Table 10.1). The first was the 52-week, multicenter Veterans Affairs Cooperative study, which randomized 1229 men aged 45–80 years to receive placebo, terazosin, finasteride or the combination of both drugs [8]. Eligibility criteria included a mean AUA Symptom Index score of at least 8 and a mean peak urinary flow rate of 4–15 mL per second. Among the 1007 men who completed the study, the authors found that while the terazosin and combination therapy groups had similar symptomatic relief (mean changes from baseline in symptom scores were decreases of 6.1 and 6.2 points, respectively) and improved peak urinary flow rates (mean changes were increases of 2.7 and 3.2 mL per second, respectively) compared to the placebo and finasteride groups, the combination of terazosin and finasteride offered no additional benefit over terazosin alone with respect to those outcomes.
Table 10.1 Randomized clinical trials comparing combination medical therapy to placebo or an active control
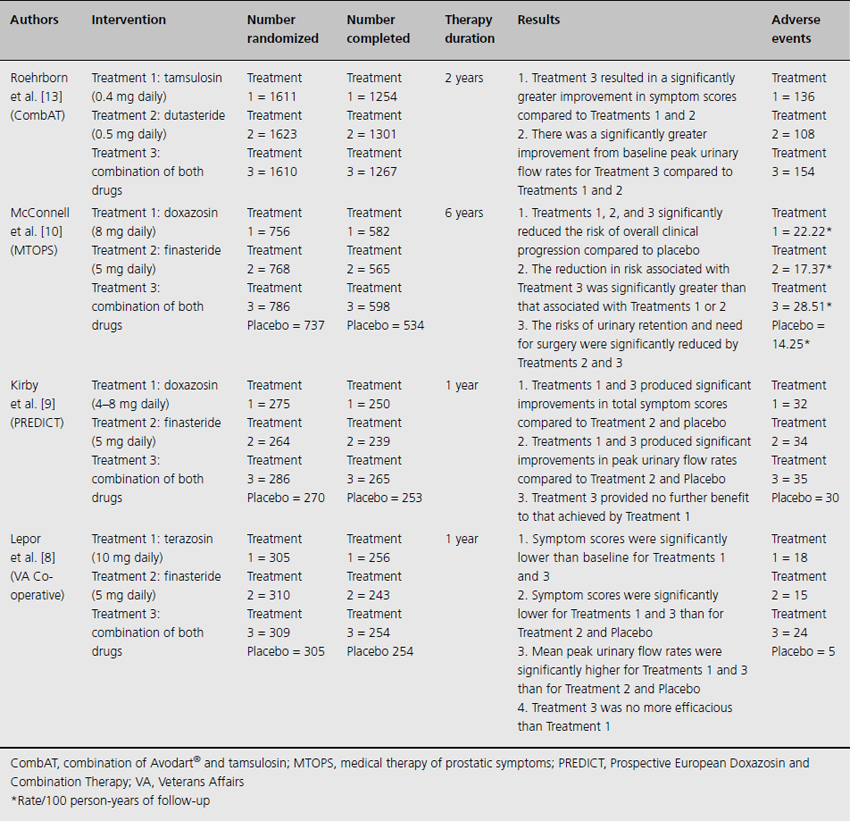
The Prospective European Doxazosin and Combination Therapy trial followed [9]. This 52-week, multicenter study randomized 1095 men aged 50–80 years to treatment with doxazosin, finasteride, doxazosin plus finasteride or placebo. Patients with a total International Prostate Symptom Score (IPSS) of at least 12 and a mean peak urinary flow rate between 5 and 15 mL per second were eligible for inclusion. Although only 771 subjects (71%) completed the study, the discontinuations were evenly distributed amongst all four groups. Compared with the placebo and finasteride monotherapy groups, both the doxazosin and doxazosin plus finasteride groups experienced similar and statistically significant improvements from baseline in urinary flow rates (mean increases of 3.6 and 3.8 mL per second, respectively) and IPSS (mean decreases of 8.3 and 8.5 points, respectively). However, no statistically significant difference was demonstrated between the doxazosin and doxazosin plus finasteride groups.
The preceding two 52-week trials primarily assessed longitudinal changes in urinary symptom scores and flow rates. The Medical Therapy of Prostatic Symptoms trial (MTOPS) was conducted in order to examine the long-term effect of combination medical therapy on risk of clinical progression [10]. This 6-year, multicenter study randomized 3047 men aged 50 years and older to receive placebo, doxazosin, finasteride or combination medical therapy of doxazosin plus finasteride. Over a mean follow-up of 4.5 years, combination medical therapy significantly reduced the risk of overall clinical progression by 66% when compared with placebo. This reduction in risk was significantly greater than that associated with doxazosin or finasteride alone. Of note, approximately 78% of the primary outcome events took the form of improvement in AUA symptom scores. Further, doxazosin, finasteride and combination medical therapy each resulted in significant improvement in AUA symptom scores (median changes were decreases of 6.0, 5.0 and 7.0 points, respectively), with combination medical therapy superior to either monotherapy alone. Subsequent post hoc analysis of these data suggested that larger prostate volume was a strong predictor of benefit from combination medical therapy, and the risk of clinical progression amongst prostate volumes less than 25 mL was not statistically different between combination medical therapy and either monotherapy alone [11].
To further address the role of combination medical therapy for men with larger prostate glands, the Combination of Avodart® and Tamsulosin trial was conceived [12]. This 4-year, multicenter trial randomized 4844 men 50 years and older to receive dutasteride and tamsulosin-matched placebo, tamsulosin and dutasteride-matched placebo or combination medical therapy. Men with prostate volumes of 30 mL or greater and a total IPSS of at least 12 were eligible for inclusion. The investigators recently reported the results of a preplanned 2-year interim analysis. Combination medical therapy was associated with a significantly greater decrease from baseline in symptom score compared with dutasteride (at month 3) and tamsulosin group (at month 9) alone [13]. Moreover, increases in peak urinary flow rates from baseline were significantly greater for combination medical therapy when compared with either monotherapy.
Comment
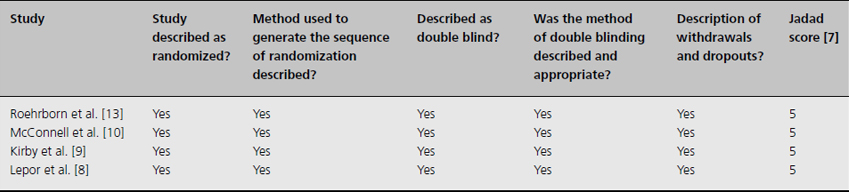
The reviewed trials of combination medical therapy are of substantially high quality (Table 10.2). In accordance with the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) Working Group statement [14], the published data provide strong support for long-term combined use of doxazosin and finasteride to reduce the risk of BPH progression (1A recommendation). And, in men with large glands (volumes greater than 30 mL), there is strong evidence to suggest that combined use of tamsulosin and dutasteride provides added symptom amelioration compared with either therapy alone. It remains uncertain whether there is a “class effect” (i.e. ability to generalize these findings to other pharmacotherapies within the same classes).
Table 10.2 Study quality assessment using Jadad scores for combination medical therapy trials
Clinical question 10.2
Is there a role for antimuscarinic agents in the treatment of male LUTS associated with BPH?
Background
Overactive bladder syndrome, characterized by urinary urgency and increased day and night-time micturition frequency [15], may co-exist with bladder outlet obstruction caused by BPH or may be secondary to the obstruction itself [16]. When the former situation occurs, treatments targeted at the prostate exclusively may not relieve overactive bladder symptoms [17]. In the latter scenario, some men will not respond to antimuscarinics alone. This is the rationale behind combining antimuscarinic agents with alpha-1-adrenergic antagonists for the management of men with overactive bladder and other LUTS.
Literature search
The English-language literature was searched for human studies relating to antimuscarinic therapy for the treatment of male LUTS associated with BPH using the Medline database from 1998, the year that tolterodine earned US Food and Drug Administration approval, to 2008. The search was conducted by exploding and combining the following MeSH terms: “muscarinic antagonists and benign prostatic hyperplasia,” “adrenergic alpha-antagonists and benign prostatic hyperplasia,” “muscarinic antagonists and luts,” and “adrenergic alpha-antagonists and luts.” Only randomized controlled trials comparing combination medical therapy, with antimuscarinics and adrenergic alpha-antagonists, with placebo or an active control were eligible for inclusion in this systematic review. Initially, 63 citations were identified in the electronic database search. Articles excluded were nonresearch reports (e.g. editorials and commentaries), studies on the wrong topic (e.g. trials involving different interventions and observational studies) or studies which did not include clinical outcomes. To judge quality of the clinical trials, a numerical score between 0 and 5 (0 being the weakest and 5 being the strongest) was assigned according to the validated scale reported by Jadad et al. [7].
The evidence
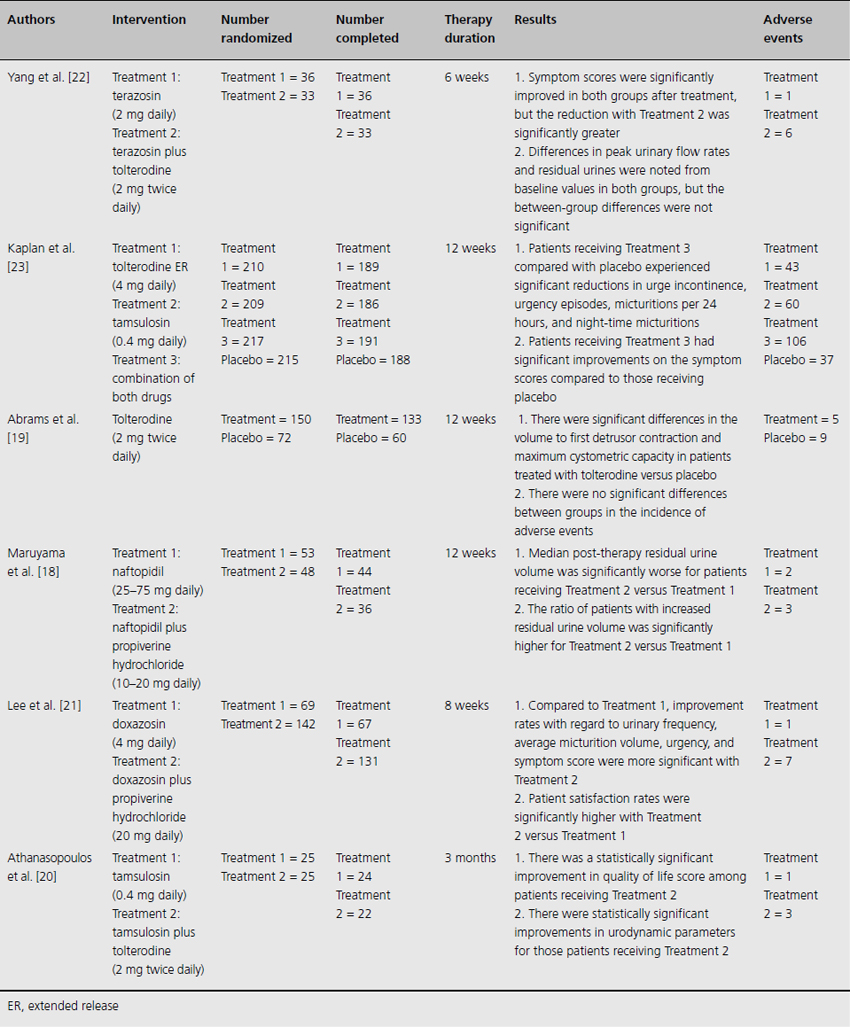
The literature search yielded six randomized controlled trials that were reviewed in detail (Table 10.3). First, in terms of safety, there exists the theoretical risk that the inhibitory effect of antimuscarinics could aggravate the voiding difficulties of men with concomitant bladder outlet obstruction, causing acute urinary retention. These concerns were echoed by a recent, multicenter Japanese study [18]. In total, 101 men with clinically diagnosed BPH and storage symptoms were randomized to receive the alpha-1-adrenergic antagonist naftopidil or naftopidil plus the anticholinergic agent propiverine hydrochloride for 12 weeks. After completing treatment, the median postvoid residual was significantly higher in the combination group compared with the naftopidil monotherapy group (45.0 versus 13.5 mL) but there were no cases of acute urinary retention. In that same year, results were also reported from a multicenter, double-blind trial that randomized 222 men 40 years and older with confirmed overactive bladder and bladder outlet obstruction to receive tolterodine or placebo for 12 weeks [19]. This study found that the mean change from baseline postvoid residual urine was significantly greater with tolterodine than placebo (49 versus 16 mL) although the clinical significance of this difference was uncertain. There were no significant between-group differences in the incidence of adverse events. Moreover, urinary retention was only reported for one subject who had been treated with placebo.
Table 10.3 Randomized clinical trials comparing an antimuscarinic agent (alone or combined with an alpha-1-adrenergic antagonist) to placebo or an active control
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








