Although the standard approach of retroperitoneal lymphadnectomy (RLA) is open surgery, laparoscopy is an emerging technique in urology and reports of laparoscopic RLA are increasing. This article presents the indications, technique, and outcome of RLA as primary treatment and post–cisplatin-based chemotherapy by means of laparoscopy. In expert hands RLA is minimally invasive and reduces morbidity in comparison with open surgery, with the same oncologic outcome. However, patient selection is crucial.
Key points
- •
In patients with nonseminomatous germ cell tumor (NSGCT), clinical stage I retroperitoneal lymphadenectomy (RLA) is considered the only method that can immediately and reliably identify lymph nodes suspected of metastatic involvement.
- •
In well-defined residual masses smaller than 5 cm, strictly unilateral before chemotherapy, a modified unilateral template does not interfere with oncologic outcome.
- •
Recommendation for minimally invasive RLA and postchemotherapy RLA (PC) can only be given to tertiary centers with experience in laparoscopy and managing testicular cancer. No recommendation can be given for laparoscopic bilateral PC-RLA because of the lack of available data.
Introduction
Testicular cancer typically occurs in men between 15 and 35 years of age. The incidence is low, with 3 to 10 new cases per 100,000 men per year in the Western world. In total, testicular cancer represents 5% of urologic tumors and only 2% of all human malignancies. On the other hand, the incidence of germ cell tumors (GCTs) has been rising over the last 30 years in industrialized countries. GCTs are divided in 2 major groups, seminomatous germ cell tumors (SGCTs) and nonseminomatous germ cell tumors (NSGCTs), consisting of teratoma, embryonal carcinoma, yolk sac tumor, and choriocarcinoma.
Testicular cancer is eminently treatable with radiotherapy and chemotherapy, with excellent cure rates even in advanced cases provided that correct staging, early adequate treatment, and strict follow-up is carried out. If indicated, RCA in clinical stage I (CS I) or postchemotherapy (PC) is done as an open procedure at most centers. Nowadays, with the increased routine use of laparoscopy, the minimally invasive approach is gaining more interest. The first laparoscopic RLA was described for clinical stage I cancer in 1995 by Janetschek and colleagues, who reported a case of laparoscopic PC-RLA in a patient with a left-sided stage IIb tumor. The operation was successful without major complications.
Introduction
Testicular cancer typically occurs in men between 15 and 35 years of age. The incidence is low, with 3 to 10 new cases per 100,000 men per year in the Western world. In total, testicular cancer represents 5% of urologic tumors and only 2% of all human malignancies. On the other hand, the incidence of germ cell tumors (GCTs) has been rising over the last 30 years in industrialized countries. GCTs are divided in 2 major groups, seminomatous germ cell tumors (SGCTs) and nonseminomatous germ cell tumors (NSGCTs), consisting of teratoma, embryonal carcinoma, yolk sac tumor, and choriocarcinoma.
Testicular cancer is eminently treatable with radiotherapy and chemotherapy, with excellent cure rates even in advanced cases provided that correct staging, early adequate treatment, and strict follow-up is carried out. If indicated, RCA in clinical stage I (CS I) or postchemotherapy (PC) is done as an open procedure at most centers. Nowadays, with the increased routine use of laparoscopy, the minimally invasive approach is gaining more interest. The first laparoscopic RLA was described for clinical stage I cancer in 1995 by Janetschek and colleagues, who reported a case of laparoscopic PC-RLA in a patient with a left-sided stage IIb tumor. The operation was successful without major complications.
Indications for retroperitoneal lymphadenectomy
Clinical Stage I Nonseminomatous Germ Cell Tumor
CS I NSGCT is defined as tumor involvement that is limited to the testis, normal serum tumor markers after inguinal orchiectomy, and no retroperitoneal lymph node metastasis. However, up to 30% of these patients have occult metastases and will relapse if only surveillance is chosen after orchiectomy. In large studies with high patient numbers, 80% of relapses occur during the first year of follow-up.
Several studies have shown that vascular invasion of the tumor is a reproducible risk factor for relapse in CS I. For this reason, the European Association of Urology (EAU) and the European Germ Cell Cancer Consensus Group (EGCCCG) recommend risk-adapted treatment in their guidelines. For patients with vascular invasion, PEB (cisplatin/etoposide/bleomycin) chemotherapy is the treatment of choice, and for those without vascular invasion surveillance should be chosen.
RLA in CS I patients is preserved for those who are not suitable for chemotherapy or surveillance in the risk-adapted treatment according to EAU guidelines. Also, the EGCCCG suggest RLA only for patients who do not agree to or qualify for the aforementioned options. These recommendations are in contrast to those in the United States. The National Cancer Institute suggests postorchiectomy surveillance as the standard treatment option for CS I with no vascular invasion. On the other hand, RLA is considered the only method that can immediately and reliably identify lymph nodes suspected of metastatic involvement without the potential for false-positive results. If no retroperitoneal metastases are found, only 10% of these patients will relapse, and therefore no further treatment is needed.
In the guidelines of the National Comprehensive Cancer Network (NCCN), RLA-CS I has to be done within 4 weeks after imaging, with normal tumor markers not older than 7 days. This strict regime is chosen to ensure correct clinical staging. RLA in NSGCT CS I has proved to be the most sensitive and specific method for testicular cancer staging in helping to choose the best treatment option for the patient. In 30% of patients testicular metastases are found, which leads to an upgrading to stage II disease.
Clinical Stage IIa Nonseminomatous Germ Cell Tumor Without Elevated Tumor Marker: S0
In all advanced stages of NSGCT, initial cisplatin-based chemotherapy is the standard of care; this is one of the few treatment recommendations to reach general consensus in all guidelines. Only in patients with very small lymph nodes (<2 cm) and negative tumor markers does the EAU suggest surveillance for 6 weeks, at which point a computed tomography (CT) scan should be repeated to clarify whether the lesion is stable, shrinking, or growing. A shrinking lesion is due to a nonmalignant origin, so no further treatment is needed. A stable or progressive lymph node without marker evaluation indicates teratoma or tumor. In this case, nonseminomatous RLA should be performed. On the other hand, in patients with simultaneous increase of tumor markers surgery is obsolete. These patients require PEB chemotherapy according to the International Germ Cell Cancer Cooperative Group (IGCCCG) treatment algorithm.
Indications for postchemotherapy retroperitoneal lymphadenectomy
Postchemotherapy Retroperitoneal Lymphadenectomy in Advanced Seminomas
A residual tumor after cisplatin-based chemotherapy in advanced stages of seminoma should not be primarily resected, irrespective of the size. A residual tumor smaller than 3 cm almost never contains viable cancer. Therefore, PC-RLA is not indicated in these cases. After cisplatin-based chemotherapy in advanced seminoma, viable cancer was observed histologically only in 12% to 30% of patients after PC-RLA, even though the residual tumor was larger than 3 cm. Hence PC-RLA is an overtreatment in about 80% of patients, harming them with needless morbidity.
To solve this problem, 18 F-labeled fluorodeoxyglucose PET (FDG-PET) scanning was included in the EAU guidelines in 2005, based on the SEMPET trial. FDG-PET was affiliated into the EAU guidelines for patients with residual tumors larger than 3 cm to clarify viability. However, the scan must not be performed before 6 weeks after the last course of chemotherapy to reduce false-positive rates. After this period, false positives are rare on FDG-PET. In patients with a positive FDG-PET scan, PC-RLA is indicated.
FDG-PET is an option in patients with residual tumors smaller than 3 cm according to EAU guidelines. No further treatment besides observation is necessary in patients with a negative FDG-PET scan.
Postchemotherapy Retroperitoneal Lymphadenectomy in Advanced Nonseminomatous Germ Cell Tumor
Overall, only 10% of residual masses contain viable cancer following PEB induction chemotherapy in NSGCT; 50% contain mature teratoma, and 40% contain necrotic-fibrotic tissue. Even today no imaging investigation, including PET, or prognosis model can predict histologic differentiation of NSGCT residual tumor. Therefore in the case of any visible residual mass and marker normalization, surgical resection is indicated in accordance with EAU guidelines.
However, there is still an increased risk of viable cancer or teratoma in patients with residual tumor smaller than 1 cm. Mature teratoma was found in up to 22% and viable cancer in 9.4%. In cases with teratoma in the primary orchiectomy specimen, these numbers increase to 41% and 16%. Therefore, PC-RLA for residual tumors smaller than 1 cm may be considered for patients with teratoma in their primary histology. However, PC-RLA in this setting is controversial.
This controversy begins with the critical size of lymph nodes in the retroperitoneum. Today there is no clear-cut consensus on nodal size. Therefore, it is mandatory that an expert surgeon reviews the prechemotherapy and postchemotherapy imaging to determine the extent and size of residual disease in relation to the initial tumor extension. In contrast to the aforementioned increased risk in small residual tumors, the EGCCCG and Ehrlich and colleagues from Indiana University recommended surveillance of patients with residual tumors smaller than 1 cm. Ehrlich and colleagues published data from 141 patients, and found relapse in only 12 patients (9%) after a long median follow-up of 15.5 years. Eight patients currently have no evidence of disease. However, 4 patients died of testicular cancer. The estimated 15-year recurrence-free survival (RFS) and cancer-specific survival (CSS) rates were 90% and 97%, respectively. The initial IGCCCG risk classification was found to be the only predictor of relapse and CSS.
Technique
Template
The anatomic extent of RLA has been under consideration for many years. The traditional approach was to perform a full bilateral template dissection. In the 1980s this approach was the choice for patients with high-volume disease. However, the bilateral template leads to damage of the sympathetic trunks, hypogastric plexus, and postganglionic efferent nerves. To reduce morbidity, a modified unilateral template dissection was developed.
A small residual tumor is the best indication for laparoscopic RLA because the dissection can be restricted to a unilateral template. Such a template can be chosen because the primary landing site of lymph node metastasis of a testicular cancer is unilateral. The primary tumor site of the testicle does not make a difference. Therefore, Weissbach and colleagues defined the extent of surgery in a unilateral template for both sides. Studies have also shown that the primary landing site is always ventral to the lumbar vessels. Regarding the extent of retroperitoneal metastasis, the tumor spreads to the contralateral side or behind the aorta and vena cava.
To clarify the best indication for a unilateral template, several investigators published data comparing unilateral templates with bilateral surgery. The Cologne Study Group assessed 152 patients who underwent PC-RLA. A modified template was chosen for residual tumors smaller than 5 cm in 98 patients. A full bilateral template resection was done in 54 patients with larger lesions. Eight recurrences (5.2%) were observed after a mean follow-up of 39 months. All but one recurrence were outside of a bilateral template. Hence the investigators did not find a correlation with the extent of surgery. In 2014, Vallier and colleagues validated these selection criteria in a prospective study.
Bilateral nerve-sparing PC-RLA can also be done safely by means of laparoscopy, as reported by Steiner and colleagues. The surgery was successfully finished in all 19 of their patients and no conversion was necessary, and there was no recurrence after a mean follow-up of 17.2 months. Another study compared the unilateral template with a bilateral template in PC-RLA. All operations were done laparoscopically, and 19 patients with a unilateral template were compared with 20 patients with a bilateral resection. The investigators did not find any difference in oncologic outcome and, surprisingly, morbidity.
However, selection for a unilateral template is crucial. Not only the size of the residual tumor is important; it is also important to focus on tumor size and unilaterality before chemotherapy. A safe but too restrictive approach was chosen by some investigators, who decided to include only CS IIb (tumors <5 cm before chemotherapy) in unilateral template dissection.
In the United States the unilateral left template often includes the interaortocaval space, in contrast to the template described by Weissbach and colleagues ; this leads to damage of both sympathetic nerves ( Fig. 1 ). Donohue and coleagues first described nerve sparing using such a large left template. However, more recently they changed their template in terms of sparing the interaortocaval space. Hence, nerve sparing was provided by the template without compromising oncologic outcome.
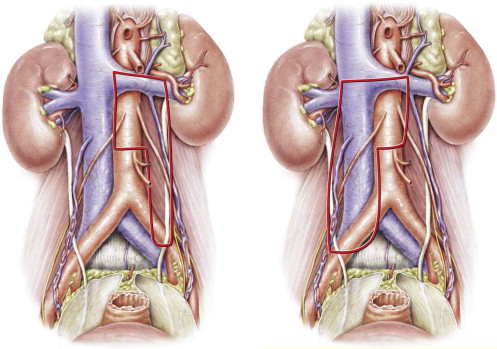
The data presented indicate that it is not always necessary to choose a full bilateral template. In well-defined residual masses smaller than 5 cm and strictly unilateral before chemotherapy, a modified unilateral template does not interfere with oncologic outcome. Moreover, full bilateral PC-RLA can be done laparoscopically, although it should be mentioned that in this procedure the position of the patient has to be changed with consequent loss of the advantages of laparoscopy.
Approach
Laparoscopic Transperitoneal
Most published data concerns a transperitoneal approach with the patient in lateral decubitus position. This approach provides exposure comparable with open surgery. A wide dissection and complete displacement of the bowel is crucial to obtain good exposure of the retroperitoneum. There is no difference between RLA and PC-RLA in terms of exposure. On the other hand, laparoscopic PC-RLA is one of the most technically challenging procedures because of the fibrosis and desmoplastic reaction caused by the chemotherapy, especially in seminoma where severe fibrosis can be observed. However, less desmoplastic reaction is found in residual tumors caused by teratoma.
Laparoscopic Extraperitoneal
The first data on the laparoscopic extraperitoneal approach were published by LeBlanc and coleagues in 2001, in which the approach was used in CS I patients. Nowadays it is also used for PC-RLA by several groups. In contrast to the transperitoneal approach, the patient is in supine position. The surgical team is located on the ipsilateral side. Just as in retroscopic nephrectomy, a sufficient extraperitoneal space is developed by blunt dissection and insufflation, or a distending balloon is used. However, a major concern is that in contrast to a transperitoneal approach, chylous ascites in extraperitoneal RLA will result in large lymphoceles.
Follow-up
There is no consensus on the exact protocol for follow-up. All follow-up recommendations suggest tumor markers and CT scans as the methods of choice. The recurrence rate after RLA in CS I NSGCT patients is low. Therefore the EAU, in contrast to surveillance policy in CS I, recommend only 1 CT scan per year to avoid unnecessary radiation, increasing the risk of a radiation-induced second cancer. Of course, CT could be replaced by MRI to reduce radiation depending on national and institutional facilities.
Certainly CSS in advanced GCT correlates with the extent of disease and the primary response to therapy. Also, the primary objective of follow-up is to detect relapse in these patients as soon as possible. Therefore, CT scans are recommended twice a year in the first 2 years. Tumor markers should be determined every 3 months.
Outcomes
Complications
To summarize complications arising from minimally invasive RLA-CSI or PC-RLA is difficult because the first reported data were published 20 years ago, so complications are not classified according to the Clavien system in every publication. Certainly complication rates are decreasing with experience. Overall complication rates were described in early series in up to 46.7% of RLA-CS I and 57.1% of PC-RLA procedures. These numbers are decreasing in later series, to 15.6% on average.
In RLA CS I, vascular injury and hemorrhage were the most commonly reported complications. Clavien grade IV complications were described rarely. In PC-RLA, however, injury to the renal artery requiring vascular bypass or nephrectomy, duodenal perforation, intestinal segment resection because of intestinal lesion, and transection of the iliac artery have been described. Conversion to open surgery should not been seen as a complication per se; furthermore, it could be due to wrong patient selection. The highest conversion rate was described by Rassweiler and colleagues in 7 of 9 patients. In recent series only 1.1% for RLA and not a single conversion for PL-RLA were described.
A frequent postoperative finding is chylous ascites. In most of these cases, conservative treatment with a low-fat or medium-chain triglyceride diet is effective. As mentioned earlier, the transperitoneal approach should prevent lymphocele formation. However, 2 symptomatic lymphoceles requiring drainage after PC-RLA have been described. By contrast, for the retroperitoneal approach lymphorrhea rates of 30%–40% have been reported.
The sympathetic nerves are crucial for antegrade ejaculation. On full bilateral dissection, these nerves are destroyed. Therefore, nerve-sparing techniques are mandatory to preserve antegrade ejaculation. Steiner and colleagues have published data showing that nerve sparing is also feasible in laparoscopy. Using the modified template for unilateral RLA, nerve sparing is provided by the template as mentioned earlier ( Tables 1 and 2 ).




