outer cell membrane (approximately 100 ns for mammalian cells), there is interaction of the electric field with subcellular structures. Cell survival is inversely proportional to the electric field generated and by manipulating the pulse duration, the electric field intensity, and the number of pulses, it is possible to alter the effects on the target cells. The pulsed electric fields increase the permeability of the cell membrane by a process known as electroporation, a process that can be reversible or irreversible depending on the combination of the variables above [41]. Reversible electroporation temporarily makes the cell membrane more permeable [42]. The cell can survive this insult [43], and it has been employed in electro chemotherapy, to facilitate the uptake of chemotherapeutic agents into cells [42, 44], and gene therapy. Irreversible electroporation results in the permanent permeabilization of the cell membrane, which disrupts cell homeostasis and leads to cell death [42, 43]. In vitro, it has commercial application and has been used by the food industry to sterilize and pre-process food since 1961 [41]; it can also be used to sterilize water, because the process destroys bacteria and yeasts. In vivo, the irreversibly permeabilized cells are left in situ and are removed by the immune system [44] (See Fig. 10.3).
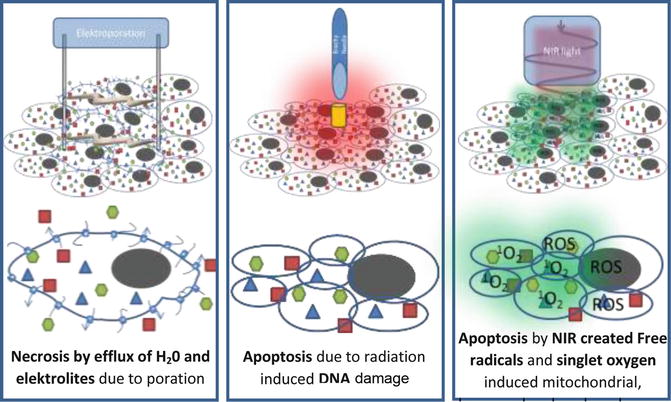

Fig. 10.3
Therapies based on non-thermal ablation method. From left to right: irreversible electroporation, brachytherapy, photodynamic therapy
Applications and Outcome
IRE has been shown to effectively ablate tumor cells in vitro, in small and large animal experiments [43, 45, 46] and in a recent safety study on the IRE of focal liver, kidney and lung tumors [47, 48]. There are two main factors driving research into IRE as a treatment modality. First, tumor ablation experiments in animals and humans have shown that connective tissue structure is preserved and there is no damage to associated blood vessels, neural tissue, or other vital structures [43, 46, 49]. Second, IRE may ablate below the thermal damage threshold of 50 °C and there is no “heat sink” effect, a factor that decreases the effectiveness of other ablation therapies such as RFA near major vessels [44, 50–52]. It is anticipated that the preservation of surrounding tissue will reduce treatment-induced side effects inherent in current prostate cancer therapies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








