Chapter 21 Minimally Invasive Splenectomy for Massive Splenomegaly
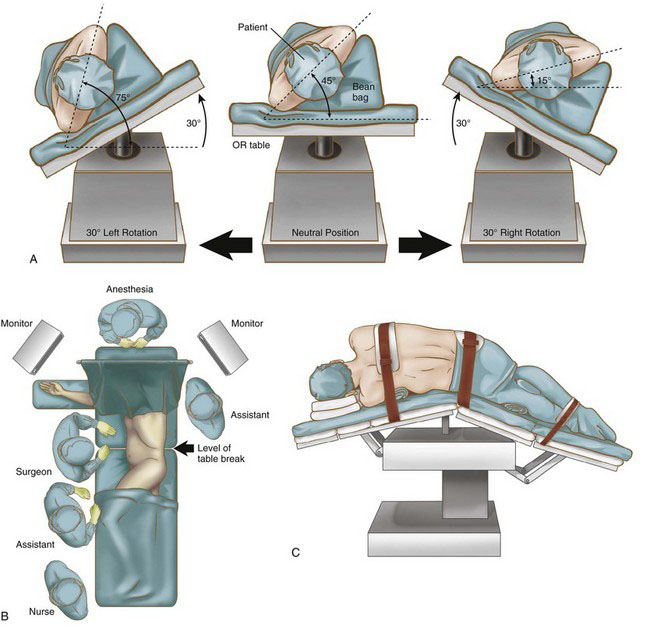
Patient positioning in the operating suite
Laparoscopic splenectomy may be performed using a lateral decubitus, semi-decubitus, or supine approach, depending on surgeon preference, spleen size, patient characteristics, and the need for any concomitant procedures. The supine position provides access to the omental pouch, excellent visualization of the splenic hilum, and access to the rest of the abdomen if a concomitant procedure is planned. The decubitus position can facilitate bowel retraction away from the operative field, but full lateral decubitus alone is not recommended for a massive spleen because it will sag into the right upper quadrant, blocking the view of the hilum and making splenic manipulation extremely difficult. We prefer the semi-decubitus position for a massive spleen because the operating table can then be rotated to place the patient’s body in the supine or decubitus position (or anywhere in between), depending on surgeon need at any given point in the procedure (Fig. 21-1A).
Using a bean bag, foam wedges, or other stabilizing device, the patient is positioned with the left side up at about a 45-degree angle (Fig. 21-1B). Cushions are place under the knees and ankles of both legs. The right arm is extended onto an arm board, and the left arm is elevated and secured in a flexed position over the patient’s head. The patient is secured to the operating table with straps across the chest, hips, and legs to permit extreme rotation. The table is flexed to expand distance between the iliac crest and costal margin, and then reverse Trendelenburg tilt is applied (Fig. 21-1C). The monitors are placed on either side of the patient’s head. The surgeon and the camera operator stand on the patient’s right side, and the first assistant stands on the left, as shown in Figure 21-1B.
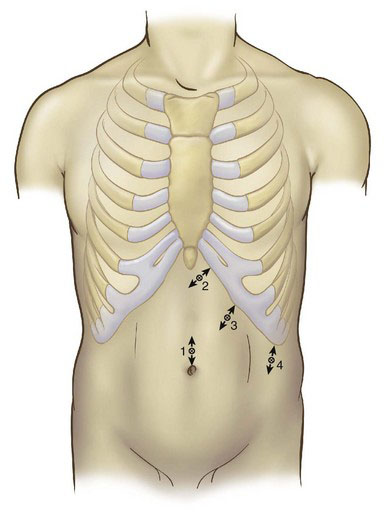
Positioning and placement of trocars
Four ports are used, as shown in Figure 21-2. A 10-mm camera port is inserted at the umbilicus using the open technique. If the patient is large or tall, or if the splenomegaly is moderate, then this initial port may be placed superior to or to the left of the umbilicus, or both. Under laparoscopic guidance, a 5-mm port is placed in the subxiphoid area, a 10-mm port is placed variably under the costal margin along the midclavicular line, and a 10-mm port is placed in the left axillary line, halfway between the costal margin and the iliac crest. The surgeon should adjust port placement to accommodate organ size. In massive splenomegaly, the subcostal ports are positioned 4 cm below the medial and inferior tip of the spleen, parallel to the left costal margin, yet still allowing the instruments to reach the diaphragm. Occasionally the most lateral port cannot be placed below the spleen; in such a case, the port should be placed as low as possible. Infiltration with local anesthesia before insertion of each trocar helps reduce postoperative pain and confirms the precise location of each port.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree