Background
In the United States of America and Europe, between 50,000 and 70,000 new cases of bladder cancer are diagnosed each year. It is the second most common urogenital cancer and predominantly affects elderly men and women with a ratio of 3:1. earlystage superficial bladder cancer, confined to the urothelium, can often be managed with serial resection and intravesical therapy, whist the standard treatments for invasive bladder cancer are cystectomy or radiotherapy, often combined with neo-adjuvant chemotherapy. Overall 5-year survival rates for muscle-invasive disease are between 10% and 40% depending on the depth of tumor penetration and the extent of visceral involvement. For patients who fail these primary therapies and develop metastases or those who are diagnosed with metastatic bladder cancer, the prognosis is poor, with less than 5% alive at 5 years. For patients with metastatic bladder cancer, systemic chemotherapy is the standard treatment.
This chapter will review the evidence on the efficacy and toxicity of chemotherapeutic regimes in the treatment of metastatic bladder cancer.
Literature search
A search strategy was developed using the Cochrane search strategy for randomized trials combined with thesaurus and text words for chemotherapeutic regimes and metastatic bladder cancer. Initially Medline was searched from 1966 to 2008. The search strategy was then modified to search EMBASE, Web of Science and the Cochrane Library.
Clinical question 33.1
Is cisplatin alone as effective as cisplatin in combination with methotrexate, cyclophosphamide or adriamycin?
The evidence
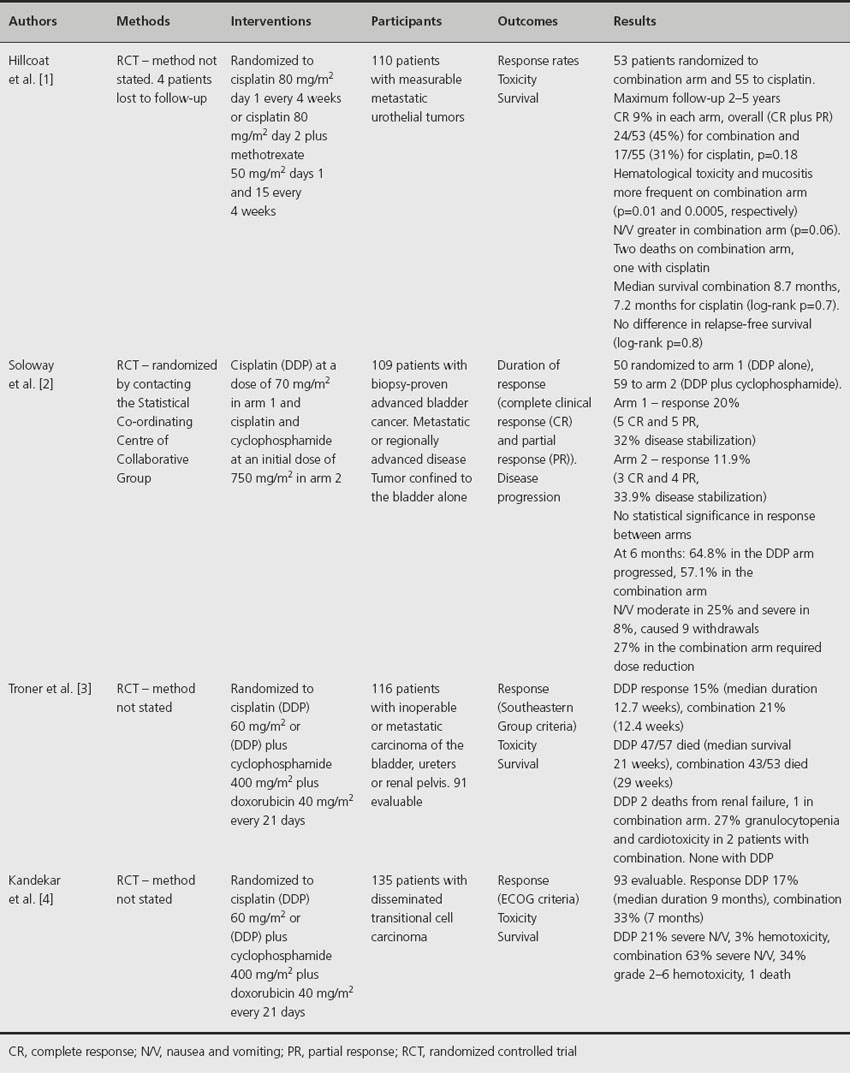
Four randomized trials have compared cisplatin alone with cisplatin plus methotrexate, cyclophosphamide or adriamycin (Table 33.1) [1–4]. A randomized trial of 53 patients receiving either cisplatin alone or in combination with methotrexate reported no difference in clinical response or survival [1]. In another study of 109 patients randomized to either cisplatin alone or combined with cyclophosphamide, no significant difference in response rate was seen between the two arms [2]. Similarly, when cisplatin alone was compared to cisplatin plus adriamycin and cyclophosphamide, there was no significant difference in response rate, response duration or survival [3]. Notably, the combination regime in this study was more toxic, with 27% of patients having granulocytopenia compared to none with cisplatin alone. In a study of 135 patients with disseminated transitional cell carcinoma [4], 17% of evaluable patients had a partial or complete remission with cisplatin alone compared to 33% for those receiving a combination of cisplatin, doxorubicin plus cyclophosphamide (p = 0.09). Crude median survival was slightly better with the combination regime (6 months versus 7.3 months, p = 0.17), but was significantly more toxic; 34% developed grade 3 or 4 hematological toxicity compared to 3% for cisplatin alone.
Table 33.1 Effect of cisplatin alone or in combination with methotrexate, cyclophosphamide or adriamycin on clinical outcomes
Comment
In small randomized trials, single-agent cisplatin induces similar response rates and overall survival estimates when compared to cisplatin combined with methotrexate or cyclophosphamide, although the combination of cisplatin, adriamycin and cyclophosphamide appears to be more active. The combination regimes were significantly more toxic (evidence and recommendation Grade 1A).
Clinical question 33.2
Does the combination of cisplatin, methotrexate and vinblastine (CMV) induce higher response rates than methotrexate plus vinblastine (MV)?
The evidence
Only one study has addressed this issue. A multicenter, Medical Research Council study enrolled 214 patients with poor performance status and metastatic bladder cancer and randomized 108 to receive CMV and 106 to VM [5]. At 2 years post randomization, a total of 204 patients had died – 101 allocated to CMV and 103 allocated to VM. Statistical analysis revealed a hazard ratio of 0.68 (95% CI 0.51–0.90, p = 0.0065) indicating a 32% reduction in the relative risk of dying with CMV. This translates into an overall improvement in median survival of 2.5 months (from 4.5 to 7 months). CMV was considerably more toxic, with five treatment-related deaths (cardiovascular toxicity N 2, septicemia N 2, renal failure N 1) compared to none with VM. CVM resulted in grade 3 neutropenia or thrombocytopenia in five patients compared to none with VM. Hospitalization with systemic antibiotics was required for neutropenic fever in 11 and two patients receiving CMV or MV, respectively. Long-term toxicity presented as neurotoxicity in nine patients on CMV and one on MV.
Comment
The combination of CMV for the treatment of metastatic bladder cancer in poor performance status patients is associated with a small improvement in overall survival, but more toxicity compared to MV. No recommendations can be made.
Clinical question 33.3
Are response rates and survival superior with methotrexate, vinblastine, adriamycin and cisplatin (MVAC) compared to single-agent or combination chemotherapy?
The evidence
Three prospective randomized studies have established MVAC as a standard chemotherapeutic regime for metastatic bladder cancer (Table 33.2). In a study by Loehrer et al. [6], 246 assessable patients were randomized to either cisplatin alone or to MVAC. The combination regime significantly increased complete and partial response rates, with respective levels of 13% and 25% for MVAC and 3% and 8% for cisplatin alone. The median progression-free survival was superior with MVAC (10 versus 4.3 months) with five complete responders to MVAC still free of disease at 8.5–39.5 months. Overall survival was also significantly improved with MVAC compared to cisplatin alone (median times 12.5 versus 8.2 months, p = 0.0002). However, MVAC was more toxic, inducing significantly more severe leukopenia and fever. In an update of this study, overall survival remained significantly better with MVAC compared to cisplatin alone (log rank p = 0.00015) [7]. At 6 years, 11 patients were alive, two treated with cisplatin alone and nine with MVAC.
Table 33.2 Comparison of methotrexate, vinblastine, adriamycin and cisplatin (MVAC) combination chemotherapy with other chemotherapy regimes
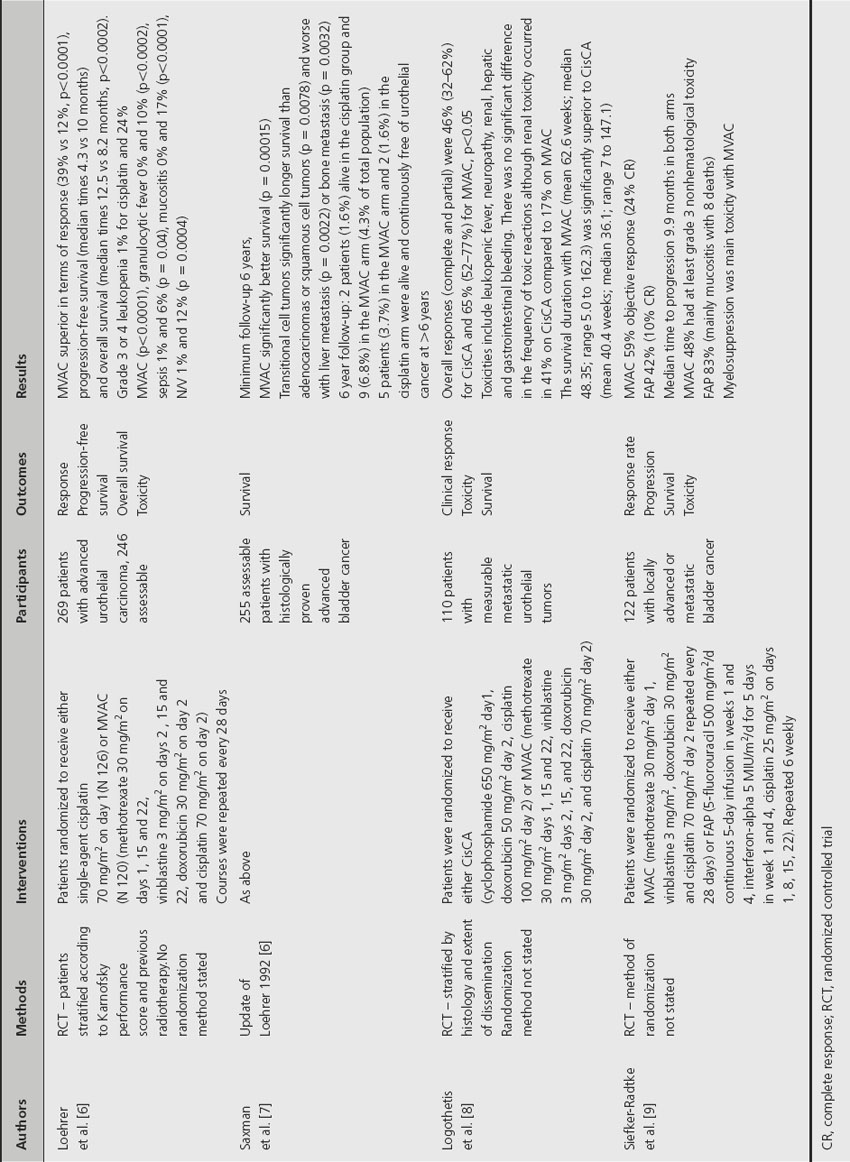
Logothetis et al. [8] compared MVAC with another cisplatin-based regime, CisCA (cisplatin, cyclophosphamide, adriamycin), in a randomized study of 110 patients with metastatic bladder cancer. Overall response was significantly better for MVAC (46% versus 65%, p = 0.05) and median survival (44.4 weeks vs 36.1 weeks).
MVAC has also been evaluated against the triplet 5-fluorouracil, interferon alpha-2b and cisplatin (FAP) in a randomized setting [9]. The objective response rate for those assigned to FAP was 42% (complete response in 10%). This compared to 59% for those receiving MVAC (complete response in 24%). The Kaplan–Meier estimate of median survival was 12.5 months for both groups (no p value given).
Comment
The evidence suggests that, in terms of response rates and overall survival, MVAC is superior to single-agent cisplatin, CisCA and FAP (evidence and recommendation Grade 1A).
Clinical question 33.4
Does increasing the dose-intensity of MVAC improve overall survival?
The evidence
The standard dose regime of MVAC was compared to high-dose intensity MVAC plus granulocyte colony-stimulating factor (HD-MVAC) in an international randomized EORTC phase III study [10]. Two hundred and sixty-three patients with advanced bladder cancer were randomized. An objective response (complete and partial) was seen in 72% of patients on HD-MVAC compared to 58% with the standard MVAC. Median time to progression was significantly improved with the HD-MVAC regime (hazard ratio (HR) 0.73, 95% CI 0.56–0.95, p = 0.017). At a median follow-up of 7.3 years, the median overall survival was 15.1 months with HD-MVAC versus 14.9 months for MVAC (no p value given). The 5-year survival rate was 21.8% (95% CI 18.4-–34.0) and 13.5% (95% CI 7.4–19.6) for the HD-MVAC and MVAC regimes, respectively. There was one toxic death in both arms of the study. Grade 4 leukopenia was worse with MVAC (16% versus 8%), as was neutropenic fever (26% versus 10%), although grade 4 thrombocytopenia was more evident with HD-MAVAC (6% versus 11%).
Comment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








