CHAPTER 26 Minimally Invasive Low Anterior Resection with Total Mesorectal Excision for Malignancy
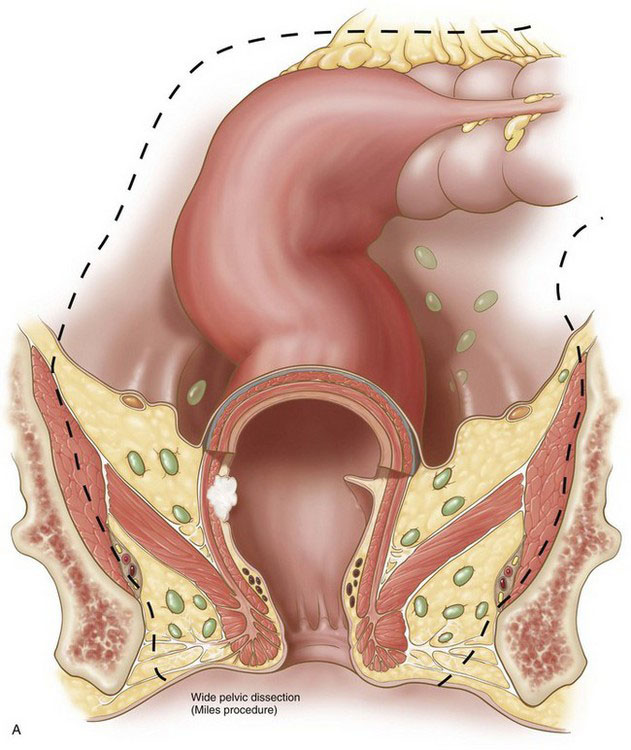
Current resective procedures for rectal cancer can have their origins traced to a radical pelvic extirpation described by Ernest Miles in the early 1900s, an operation now commonly known as the abdominal-perineal resection (APR). In Miles’ original description of this operation, a cylinder of tissue was rapidly excavated from the pelvis (Fig. 26-1A), without preservation of neural structures. Five of his first 12 patients died, though none from hemorrhage. In addition, local recurrence was problematic in Miles’ early experience with this technique. Nevertheless, the Miles procedure did represent an advance in rectal cancer surgery that, before this time, had been operated from a perineal approach.
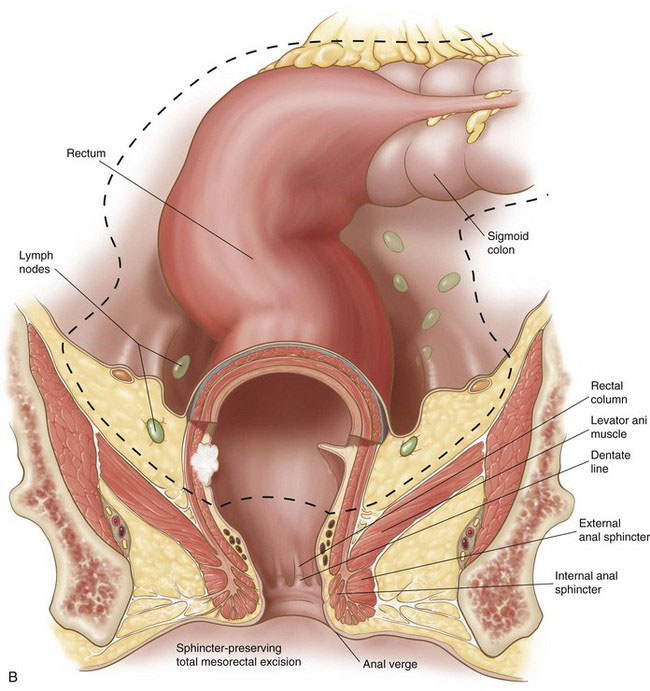
Although the rate of anastomotic reconstruction for rectal cancer resection has increased, the morbidity from these procedures, in terms of sexual and urinary dysfunction, has remained high. In the 1980s, Heald and colleagues demonstrated that sharp dissection of the mesorectum with preservation of known neural structures in the pelvis and intra-abdominal anastomosis (Fig. 26-1B) could produce excellent survival and recurrence rates without the associated morbidities. The procedure did require more time to perform than the cylindric resection shown in Figure 26-1A. The goal of Heald’s procedure, however, was to remove all of the node-bearing mesorectum, without injuring important autonomic and sensory nerves. Heald’s technique of total mesorectal excision (TME) has now become generally accepted among colorectal surgeons and is emphasized in this chapter.
Operative indications
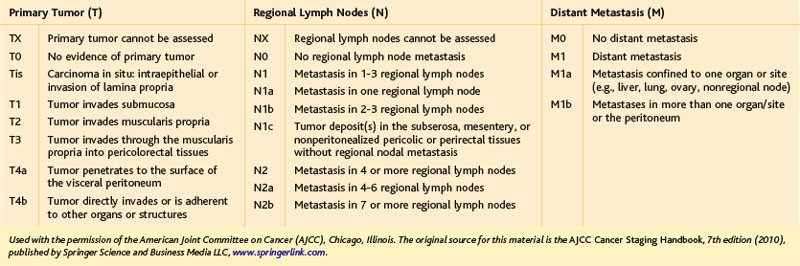
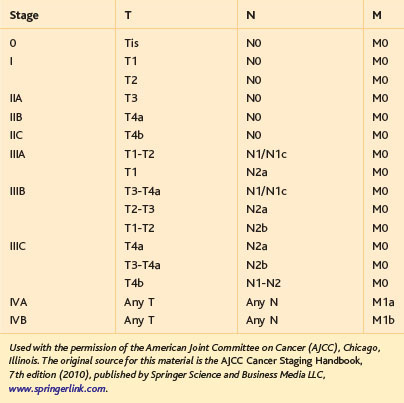
The primary indication for minimally invasive LAR with TME is localized, resectable adenocarcinoma of the rectum. In terms of the commonly used TNM classification system of the American Joint Committee on Cancer (AJCC), rectal tumors amenable to LAR with TME would include T1-3, N0-2 lesions or, generally speaking, stages I to III (Tables 26-1 and 26-2). It should be noted that in 2012, some guidelines (including the National Comprehensive Cancer Network) and experts still consider minimally invasive LAR for rectal cancer to be an experimental procedure, only to be performed within the confines of a clinical trial. As evident from the surgical literature, however, this procedure has been performed commonly (outside of trials) for rectal cancer in the Americas, Europe, and Asia since the mid-1990s. LAR for localized, resectable rectal cancer is intended to be curative; in some circumstances, however, palliative transabdominal resection for metastatic rectal cancer may be performed.
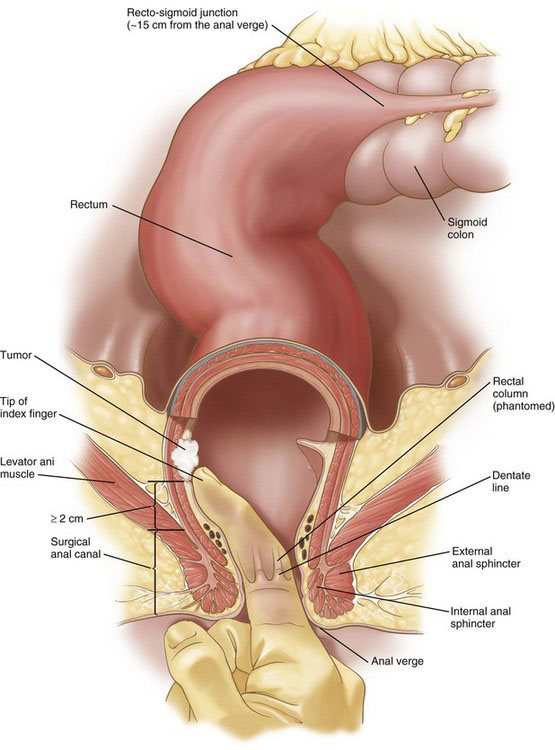
The resectability and the need for neoadjuvant therapy of a rectal cancer is dependent on the distance of the tumor from the anal sphincteric complex (Fig. 26-2) and the depth of tumor invasion (the T classification; see Table 26-1), and whether there are radiologically positive lymph nodes in the mesorectum (the N classification; see Table 26-1). Tumor location, depth of invasion, and nodal status are evaluated with computed tomography (CT), endorectal ultrasound (EUS), and/or magnetic resonance imaging (MRI), as discussed in the Preoperative Evaluation, Testing, and Preparation section.
A T1-2, N0 rectal cancer should undergo resection as primary therapy (Fig. 26-3). If the primary tumor is T1, N0, has a dimension of less than 3 cm, has favorable histopathology, and is within 8 cm of the anal verge (see Fig. 26-2), then local excision also may be acceptable treatment, particularly in the patient with increased operative risk or limited survival. Favorable histopathology has been defined as the absence of (1) perineural and lymphovascular invasion, (2) positive margins, and (3) poor differentiation. Recurrence appears to be higher after transanal compared with transabdominal resection of T1 lesions, and radiologically silent N1 disease may be missed by transanal excision of T1 tumors in up to two thirds of cases. As of 2012, the guidelines for transanal excision are in flux.
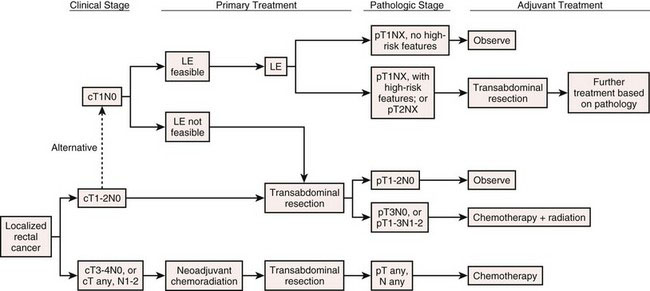
FIGURE 26-3 Algorithm for treatment of localized rectal cancer. See Table 26-1 for TNM staging definitions. LE, local excision.
(© National Comprehensive Cancer Network, Inc. 2010, all rights reserved.)
If the tumor is less than 5 cm from the anal verge, then abdominoperineal resection generally is recommended. This usually means that the tumor is within 1 cm of the sphincteric complex (see Fig. 26-2). Sphincter-preserving operations for tumors at or below this level are experimental and will not be covered in this chapter. Tumors that are more than 5 cm from the anal verge generally are amenable to LAR.
If the rectal cancer has been staged at T3-4 or N1-2, then the patient should undergo neoadjuvant chemoradiation (see Fig. 26-3). This treatment typically has involved continuous intravenous 5-fluorouracil along with 45 to 50 Gy (fractionated) of pelvic radiation. This neoadjuvant chemoradiation requires about 6 weeks to complete. The transabdominal resection then should be performed 5 to 10 weeks after completion of the chemoradiation. A T1-2 tumor that is too close to the sphincter mechanism to permit a sphincter-preserving procedure also may undergo neoadjuvant chemoradiation in an attempt to make sphincter preservation possible, but the efficacy of this strategy is not clear.
Contraindications
The contraindications to LAR for rectal cancer include (1) tumor invasion into the sphincteric complex; (2) tumor invasion into the pelvic sidewall, bladder (see later), or prostate (i.e., a T4b lesion); (3) tumor less than 2 cm from the sphincteric complex (see Fig. 26-2); (4) fecal incontinence; (5) unresectable M1 disease with minimal or no pelvic symptoms; and (6) medical contraindication to a major surgical procedure. A tumor that invades the sphincteric complex should be treated with an APR. As alluded to previously, there have been reports of LAR for tumors located less than 2 cm from the sphincteric complex, using an intersphincteric dissection (en bloc excision of the internal sphincter, which can be performed robotically), with a coloanal anastomosis performed from the perineum. With preoperative chemoradiation, a high rectal cancer with bladder involvement may be resectable with an LAR and concomitant cystectomy.
Preoperative evaluation, testing, and preparation
If the patient with rectal cancer is a candidate for anterior resection of the rectum, then the surgeon should perform her or his own proctoscopy with digital rectal examination. Ideally, this should be done on a day before the actual resection. The surgeon should determine tumor diameter, fixation, circumferential location, and the distance of the tumor from the anal verge or sphincteric complex. Traditionally, the relevant distance has been that from the tumor to the anal verge, as measured with a proctoscope or a finger (see Fig. 26-2). As indicated earlier, the minimal distance (tumor to anal verge) for the safe performance of an LAR is about 5 cm. The precise location of the anal verge can vary with sphincter contraction, introduction of an endoscope, and so forth, so the surgeon should be cautious when determining this distance in tumors of the lower rectum.
If the tumor is within reach of the surgeon’s examining finger, then perhaps a more reliable (and relevant) distance to measure would be that between the sphincter mechanism and the tumor (see Fig. 26-2). This distance can be determined by sliding the index finger of the other hand into the anal canal to palpate the ring of the sphincteric complex while the tip of the examining finger is placed into the sulcus between the tumor and the rectal mucosa. Using the finger on the ring of the sphincteric complex, the surgeon touches the point on the examining finger where it crosses the sphincter, withdraws the fingers, and thereby can obtain a direct measure of tumor-sphincter distance. Of note, it is easy to stretch the rectal mucosa and overestimate the tumor-sphincter distance, so the surgeon should not apply vigorous digital pressure. A minimum of 2 cm would be needed between the tumor sulcus proximally and the top of the sphincteric complex distally (i.e., not the anal verge) for the safe performance of an LAR. Of note, the critical distance of the tumor to the anal verge versus the sphincters obviously will not be the same, so the surgeon should use consistent terminology.
Patient positioning in the operating suite
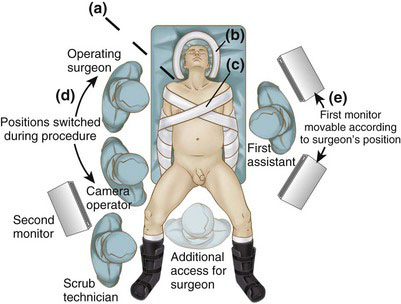
General endotracheal anesthesia is administered, and an orogastric tube and Foley catheter are inserted. A central venous catheter and arterial line are optional, depending on the patient’s underlying comorbidities. Sequential compression devices are placed on both lower extremities, and the patient is placed in the low lithotomy position with both arms tucked (Fig. 26-4). The angle of thigh flexion should be less than 15 degrees to avoid interference with instrumentation during the splenic flexure mobilization. The buttocks should overhang the edge of the table to facilitate intraoperative sigmoidoscopy and transanal stapler insertion. The legs and feet are padded and strapped to the stirrups to secure the lower half of the patient during extremes of table maneuvering. The upper torso is secured to the operating table using two adjustable soft straps in an X configuration (see Fig. 26-4). The anesthesiologist should secure the head so that it will not roll during extremes of lateral tilt.
If the patient has an ample panniculus, then it may be helpful to place an additional soft strap underneath the panniculus at the level of the anterosuperior iliac spines (see Fig. 26-4) to prevent the patient from rolling off the table during lateral tilt. The Foley catheter is taped to the leg, away from the perineum. In men, the scrotum may need to be suspended away from the perineum with a single stay suture (or taped) to the medial thigh. Before the sterile preparation and draping, the operating table is placed through the extremes of rotation and Trendelenburg position to ensure that the patient is immobilized and that surrounding equipment will not hinder table movement. In addition, the surgeon should make sure that a segment of left-sided bed rail is available for clamping of the post for a self-retaining retractor, either for the laparoscopic pelvic dissection or in case of open conversion.
The surgeon stands on the patient’s right, the first assistant stands opposite to the surgeon, the camera operator stands on the right cephalad to the surgeon, and the scrub technician is positioned off the right leg (see Fig. 26-4). Two monitors are used, one on each side of the patient; the surgeon’s monitor (on the patient’s left) may need to be repositioned according to where the dissection occurs (e.g., cephalad for the splenic flexure mobilization, caudad for the mesorectal excision). The entire right side of the patient, from the head to the foot, should be free of obstruction (e.g., intravenous poles, anesthesia machine, energy units) to allow the surgeon unencumbered movement.
Positioning and placement of trocars
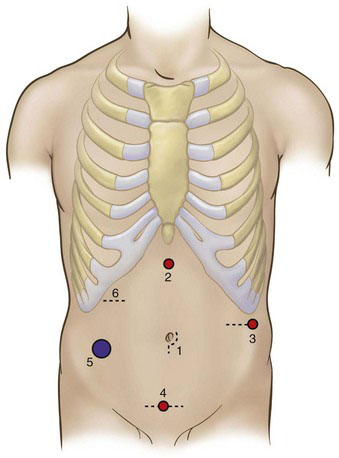
At a minimum, minimally invasive LAR requires access to both lower abdominal quadrants. If mobilization of the splenic flexure or fecal diversion is necessary, then three or all four quadrants will need to be accessible. In most patients, initial access may be obtained in the infraumbilical position with open insertion of a blunt 10-mm cannula (Hasson type), and this port subsequently will be used for the camera (Fig. 26-5). Alternatively, peritoneal access may be obtained with a Veress needle or with an optical trocar. If the position of the umbilicus has shifted inferiorly because of obesity, then it may be better to place the camera in the midline midway between the xiphoid process and the symphysis pubis. If there is an increased risk that the procedure may need to be converted to open (e.g., patient’s BMI >35), then it would be prudent to use a vertically oriented incision for the camera port.
After the camera port has been inserted and the abdomen has been insufflated to 12 to 15 mm Hg, four additional ports (2 through 5) are placed in the diamond configuration, as shown in Figure 26-5. Although there is no absolute or constant arrangement for trocar placement, the positioning in Figure 26-5 is a general guideline that can be modified based on the patient’s body habitus, previous surgical scars, and so forth. The diamond configuration allows reasonable access to all four quadrants of the abdomen.
Because the shape and anatomic relations of the abdominal wall can be distorted with distention, the actual insertion point for ports 2 through 5 should be determined by the surgeon only after the abdomen has been fully insufflated. If the abdominal wall is not excessively thick, then laparoscope transillumination should be used at each port site to identify and avoid laceration of abdominal wall vessels. The port size at positions 2, 3, and 4 in Figure 26-5 initially can be 5 mm, and then upsized to 12 mm later if necessary. The port at position 5 (superior to the right anterosuperior iliac spine) should be 12 mm to accommodate the endoscopic stapler-cutter, and should be positioned as lateral and inferior as possible to facilitate placement of the stapler across the distal rectum. Furthermore, if the surgeon encounters difficulty during the pelvic dissection, then she or he should not hesitate to place an additional port (typically in the right mid-abdomen) to improve pelvic retraction, instrument triangulation, and so forth.
Operative technique
Surgical Anatomy
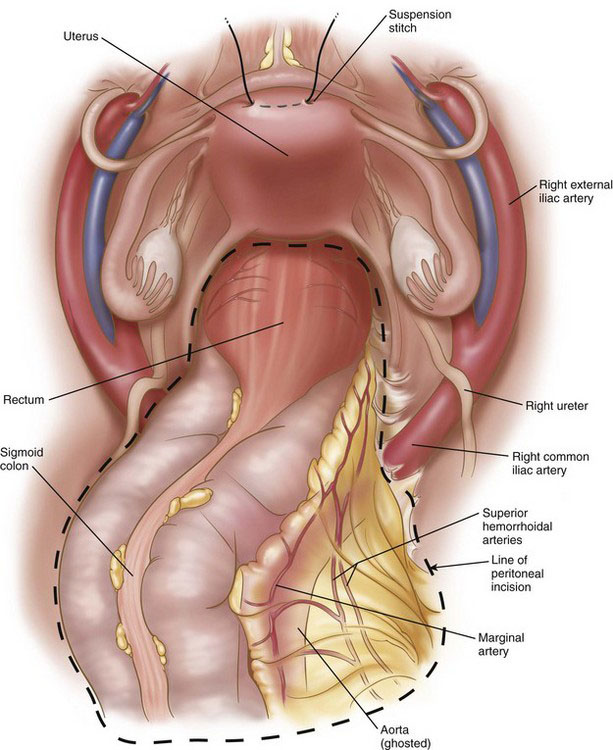
Definitions and descriptions of pelvic anatomy are somewhat arbitrary and have been debated in the surgical and anatomic literature for over 100 years. The following is an abbreviated synthesis of current opinions; this is not intended to be a final statement on the relevant pelvic anatomy. The rectum is about 12 to 15 cm long (see Fig. 26-2), depending on how the measurements are taken, and is arbitrarily divided into upper, middle, and lower thirds. The rectosigmoid junction is near the sacral promontory, typically an angled region of the large bowel where the epiploic appendices of the sigmoid colon become less prominent and where the longitudinal bands of the taenia coli diverge and coalesce, encircling the rectum. The anterolateral portion of the upper to mid rectum is covered with peritoneum, whereas the remainder is extraperitoneal. The peritoneum reflects off the mid rectum onto the bladder or the uterus, forming the rectovesicular pouch (in the male) or rectouterine pouch (in the female; sometimes known as the pouch of Douglas) or, simply, the peritoneal reflection.
The arterial blood supply to the rectum derives mainly from the superior hemorrhoidal (rectal) branches of the inferior mesenteric artery and, to a lesser extent, from the middle and inferior hemorrhoidal arteries, which derive from the internal iliac (hypogastric) arteries (Fig. 26-6). The middle hemorrhoidal vessels were described in older surgical literature to run in a structure called the lateral ligament (discussed under Operative Technique), but this arrangement has been questioned by numerous anatomic studies, and the presence of the middle hemorrhoidal vessels now is considered inconstant. Although the inferior (and middle) hemorrhoidal vessels are the primary supply to the anal canal, these vessels have an anastomotic network that can support a long rectal stump after ligation of the superior hemorrhoidal vessels.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree