Minimally Invasive Nephroureterectomy
ALON Z. WEIZER
JEFFREY S. MONTGOMERY
EPIDEMIOLOGY
Upper tract urothelial carcinoma (UTUC) is a rare entity representing approximately 5% of all urothelial carcinomas (1). Based on Surveillance, Epidemiology, and End Results (SEER) data from 1973 to 2005, the incidence of UTUC has increased from 1.88 to 2.06 cases per 100,000 person-years. This includes an increase in ureteral tumors from 0.69 to 0.91 cases per 100,000 person-years and a slight decrease in renal pelvic tumors from 1.19 to 1.15 cases per 100,000 person-years over this time period. The increased incidence is associated with an increase in the median age at diagnosis (from 68 to 73 years old) and an increase detection of in situ disease without dramatic increases in regional or distant disease (2). Despite this, the literature suggests that approximately 60% of UTUC are invasive at the time of diagnosis (3). Roughly 2% to 5% of patients have bilateral UTUC on presentation, and up to 4% of patients will develop a contralateral UTUC at some point in their course. Although the incidence of UTUC has increased, other studies have suggested that survival has not improved. Eylert et al. (4) combined multiple data sets in England from 1985 to 2010 and found that an increased number of patients were diagnosed at more advanced stages with a decrease in 5-year overall survival from 60% to 48% over the study period.
Explanations for the increased incidence of UTUC include increased survival among patients with bladder cancer who are at risk of developing metachronous UTUC (incidence in bladder cancer patients is 0.75% to 6.4% after cystectomy) (5), the increased use of intravesical therapy especially in the setting of carcinoma in situ, and improved detection with current crosssectional imaging and endoscopic technology. However, because there has been little change in the management of UTUC (nephroureterectomy remains the mainstay of treatment with
limited data on the use of perioperative chemotherapy), the increased incidence has not been associated with a decline in mortality.
limited data on the use of perioperative chemotherapy), the increased incidence has not been associated with a decline in mortality.
RISK FACTORS
Patients who develop UTUC share many of the same characteristics as those who develop bladder cancer. These include exposure to tobacco (6) and environmental/occupational exposures including aromatic amines (7). Several specific risk factors include chronic inflammation caused by nephrolithiasis or recurrent ascending urinary tract infections. Congenital or acquired obstruction (as seen in patients with chronic nephrolithiasis and ureteral stricture) may increase the risk of developing UTUC. Due to the rarity of the disease, it is unclear whether congenital abnormalities of the upper urinary tract such as ureteral duplication or ectopic kidneys predispose to UTUC. An increased incidence of UTUC in parts of Taiwan has been associated with blackfoot disease and exposure to arsenic in drinking water (7). Aristolochia fangchi and Aristolochia clematis contain aristolochic acid, which is found in certain Chinese herbal preparations (8) and is also endemic to the Balkans. This has been associated with UTUC and may play a role in the development of Balkan nephropathy (9). Finally, patients with hereditary nonpolyposis colon cancer (HNPCC) have an increased risk of UTUC and bladder cancer; this population requires screening for urothelial carcinoma (10).
DIAGNOSIS
The diagnosis of UTUC can be challenging. In patients without a prior history of bladder cancer, signs and symptoms typical of UTUC, including hematuria, flank pain, fever/infection, and hydronephrosis, can mimic nephrolithiasis. This becomes more confusing when patients present with a stone due to urine stasis caused by an obstructing tumor. Because the standard imaging for stone disease is a noncontrast computerized tomography (CT) scan, an UTUC may not be apparent unless it is causing a contour deformity of the kidney or the ureter. In addition, a calcified tumor may be interpreted as a stone, and if treated with shock wave lithotripsy as opposed to endoscopic management, a delay in diagnosis occurs.
In the absence of symptoms, hematuria should be evaluated according to the current American Urological Association hematuria guidelines with a CT urogram, cystoscopy, and urine cytology (11). Even in the setting of symptoms suggestive of nephrolithiasis, consideration should be made for obtaining a urine cytology, especially if the patient has risk factors for bladder cancer (although nephrolithiasis can cause cytologic atypia). Pursuit of cross-sectional imaging with a contrastenhanced excretory phase or endoscopic visualization should be considered if symptoms persist. Finally, recalcitrant irritative voiding symptoms presumed to be caused by a urinary tract infection treated with a course of culture-directed antibiotics warrant further evaluation for urothelial carcinoma with cystoscopy, upper tract imaging, and cytology. For patients who cannot undergo CT with contrast due to compromised renal function, magnetic resonance (MR) urogram can be considered if their estimated glomerular filtration rate (eGFR) is ≥30 mL per minute. For those with eGFR <30 mL per minute, magnetic resonance imaging (MRI) of the abdomen/pelvis without gadolinium, noncontrast CT scan, or renal ultrasound can be informative but should be coupled with retrograde pyelograms and possible ureteroscopy to complete a full evaluation of the urinary tract.
Several options for diagnosis exist if a patient has a filling defect in the upper urinary tract or positive urine cytology without visible signs of disease in the bladder on cystoscopy. We believe that a filling defect on imaging studies with a positive selective cytology from that upper urinary tract is adequate to make the diagnosis of UTUC. Ureteroscopy with biopsy can still be performed but may not be necessary in the setting of a large filling defect and positive selective cytology. Alternatively, image-guided percutaneous biopsy can be considered for bulky upper tract masses. Although some contend that tumor seeding of the biopsy tract is a possibility, there may be a risk of lymphatic dissemination of tumor due to use of pressurized irrigation with ureteroscopy (12). We have not seen tumor tract seeding when employing a biopsy sheath during percutaneous biopsy. For patients with a positive cytology but without visible signs of disease, random bladder and prostatic urethral biopsies should be obtained in addition to ureteroscopy with ureteral barbotage cytology to rule out the possibility of occult disease in these locations. Use of ureteral brushes or other biopsy tools are helpful in obtaining an adequate cytologic or tissue diagnosis in the upper urinary tract.
Patients with UTUC should undergo additional staging to assess for metastatic disease. This includes a chest X-ray or chest CT, complete blood count, and comprehensive metabolic panel (including liver function tests and an alkaline phosphatase). These can help guide the need for additional imaging including nuclear medicine bone scan to assess for bony metastasis and chest CT if the chest X-ray is concerning. Symptoms of bone pain may also guide the use of a bone scan, regional plain films, or selective MRI or CT depending on location.
Patients with a prior history of bladder cancer warrant special consideration. Microscopic or gross hematuria, persistent urinary symptoms, or abnormalities on urinalysis despite treatment for urinary tract infection warrant additional evaluation. Urine cytology should be part of standard surveillance for patients with a history of bladder cancer. For patients with an intact bladder and prior endoscopic and/or intravesical bladder therapy, upper tract imaging should be performed prior to any biopsy. In situations with no obvious bladder recurrence, endoscopic evaluation should include bladder and prostatic urethral biopsies as well as retrograde pyelograms, selective upper tract cytology, and ureteroscopy. Cystectomy patients, especially with a history of carcinoma in situ, should undergo a CT or an MR urogram if possible. If this is not possible due to poor renal function, MRI of the abdomen and pelvis, loopogram (for ileal conduit), or cystogram (for neobladder) may help identify an upper tract lesion. Upper urinary tracts not accessible by retrograde ureteroscopy may warrant a percutaneous antegrade approach to identify disease.
ALTERNATIVE THERAPY
Nephroureterectomy is considered the standard treatment for UTUC. However, there are several patient and disease factors that obligate alternative or interim approaches. There is a large percentage of UTUC patients with compromised renal
function (13), and nephroureterectomy may necessitate dialysis. Renal function may be compromised by medical renal disease related to smoking, hypertension, diabetes, or cardiovascular disease or may be due to prior surgical intervention for upper tract disease. In addition, the disease itself may influence initial treatment strategies. Because of the difficulty in accurately assessing the stage (i.e., depth of invasion) of UTUC, grade is used to assess the potential aggressiveness of the disease. Patients with low-grade focal tumors of the renal pelvis or ureter may be managed with endoscopic approaches. Ureteroscopy is often the most straightforward option. There are various tools that can be used for both resection and ablation of these lesions. In order to get adequate tissue for diagnosis, we tend to use an access sheath and backload the BIGopsy device (Cook, Bloomington, Indiana). This is especially useful for proximal ureteral and renal pelvis tumors. For more distal tumors, a semirigid ureteroscope is used with a biopsy forceps. We often combine biopsy with ureteral wash cytology, and for flat lesions, ureteral brushings. Both a holmium laser as well as ureteroscopic Bugbee electrode (2Fr) can be used for lesion ablation. For larger low-grade collecting system tumors, percutaneous resection can be considered because the risk of tumor seeding is low with these tumors. Access is achieved in the identical fashion to percutaneous nephrolithotomy. For lower and interpolar lesions, direct access to the tumor with the use of a resectoscope can allow for complete endoscopic removal and hemostasis. However, occasionally due to angulation and narrow calyceal diameter, the tumor cannot be accessed with a resectoscope and a flexible nephroscope with biopsy forceps, laser, or Bugbee are needed to manage the lesion. In patients with previous urinary diversion, antegrade percutaneous renal access may be necessary if retrograde access is not possible.
function (13), and nephroureterectomy may necessitate dialysis. Renal function may be compromised by medical renal disease related to smoking, hypertension, diabetes, or cardiovascular disease or may be due to prior surgical intervention for upper tract disease. In addition, the disease itself may influence initial treatment strategies. Because of the difficulty in accurately assessing the stage (i.e., depth of invasion) of UTUC, grade is used to assess the potential aggressiveness of the disease. Patients with low-grade focal tumors of the renal pelvis or ureter may be managed with endoscopic approaches. Ureteroscopy is often the most straightforward option. There are various tools that can be used for both resection and ablation of these lesions. In order to get adequate tissue for diagnosis, we tend to use an access sheath and backload the BIGopsy device (Cook, Bloomington, Indiana). This is especially useful for proximal ureteral and renal pelvis tumors. For more distal tumors, a semirigid ureteroscope is used with a biopsy forceps. We often combine biopsy with ureteral wash cytology, and for flat lesions, ureteral brushings. Both a holmium laser as well as ureteroscopic Bugbee electrode (2Fr) can be used for lesion ablation. For larger low-grade collecting system tumors, percutaneous resection can be considered because the risk of tumor seeding is low with these tumors. Access is achieved in the identical fashion to percutaneous nephrolithotomy. For lower and interpolar lesions, direct access to the tumor with the use of a resectoscope can allow for complete endoscopic removal and hemostasis. However, occasionally due to angulation and narrow calyceal diameter, the tumor cannot be accessed with a resectoscope and a flexible nephroscope with biopsy forceps, laser, or Bugbee are needed to manage the lesion. In patients with previous urinary diversion, antegrade percutaneous renal access may be necessary if retrograde access is not possible.
Patients with focal but high-grade tumors may benefit from endoscopic therapy in addition to adjuvant intraurinary chemo/immunotherapy to reduce the risk of recurrence and progression. Although several approaches have been described, we believe that placement of a nephrostomy tube and antegrade instillation is the most reliable approach. Other options include placement of an indwelling ureteral stent with intravesical instillation (relying that the medication will reflux into the upper tract) or retrograde placement of an externalized ureteral catheter prior to each treatment with instillation of the medication via this catheter. This strategy is both cumbersome and time-consuming for the urologist and the patient. Similar to high-grade, non-muscle-invasive bladder cancer, the preferred intraurinary treatment for high-grade papillary tumors and carcinoma in situ upper tract disease is bacille Calmette-Guérin (BCG). Approximately, 30% of patients recur following upper tract instillation with a median follow-up of >20 months in a review by Carmignani et al. (14). Options for second-line therapy for those who fail an initial induction course of BCG include a second induction course of BCG (+/- interferon) or instilled chemotherapy (e.g., mitomycin, gemcitabine, docetaxel). Managing high-grade disease conservatively, however, carries risk of incomplete treatment and disease progression and therefore should be driven by patient life expectancy, extent of disease, renal function, and potential additional morbidity of nephroureterectomy. The risk of recurrence is high (as much as 80%) making nephroureterectomy the preferred treatment option for patients with high-grade disease (14).
When endoscopic management is performed for UTUC, a strategy of surveillance with endoscopy is necessary to evaluate for recurrence and metastases. Patients should undergo three-dimensional cross-sectional imaging at least yearly to evaluate for possible metastases. Patients managed conservatively at first do not appear to have worse outcomes when compared to patients managed with nephroureterectomy initially (15).
Patients with bilateral high-grade upper tract disease present a unique challenge and often require bilateral nephroureterectomy. Although recent data suggests that patients requiring dialysis for postsurgical indications have better outcomes than those requiring dialysis for medical renal disease, the substantial effect that dialysis has on patient quality of life needs to be considered (16). Also, with a history of UTUC, patients must be free of disease recurrence for at least 2 years prior to being listed for renal transplant.
Lesions of the distal or midureter may be managed by segmental resection with data suggesting similar cancer control to nephroureterectomy (17). Details of this approach are discussed elsewhere in this book. However, we have found that robotic-assisted approaches are effective with good outcomes for patients undergoing distal ureterectomy, psoas hitch, and/or Boari flap reconstruction with the ability to remove as much as two-thirds of the ureter. In addition, lymphadenectomy can be performed at the time of the procedure. In patients with bulky or locally advanced disease, neoadjuvant chemotherapy should be considered as the presence of both kidneys and preserved renal function allows for optimal chemotherapy dosing (18). However, there is currently no prospective, randomized evidence to support this approach. The same cisplatin-based regimens that are used for invasive bladder cancer are typically employed in this setting.
Finally, in those patients who have unresectable disease or are poor surgical candidates, palliative management should be driven by patient goals and symptoms. Early referral to specialists in palliative care is important to avoid unnecessary interventions. The most common symptoms seen in patients with locally advanced disease are pain and hematuria, with or without clot retention and the need for blood transfusion. Palliative radiation can effectively manage pain and gross hematuria caused by unresectable UTUC in select patients. Given the extensive vascular network supplying the renal pelvis and ureter, renal angioembolization often fails to control hematuria arising from UTUC.
INDICATIONS FOR SURGERY
After considering the treatment alternatives presented earlier, patients with localized high-grade Ta-T4 urothelial carcinoma are suitable candidates for nephroureterectomy. The decision on how best to approach nephroureterectomy depends on the extent of the disease and the skillset of the surgeon. There are no absolute indications for open versus a minimally invasive (i.e., laparoscopic, hand-assisted, or robotic) approach. However, there are several clinical circumstances that can help guide this decision. Most of the published literature indicates that the oncologic outcomes for open and minimally invasive nephroureterectomy are similar. An open approach has traditionally been advocated for patients with significant pulmonary disease due to the concern for absorption of carbon dioxide during laparoscopy that would result in patient hypercarbia. There are two issues with this teaching. First, the open incisions used for nephroureterectomy can result in significant abdominal pain that can compromises the depth of patient inspiration
during recovery. Second, a hand-assisted approach in this patient population can hasten the procedure and allow the insufflation pressure to be reduced (10 mm Hg), limiting carbon dioxide absorption while still providing patients the recovery benefits of laparoscopy. Patients who cannot tolerate a handassisted nephroureterectomy are likely not appropriate surgical candidates. Other relative indications for open surgery include bulky disease, presence of lymphadenopathy, as well as prior extensive endoscopic management that could result in significant perinephric inflammation. Although these procedures are more challenging, we believe they are still amenable to minimally invasive approaches at the discretion of the surgeon.
during recovery. Second, a hand-assisted approach in this patient population can hasten the procedure and allow the insufflation pressure to be reduced (10 mm Hg), limiting carbon dioxide absorption while still providing patients the recovery benefits of laparoscopy. Patients who cannot tolerate a handassisted nephroureterectomy are likely not appropriate surgical candidates. Other relative indications for open surgery include bulky disease, presence of lymphadenopathy, as well as prior extensive endoscopic management that could result in significant perinephric inflammation. Although these procedures are more challenging, we believe they are still amenable to minimally invasive approaches at the discretion of the surgeon.
Selecting a hand-assisted, conventional laparoscopic, or robotic approach also depends on the surgeon’s experience, but patient and disease characteristics influence this decision. In general, we employ a hand-assisted approach when patient comorbidities dictate an expeditious procedure or when tactile perception would be advantageous (e.g., bulky specimens or perirenal inflammation). For patients with renal pelvis tumors, we often combine an endoscopic approach for the management of the bladder cuff with a hand-assisted approach. An endoscopic bladder cuff can also be used with a conventional laparoscopic approach. We do not recommend performing an endoscopic bladder cuff for distal ureteral tumors because of risk of tumor spillage. In the case of large distal tumors, combining a conventional laparoscopic approach for the nephrectomy portion with a lower midline or Gibson incision to perform an open distal ureteral dissection and bladder cuff avoids a single large incision or two separate incisions. This is especially useful when managing upper tract recurrences in patients with a urinary diversion where the distal ureteral anatomy is often distorted and prior surgery creates scarring around structures such as the iliac vessels or bowel.
For most patients, robotic-assisted nephroureterectomy is our preferred approach. The advantages of this approach include (a) techniques have been described that obviate patient repositioning during the case (although we do reposition the robot but not the patient), (b) the location of the extraction incision can be tailored to the patient, (c) a robust lymphadenectomy can be performed, and (d) a watertight bladder closure can be achieved once the distal ureter and bladder cuff are excised. In our experience, the primary disadvantage of the robotic approach is longer average operative times compared to laparoscopic approaches. The appropriate nephroureterectomy approach is the one that best allows the individual surgeon to adhere to oncologic principles.
SURGICAL TECHNIQUE
Patient Preparation
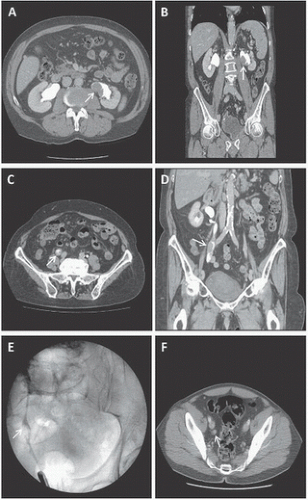
Although there are some differences in patient positioning and surgical technique by approach selected, patient preparation is similar for all patients undergoing minimally invasive nephroureterectomy. Staging is performed for all patients as described earlier including cross-sectional imaging combined with upper tract opacification (Fig. 7.1). This allows for the accurate anatomic evaluation of any upper tract disease, definition of the renal hilar vascular anatomy as well as the presence of lymphadenopathy or solid organ metastases. In addition, patients undergo preoperative lab evaluation with a comprehensive metabolic panel including liver function tests, complete blood count, coagulation panel, electrocardiogram, and blood type and screen. We do not cross-match for blood products given the low incidence of blood transfusion associated with this procedure. In addition, patients undergo preoperative evaluation by anesthesiology with directed evaluation by cardiology, pulmonology, or other medical services as indicated. Mechanical bowel preparation is not necessary; a clear liquid diet beginning 24 hours prior to surgery with nothing by mouth 4 hours prior to surgery is recommended.
Obtaining informed consent from the patient, including a description of the procedure along with a discussion of the surgical risks is a crucial step. The general risks of this procedure include bleeding requiring blood transfusion, infection (e.g., abscess, urinary tract infection, wound infection), damage to surrounding structures (e.g., bowel, blood vessels, nerves, bladder, pancreas and depending on the side of the procedure, liver or spleen), bladder urine leak requiring prolonged drainage, disease recurrence, and need for additional treatments.
Although we prefer that all other antiplatelet medications be discontinued 5 to 7 days prior to surgery, there is good data
to support continuation of at least 81 mg of aspirin throughout the perioperative period in patients with prior cardiac stenting. In fact, discontinuation of aspirin likely increases the risk of a cardiac or thromboembolic events in surgical patients (19).
to support continuation of at least 81 mg of aspirin throughout the perioperative period in patients with prior cardiac stenting. In fact, discontinuation of aspirin likely increases the risk of a cardiac or thromboembolic events in surgical patients (19).
It is critical to ensure that any urothelial disease within the bladder is controlled prior to nephroureterectomy. Although cystoscopy can be performed at the time of the nephroureterectomy to evaluate the bladder, if disease is found, there is limited ability to determine whether the bladder pathology would influence surgical decision making. As such, we recommend office cystoscopy at least 2 weeks prior to surgery so that bladder tumors can be resected and staged. In addition, some groups have reported on the use of perioperative intravesical mitomycin at the time of nephroureterectomy to reduce the risk of bladder recurrence, which has been reported to be as high as 30% (20).
Patient Positioning
A surgical beanbag is placed on the operating table with a drawsheet above and below the beanbag to facilitate proper positioning. The patient is initially in the supine position on the table. Intravenous antibiotics to cover skin flora are administered within 1 hour of surgical incision. We routinely use 5,000 units of subcutaneous heparin in addition to lower extremity sequential compression devices for venous thromboembolic prophylaxis. After induction of general anesthesia, a Foley catheter is placed as well as an orogastric tube to minimize the risk of injury to the viscera when accessing the abdominal cavity. We ensure that the circulating nurse has access to the catheter in the event that the bladder needs to be filled during the procedure. In cases where an endoscopic bladder cuff approach is used, the Foley catheter is placed following this portion of the procedure.
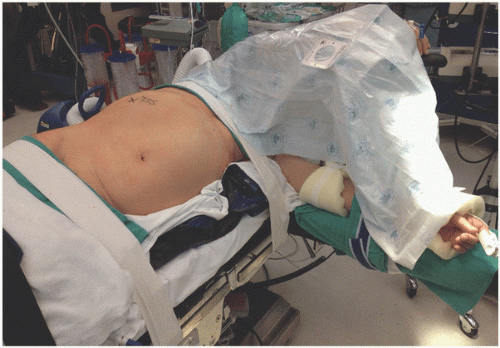
Prior to positioning, it is important to assure that there are sufficient members of the operative team available to lift and secure the patient. The patient is brought to a modified lateral decubitus position with the operative side elevated at a 45- to 60-degree angle from the horizontal plane. The downside knee is flexed at the knee and hip, the upside leg is straight and parallel to the floor, and pillows are placed between the legs and also on the table to support the upside ankle. Some surgeons choose to position the patient’s iliac crest over the table break and mildly flex (15 to 30 degrees) the table to lengthen the space between the costal margin and iliac crest. An axillary roll is generally not necessary, but it should be assured that the downside axilla is not compacted against the operating table to avoid a brachial plexus injury. The upside arm is secured on an elevated arm board in an anatomic position, assuring that it will not obstruct the movement of the surgical instruments during the case. The downside arm is supported with a table-level, detachable arm board. Assure that there are no tubes or cords under the patient or under any securing tape or straps because these can cause pressure necrosis during the case. The beanbag is then formed along the patient’s sides to support this position, and air is evacuated from the beanbag to secure it in place. All pressure points are padded and 3-inch padded cloth tape is brought over the hips and across the chest just below the axilla to firmly secure the patient to the table but avoiding compression (Fig. 7.2). The surgical, anesthesia,
and nursing teams should evaluate the patient to assure proper positioning and support. The abdomen, flank, and lower chest are sterilely prepped and draped so that the boundaries of the operative field include the midchest, superiorly; posterior axillary line, laterally; beyond the umbilicus, medially exposing the midline; and the pubic symphysis, inferiorly. Prior to the initial incision, a surgical verification is performed to confirm the correct patient, surgical site (marked in the preoperative holding area), antibiotics given, appropriate equipment, surgical team introductions, fire safety assessment as well as riskbased venous thromboembolism prophylaxis.
and nursing teams should evaluate the patient to assure proper positioning and support. The abdomen, flank, and lower chest are sterilely prepped and draped so that the boundaries of the operative field include the midchest, superiorly; posterior axillary line, laterally; beyond the umbilicus, medially exposing the midline; and the pubic symphysis, inferiorly. Prior to the initial incision, a surgical verification is performed to confirm the correct patient, surgical site (marked in the preoperative holding area), antibiotics given, appropriate equipment, surgical team introductions, fire safety assessment as well as riskbased venous thromboembolism prophylaxis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree