Fig. 15.1
Cirrhotic liver with metastatic breast carcinoma. Tumor cells are seen within the fibrous septa and in the cirrhotic nodule
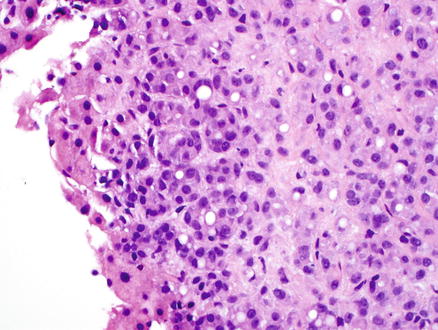
Fig. 15.2
Cirrhotic liver with metastatic breast carcinoma. High-power view showing the metastatic tumor cells
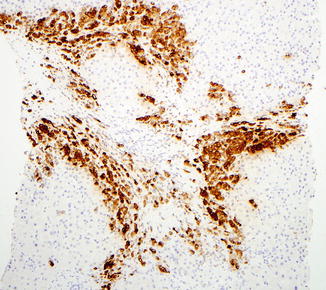
Fig. 15.3
Cirrhotic liver with metastatic breast carcinoma. Mammaglobin immunostain is positive within the tumor
Table 15.1
Summary of serum tumor markers
Serum tumor markers | Associated elevated conditions |
|---|---|
Alpha fetoprotein (AFP) | Hepatic tumors and germ cell tumors |
CA19-9 | Pancreatic, colon, and gastric carcinomas |
CA 125 | Ovarian carcinoma |
Prostatic-specific antigen (PSA) | Prostate carcinoma |
Carcinoembryonic antigen (CEA) | Colorectal carcinoma and other carcinomas |
CA 15-3 | Breast carcinoma and other carcinomas |
Neural-specific enolase (NSE) | Neuroblastoma, pheochromocytoma, small cell carcinoma, and carcinoid tumors |
Chromogranin A (CgA) | Neuroblastoma, pheochromocytoma, small cell carcinoma, and carcinoid tumors |
Beta unit of human chorionic gonadotropin (beta hCG) | Germ cell tumors |
Squamous cell carcinoma antigen (SCC) | Squamous cell carcinoma |
While immunohistochemical stains are essential in working up a liver tumor, the morphological features seen on the H&E stain should not be neglected. Using them both together is the quickest and most accurate way to get the correct diagnosis. Morphology needs be taken into account when interpreting immunostain results because making a diagnosis based only on the results of the immunostains can lead to an erroneous diagnosis. Sometimes, repeating a special stain or an immunohistochemical stain can be helpful if the results do not correlate well with the morphology.
If a tumor is both poorly differentiated and no useful clinical information is available, then the first round of immunostains should be directed at correctly identifying the broad lineage of the neoplasm, i.e., whether it is a carcinoma, sarcoma, melanoma, or lymphoma. This can be done by ordering a broad panel of immunohistochemical stains that includes markers for carcinoma (such as cytokeratin AE1/AE3, Cam5.2, and CK903), sarcoma (desmin, CD34, and CD117), melanoma (S100, Melan-A, and HMB-45), and lymphoma (CD3, CD20, and CD45). Once the broad lineage of the tumor has been identified, more specific immunostains can be ordered for further tumor subclassification and eventual accurate diagnosis.
On the other hand, if the tumor is well-differentiated and its overall lineage is evident based on the H&E findings (e.g., gland forming and mucin producing adenocarcinoma), then the immunohistochemical workup should be directed at extending the H&E findings rather than establishing the broad tumor lineage. For example, a gland forming tumor could be evaluated with CK7/CK20 and specific markers for lung, GI, breast, or prostate origin. A carcinoma with a solid or trabecular growth pattern could be worked with markers for hepatic, adrenal, renal, and neuroendocrine differentiation.
Finally, remember that immunostains that work well in a given author’s experience may be different than the ones that work best for you, depending on the availability of the stains in your laboratory, their quality, and your overall familiarity with the stains. Every laboratory has its own somewhat unique experience with a given stain, based partly on antibodies, antigen retrieval, and other local technical practices. Therefore, the published literature should be interpreted in conjunction with direct experience.
15.7 Mimickers of Hepatocellular Carcinomas
Certain tumors can histologically mimic hepatocellular carcinoma, including epithelioid angiomyolipomas, renal cell carcinomas, adrenal cortical carcinomas, neuroendocrine tumors, and melanoma. These tumors can show a trabecular or acinar or solid growth pattern that can closely resemble hepatocellular carcinoma. In addition, the tumor cells in these cases can show cytological features that further suggest hepatocellular differentiation, including eosinophilic and granular or clear cytoplasm.
Most angiomyolipomas are distinguished from hepatocellular carcinoma by their triphasic nature, but epithelioid angiomyolipomas, especially in biopsy specimens, can be difficult to distinguish from a well-differentiated hepatocellular neoplasm. Immunohistochemical stains are helpful here, as angiomyolipomas are positive for smooth muscle actin, HMB-45 and Melan-A, but negative for cytokeratin, HepPar1, and arginase-1 (Figs. 15.4, 15.5, and 15.6).
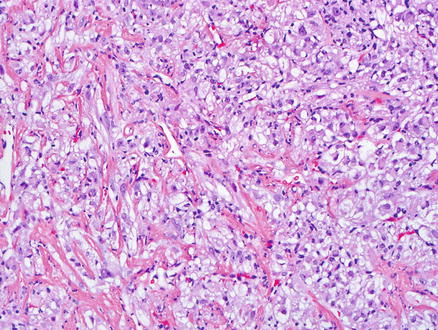
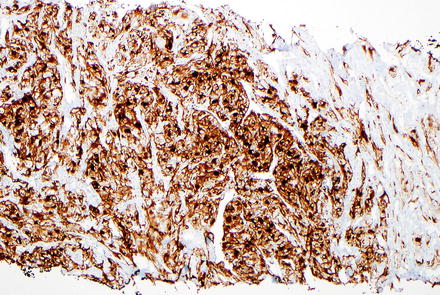
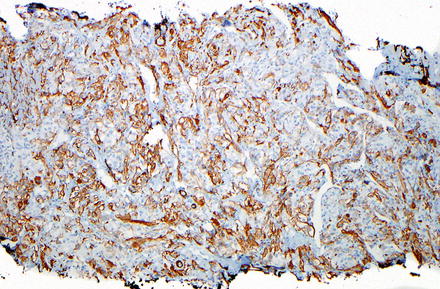

Fig. 15.4
Epithelioid angiomyolipoma. Epithelioid tumor cells with clear cytoplasm are seen

Fig. 15.5
Epithelioid angiomyolipoma. Smooth muscle actin immunostain is positive within the tumor cells

Fig. 15.6
Epithelioid angiomyolipoma. HMB-45 immunostain is positive within the tumor cells
Both clear cell and chromophobe renal cell carcinoma can be mistaken for hepatocellular neoplasms. A history of renal malignancy or the presence of a renal mass can help reach the correct diagnosis. Furthermore, some clear cell hepatocellular carcinomas have areas of more typical non-clear morphology, which can help distinguish it from metastatic clear cell renal cell carcinoma. Immunohistochemical stains are very helpful in the differential diagnosis; clear cell renal cell carcinomas are usually positive for vimentin while most hepatocellular carcinomas are either negative or show patchy staining (90 %), with the exception of poorly differentiated or spindle cell hepatocellular carcinomas which can show more diffuse reactivity for vimentin [2]. RCC antigen is negative in hepatocellular carcinoma, but RCC antigen has a lower sensitivity and specificity for metastatic renal cell carcinoma and should be interpreted cautiously [3]. On the other hand, the PAX8 immunostain is positive in the majority of metastatic clear cell renal cell carcinomas [4, 5]. However, a recent publication has shown that up to 20 % of clear cell hepatocellular carcinomas can also be positive for PAX8 [6], so other markers for hepatic differentiation also need to be examined. Clear cell renal cell carcinomas are negative for HepPar1, polyclonal CEA (canalicular pattern), and arginase-1 [7–9]. Chromophobe renal cell carcinoma, a close mimicker of hepatocellular neoplasms, is positive for PAX8 and CD117 and negative HepPar1 and arginase-1 (Figs. 15.7, 15.8, 15.9, and 15.10); this immunoprofile helps distinguish it from hepatocellular neoplasms. Of note, glypican-3 is not helpful in this differential diagnosis, as a subset of chromophobe renal cell carcinomas is positive.
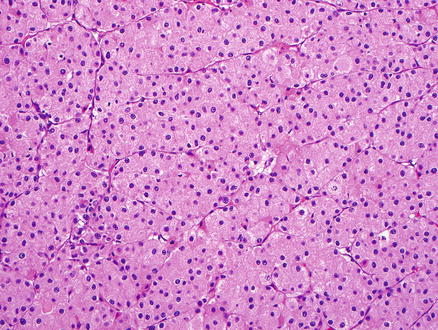
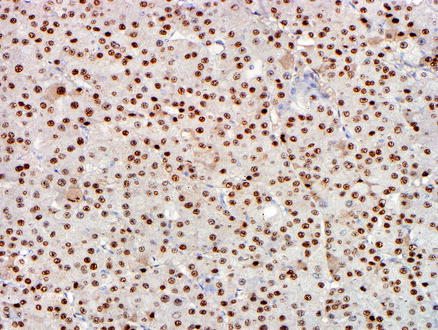
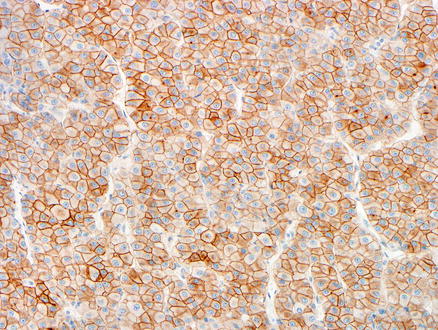
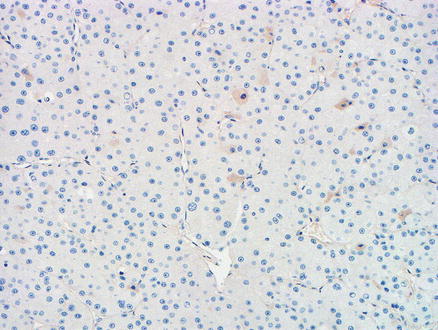

Fig. 15.7
Metastatic chromophobe renal cell carcinoma. The tumor cells have eosinophilic cytoplasm and prominent nucleoli

Fig. 15.8
Metastatic chromophobe renal cell carcinoma. A PAX8 immunostain shows nuclear positivity

Fig. 15.9
Metastatic chromophobe renal cell carcinoma. A CD117 immunostain showing membranous staining

Fig. 15.10
Metastatic chromophobe renal cell carcinoma. An arginase immunostain is negative
Adrenal cortical carcinomas can involve the liver by metastasis or direct extension and can be difficult to differentiate morphologically from hepatocellular carcinoma (Fig. 15.11). Their immunoprofile, however, is distinct from hepatocellular carcinoma; adrenal cortical carcinomas are often positive for inhibin and Melan-A and are typically negative for HepPar1 (about 5–10 % of cases can be positive), polyclonal CEA (canalicular pattern), and arginase-1 [8–11].
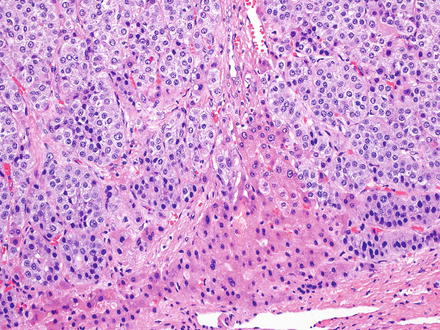

Fig. 15.11
Metastatic adrenal carcinoma. The growth pattern and cytology raises the possibility of a hepatocellular carcinoma on the H&E. Benign hepatocytes are seen in the lower portion of the image
Neuroendocrine tumors can resemble hepatocellular carcinoma. Some cases may have prominent clear cell morphology, while others will have ordinary cytology but mimic hepatocellular carcinoma with trabecular or acinar growth patterns. Neuroendocrine tumors, however, are strongly and diffusely positive for chromogranin and/or synaptophysin while they are negative for HepPar1 and arginase-1 (Figs. 15.12, 15.13, 15.14, and 15.15) [8, 9]. Hepatocellular carcinomas can show also focal neuroendocrine differentiation [12], especially when cholestatic, but will also be positive for markers of hepatic differentiation. Interestingly, neuroendocrine tumors metastatic to the liver that produce insulin can have a very distinctive feature, characterized by a marked steatosis involving only a rim of hepatocytes surrounding the neuroendocrine tumor (Figs. 15.16 and 15.17) [13].
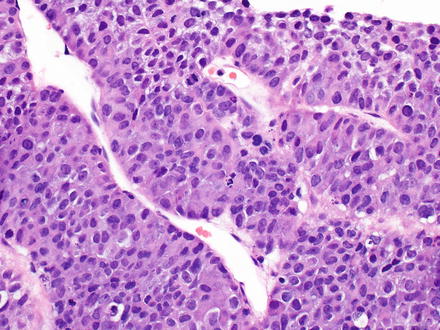
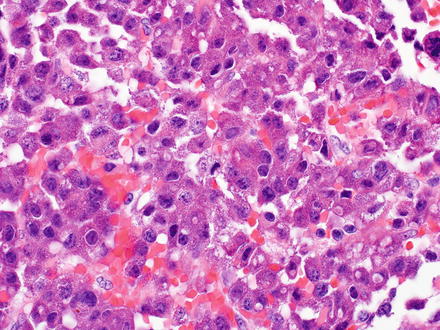
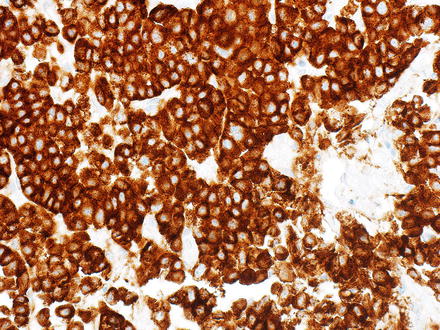
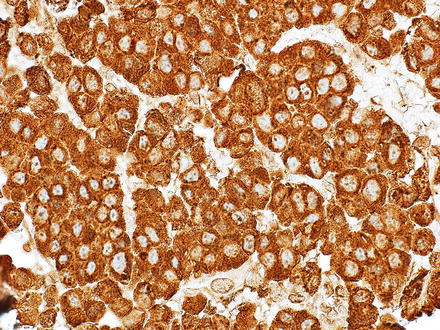
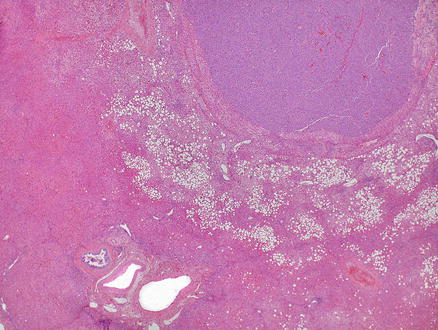
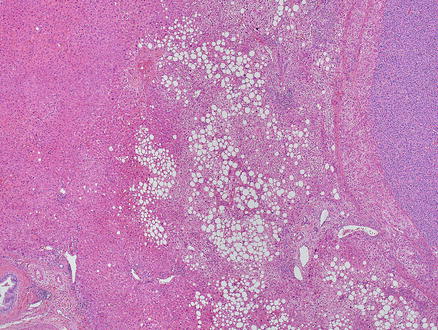

Fig. 15.12
Metastatic neuroendocrine tumor. This neuroendocrine carcinoma has a macrotrabecular growth pattern that mimics hepatocellular carcinoma

Fig. 15.13
Metastatic neuroendocrine. Eosinophilic tumor cells show acinar and trabecular growth patterns

Fig. 15.14
Metastatic neuroendocrine tumor. A chromogranin immunostain is positive (same case as Fig. 15.13)

Fig. 15.15
Metastatic neuroendocrine tumor. A synaptophysin immunostain is positive (same case as Fig. 15.13)

Fig. 15.16
Metastatic neuroendocrine tumor. An insulinoma metastatic to the liver shows a rim of marked fatty change in the liver parenchyma surrounding the lesion. Notice that the liver parenchyma away from the lesion shows no significant steatosis

Fig. 15.17
Metastatic neuroendocrine tumor. An insulinoma metastatic to the liver shows a rim of marked fatty change in the liver parenchyma surrounding the lesion. Notice that the liver parenchyma away from the lesion shows no significant steatosis (higher power of the same case in Fig. 15.16)
Finally, melanomas can mimic hepatocellular carcinoma. A history of melanoma can often guide the pathologist to the correct diagnosis. Immunohistochemistry is invaluable in this setting, as melanoma is positive for S100, Melan-A, and HMB-45 and negative for HepPar1, polyclonal CEA, and arginase-1 (Figs. 15.18, 15.19, 15.20, and 15.21). Glypican-3, however, is usually not helpful in this setting as glypican-3 is frequently positive in melanoma [14].
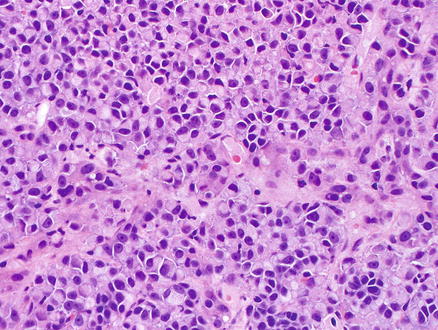
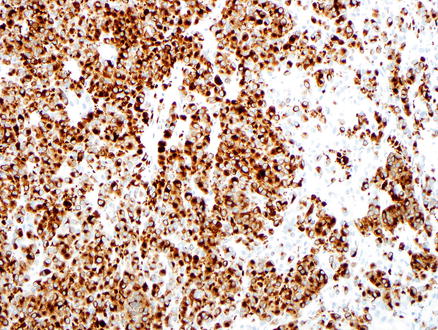
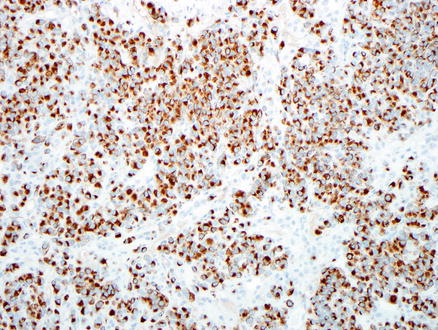
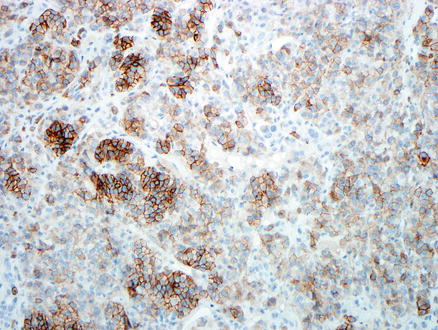

Fig. 15.18
Metastatic melanoma. Tumor cells showing eosinophilic cytoplasm, eccentrically located nuclei and prominent nucleoli

Fig. 15.19
Metastatic melanoma. A Melan-A immunostain is positive

Fig. 15.20
Metastatic melanoma. An HMB-45 immunostain is positive

Fig. 15.21
Metastatic melanoma. A CD117 immunostain is positive
There are several excellent potential panels of immunohistochemical stains, which can be useful in differentiating hepatocellular carcinoma from its metastatic morphological mimickers. If a well to moderately differentiated hepatocellular carcinoma is in the differential, than one useful approach would be to perform an immunostain for hepatic differentiation, such as HepPar-1 or arginase-1. If they are negative, then additional follow-up stains can be used to further classify the tumor. Depending on the morphology and the clinical findings, these additional stains might include PAX-8, inhibin, vimentin, S100, HMB-45, Melan-A, SMA, CD-117, polyclonal CEA, and CAM5.2 (Table 15.2). As discussed previously, an effective approach for poorly differentiated tumors is a first round of stains to determine lineage (carcinoma, sarcoma, melanoma, lymphoma), followed by additional stains to help identify the most likely site of origin.
Table 15.2
Immunohistochemical staining pattern of some tumors that can morphologically mimic hepatocellular carcinoma
Tumor | Hep | Arg | Cam5.2 | Synapt | Vim | PAX8 | p-CEA | CD117 | Inh | S100 | HMB-45 | Melan | SMA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
HCC | + | + | + | − | − | − | + (canalicular) | +/− | − | − | − | − | − |
RCC | − | − | +/− | − | + | + | − | + | − | − | − | − | − |
ACC | − | − | +/− | + | + | − | − | +/− | + | − | − | + | − |
AML | − | − | − | − | + | − | − | +/− | − | − | + | + | + |
NET | − | − | + | + | +/− | +/− | − | +/− | − | +/− | − | − | − |
Melanoma | − | − | +/− | − | + | − | − | + | − | + | + | + | − |
15.8 Distinguishing Cholangiocarcinoma and Metastatic Adenocarcinoma
Cholangiocarcinomas can assume many of the histological patterns of metastatic adenocarcinomas. However, if the bile ducts near the adenocarcinoma show high-grade biliary intraepithelial neoplasia (BilIN-3), this provides strong morphological evidence that the tumor is a primary cholangiocarcinoma rather than a metastasis. Other precursor lesions include mucinous cystic neoplasms and intraductal papillary neoplasms. However, precursor lesions are not identified in most cases. Furthermore, it is important to remember that some metastatic adenocarcinomas, such as colorectal adenocarcinomas, can have a prominent intrabiliary ductal growth pattern (“cancerization of the bile ducts”), which can closely mimic high-grade biliary intraductal neoplasia (BilIN-3).
A panel of immunostains is often required to distinguish cholangiocarcinoma from metastatic adenocarcinoma. Morphologically, adenocarcinomas arising from the upper gastrointestinal tract, pancreas, and biliary tract appear very similar to intrahepatic cholangiocarcinoma. Adenocarcinomas originating from all of these sites can be composed of small tubular or tubulopapillary structures embedded in a fibrous stroma. Therefore, distinction between intrahepatic carcinoma and metastases from upper gastrointestinal or pancreatobiliary sources is difficult, if not impossible, based on morphologic grounds alone. Furthermore, there are currently no immunohistochemical stains available in routine practice that can accurately distinguish between an intrahepatic cholangiocarcinoma and metastatic adenocarcinoma from upper gastrointestinal or pancreatic origin. In current practice, such distinctions rely first on ruling out other possible metastatic tumors and, when the morphology and immunostains are consistent with a biliary, pancreas, or upper gastrointestinal primary. When this is done, a final determination then relies on correlation with the imaging and endoscopic findings to identify the most likely site of origin. The lack of positive, affirmative immunostains or molecular assays that identifies biliary differentiation is a major weakness in our diagnostic armamentarium, one that will be hopefully rectified in the future.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








