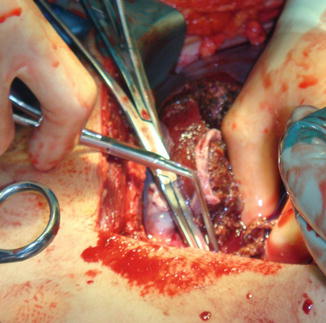
Fig. 5.1
Patient that sustained a close range shotgun blast with an AAST-OIS grade V hepatic injury and significant loss of the abdominal wall. Patient was rapidly transported to the OR and required right hepatic lobectomy
The massive transfusion protocol (MTP) must also be activated. Activation must proceed expeditiously by the leader of the trauma team, preferably the attending trauma surgeon, trauma fellow, or chief surgical resident, and the blood products must also be present in the operating room at the time the patient’s arrival [1–8, 32].
All trauma patients must be placed on the operating table in a cross position with arms extended at a 90° angle. Clearly all patients must be prepped from the neck to the mid-thighs and from side of table to side of table, thus making the thoracic cavity easily accessible if a left anterolateral thoracotomy is required for aortic cross-clamping and open cardiopulmonary resuscitation. Should this be necessary, the standard approach is used. We personally prefer to utilize a Crafoord-DeBakey aortic cross-clamp. Similarly, a median sternotomy may also be performed in the standard fashion.
Among the operating room equipment considered vital are large Poole suction devices (Fig. 5.2) in order to be able to rapidly aspirate blood contained within the abdominal cavity. Similarly, if a blood scavenging autotransfusion system is available, this must be previously set up and managed by the perfusionists so that autotransfused blood may be administered to the trauma patient intraoperatively. The trauma surgeon must work closely with the attending anesthesiologist and the anesthesia team; however, the trauma surgeon must guide the intravascular volume replacement and select the formula for replacement.
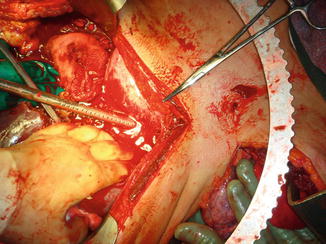

Fig. 5.2
Patient that sustained multiple gunshot wounds requiring a left anterolateral resuscitation thoracotomy and aortic cross-clamping. He recovered an excellent cardiac output and blood pressure. An exploratory laparotomy was carried out. Notice large Poole suction devices required to rapidly remove blood and temporary packing of the liver
Regardless of the choice of formulas, whether it is 1:2 or 1:1, it is clear that ample quantities of blood and blood products including fresh frozen plasma, platelets, and cryoprecipitate must be available. It is also important that the patient be monitored intraoperatively and his or her end points of resuscitation be followed by continuous point of care (POC) testing with arterial blood gases, so that the pH, bicarbonate, base deficit, and lactic acid levels can be monitored along with coagulation parameters and platelet counts. Electrolytes must also be measured including the ionized calcium, magnesium, and phosphorous while also monitoring the patient’s temperature and end-tidal CO2. If available, thromboelastography (TEG) should be used as a guide for intravascular volume replacement [1–8, 32].
Laparotomy: Initial Steps
Exposure
All trauma patients must be approached via a midline laparotomy incision extending from the xyphoid process to the symphysis pubis. The incision must be rapidly carried through into skin and subcutaneous tissue while controlling hemostasis from the transected subcutaneous vessels. We prefer to utilize either Halsted Mosquitos or Kelly clamps sequentially. This is important since many of the patients are already coagulopathic or will become coagulopathic and subcutaneous vessels are a source of fastidious bleeding. The midline fascia is sharply transected and the peritoneum is then grasped with two Schnidt clamps; utilizing a Metzenbaum scissor, it is incised. Attempts must be made to quantitate the blood loss which will be difficult by rapidly placing the two Poole suction devices in the abdominal cavity. It is important to quantitate intra-abdominal blood loss as best as possible. This measurement then serves as an objective guide for intravascular blood volume replacement [1–8].
Once the abdominal cavity is entered, large clots must be manually removed and attempts at direct visualization of the hepatic injury must be carried out. Although it has been traditional surgical lore that all four quadrants of the abdominal cavity must be packed, we do not subscribe to that notion; we prefer to directly approach the bleeding source, in this case, the liver. At this time, simultaneous compression of the injured liver with two laparotomy packs held over the anterior and posterior surfaces of the liver by an assistant will allow the operating surgeon to rapidly place a Bookwalter or Balfour retractor to allow for exposure. This allows the hands of the surgical assistants to be freed to assist the operating surgeon. Many surgical textbooks recommend that the primary operating surgeon be positioned on the left side of the patient. We do not have any strong preference as to whether we position ourselves on the right or left hand side of the table, but clearly exposure of the injured liver is of paramount importance [1–8].
Mobilization
In order to be able to assess and operatively manage complex hepatic injuries AAST-OIS grades IV and V, the liver must be adequately mobilized. To that extent, the trauma surgeon must be familiar with hepatic anatomy to promptly mobilize the liver by sharply transecting the ligaments that hold the liver in place. The first maneuver is to break the suction that exists between the dome of the right lobe of the liver and the diaphragm which is performed manually and then proceed to sharply transect the falciform ligament utilizing two Schnidt clamps while ligating the transected ligament with suture ligatures of 0 or 2-0 Silk. After the falciform ligament has been transected between clamps and ligated, we then utilize electrocautery to transect the ligament in a cephalad direction toward the dome of the liver. One notable exception is the patient with a cirrhotic liver, in which this maneuver should be avoided if at all possible.
Other important maneuvers in mobilizing the liver, depending on which lobe has been injured, include sharply transecting the triangular ligaments of either the right lobe or left lobe of the liver. Transecting the triangular ligament of the right lobe of the liver is accomplished by placing downward traction on the right lobe while digitally locating this ligament. If it is feasible, placement of an index finger under the ligament serves as a guide; Metzenbaum scissors are then utilized to transect this ligament while the liver is simultaneously rotated from right to left in the abdominal cavity. Meticulous attention must be exercised not to extend the transection of the triangular ligament into the two leaflets of the right coronary ligament without proper visualization, as the right hepatic vein is found in close proximity and may be inadvertently injured.
Similarly, to mobilize the left lobe, downward traction must be placed in the left lobe identifying the left triangular ligament which is then sharply transected in the previously described fashion. Again, caution is required so that the transection of the left triangular ligament is not extended to the left coronary ligament without visualization, as the left hepatic or the common trunk of the left and middle hepatic veins is found in close proximity and may be inadvertently injured. Similarly, the left phrenic vessels, but particularly the left phrenic vein, may be iatrogenically injured. To fully mobilize the right lobe of the liver, it is recommended that a series of folded laparotomy packs be placed behind the right lobe of the liver.
Another important maneuver which is part of mobilization consists of identifying the gastroepiploic foramen of Winslow and assessing whether it is fused or not. This is important if the trauma surgeon anticipates performing a Pringle maneuver which would be facilitated if the foramen were open.
Temporary Hepatic Packing and Compression
After the liver has been fully mobilized, compression is required to allow the anesthesiology team to restore intravascular volume losses which must be replaced. Once the injury has been identified, direct compression of the injured lobe or segment of the liver must be carried out with folded laparotomy packs (Fig. 5.3). Compression must be bimanually applied. However, should there be other intra-abdominal injuries, it is judicious to pack the liver to arrest hepatic hemorrhage temporarily while other life-threatening injuries are addressed.
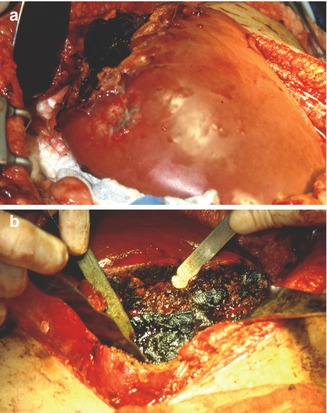

Fig. 5.3
Patient that sustained an abdominal gunshot wound resulting in a grade IV liver injury (a). Temporary intrahepatic packing was done along with an external hepatic compression (b). Notice the malleable retractors
If there are large bleeding parenchymal lacerations, intrahepatic packing must be considered. This can be performed temporarily by placing sheets of hemostatic agents such as SurgicelTM Nu Knit or plain SurgicelTM which can be applied to the denuded and injured surfaces of the intrahepatic parenchyma directly and be covered with a laparotomy pack. Alternatively, gel foam and thrombin patches may also be used during temporary closure of the hepatic laceration. To achieve closure, its edges are coapted by placement of several separate horizontal mattress Halsted sutures with 2-0 chromic sutures on a CT-1 needle to secure this intraparenchymal pack. Similarly, the liver can then be externally packed by applying several folded laparotomy packs compressing the dome of the liver superiorly and inferiorly and by placement of laparotomy packs in the subhepatic pouch of Morison while simultaneously wrapping laparotomy packs around the left lobe of the liver. These maneuvers will allow for temporary control of hemostasis while other life-threatening injuries are addressed and gastrointestinal spillage is controlled [1–8, 14–32].
Injury Identification
It is important for the trauma surgeon to identify and appropriately grade hepatic injuries utilizing the AAST-OIS. It is also important that the trauma surgeon be familiar with Couinaud’s description of hepatic segmental anatomy in order to describe accurately the location of the injury (Fig. 5.4).
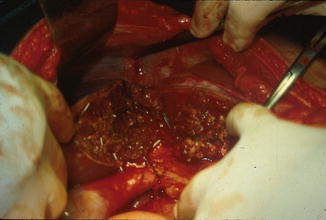

Fig. 5.4
Cantlie’s line showing the delimitation between the right and left liver, exposing the retrohepatic inferior vena cava (IVC). Notice the several clips applied in order to control hemostasis. Falciform ligament is also seen marking the left segmental fissure to the lobar fissure
At times, injuries to Couinaud’s segment I, the caudate lobe (Spiegel’s lobe), can be challenging to evaluate as they are often associated with a significant zone I supra-mesocolic and right zone II retroperitoneal hematomas. In order to identify injuries in this segment, a right-sided medial visceral rotation must be performed by sharp transection of the avascular line of Toldt which holds the right colon in place. This is carried out by a meticulous combination of blunt and sharp dissection with Metzenbaum scissors while extending this maneuver above the hepatic flexure. Gentle sweeping from the right to left of the colon, digitally or utilizing sponge sticks, will expose the infrarenal inferior vena cava (IVC).
Meticulous attention must be directed to identifying the right ureter and the right gonadal vein which drains in the anterior surface of the infrarenal IVC. An extensive Kocher maneuver must be added, also utilizing a meticulous combination of blunt and sharp dissection mobilizing the first through the third portion of the duodenum from medial to lateral which can be performed while the assistant holds gentle traction on the duodenal C-loop. These maneuvers will expose injuries to the caudate (Spiegel’s lobe). In addition, suprarenal dissection of the IVC can then be carried out utilizing a meticulous combination of blunt and sharp dissection. We prefer to separate the blood suffused tissues of the retroperitoneum utilizing either Kittner dissectors or sponge sticks while preserving the integrity of the very delicate IVC [1–8, 17–32].
After the liver has been mobilized and exposed, it is important to carefully observe and visualize the injury in order to assign an AAST-OIS injury grade. This is important, as intraoperative injury grading will lead to appropriate technique selection from the vast armamentarium of surgical techniques that a trauma surgeon must possess in order to deal with these injuries. Trauma surgeons must be cognizant of the fact that only the most complex surgical techniques will be required for the management of complex hepatic injuries grade IV or V; however, these techniques are infrequently utilized.
We prefer to grade the depth of the injury by placing the end of a scalpel which has a ruler in order to measure the depth of the injury. This must be done gently and not to cause any further bleeding. Alternatively, a sterile metal ruler can also be utilized to measure depth. Once this maneuver has been performed, the surgeon will have all the necessary information required to assign an injury grade and select the appropriate surgical technique to control the hemostasis [1–8, 17–32].
Decisions
Thoracotomy
The decision to perform a left anterolateral resuscitative thoracotomy is easy and usually dictated by the patient’s hemodynamic compromise or the onset cardiopulmonary arrest. The standard incision is utilized commencing at the fifth intercostal space from the left sternocostal margin and extending it laterally to the medial border of the left latissimus dorsi. All patients should have their left arm elevated in a cephalad direction to open the intercostal spaces. Similarly, in female patients, the left breast must be also elevated cephalad to avoid iatrogenic injury [33–42].
The incision is sharply carried through into skin and subcutaneous tissues utilizing a 10 or 21 scalpel blade. The intercostalis muscle is then identified and sharply incised for approximately 2 cm. Insertion of the index finger will allow guidance to sharply transect the three slips of this muscle utilizing Metzenbaum scissors. This incision is sharply carried through from the medial to the lateral border with simultaneous transection of the parietal pleura.
Placement of a Finochietto retractor follows completion of the incision to access the left hemithoracic cavity. At that time, evacuation of any blood is carried out. Inspection of the left lung and pericardium is also carried out. Whether or not there is an associated cardiac injury, the pericardium is then grasped with two Allis clamps anterior to the left phrenic nerve and, utilizing either a 10 or 15 scalpel blade, a 1–2 cm incision is created as the two Allis clamps lift the pericardium off the heart to avoid iatrogenic injury. At that time, Metzenbaum scissors are then inserted and the incision extended in a cephalad direction with the upper limits being the root of the ascending aorta. Similarly, the incision is then extended in a caudad direction toward the apex of the heart and any clots located are evacuated. This is an important maneuver if open cardiopulmonary resuscitation is required [33–42].
Attention is then shifted toward cross-clamping the descending thoracic aorta, another vital maneuver, to be able to redistribute the remaining blood volume to perfuse both the carotid and coronary arteries as the patient’s intravascular blood volume is rapidly replaced.
The descending thoracic aorta is located against the spinal column. The NG tube placed during the initial assessment and resuscitation will serve to identify the esophagus which crosses in the lower aspect of the left hemithoracic cavity from the right to the left. Having identified the esophagus, the aorta is palpated and then utilizing a meticulous combination of blunt and sharp dissection with Metzenbaum scissors, the circumference of the aorta is dissected. At that time, the aorta may be digitally occluded or clamped with a Crafoord-DeBakey aortic clamp to allow the anesthesiologists to replace lost intravascular blood volume. With this maneuver, the patient’s blood pressure should rise to between 80 and 100 systolic; if this occurs, it serves as a positive indicator of outcome. Similarly, this maneuver will also help in arresting arterial subdiaphragmatic hemorrhage.
The decision to perform a right anterolateral thoracotomy is more difficult. It is indicated in the presence of significant hemorrhage from the right dome of the liver especially if there has been an associated destructive injury to the right hemidiaphragm. The same previously described technique for a left anterolateral thoracotomy applies.
The decision to perform median sternotomy is a difficult one and should be considered when there is extensive injury to the dome of the liver which may involve the hepatic veins draining into the retrohepatic vena cava or an injury to the retrohepatic vena cava itself. A median sternotomy must be rapidly performed with the incision commencing at the sternal notch and ending in the xyphoid process utilizing a 10 or 21 scalpel blade and transecting the skin and subcutaneous tissues. It is important to remain in the midline of the sternum. This incision is sharply carried through skin and subcutaneous tissue and electrocautery is used to achieve hemostasis [33–42].
A meticulous combination of blunt and sharp dissection at the sternal notch must be carried out in order to open a plane to place the sternal saw. This can be carried out with Mixter right angle or Schnidt clamps. The trauma surgeon must be aware that both the innominate artery and vein are in close juxtaposition with the posterior surface of the upper sternum in order to avoid iatrogenic injury. Once this plane has been sufficiently opened, the sternal saw is placed and the anesthesiologists are asked to temporarily cease ventilation. The sternal saw is then directed from a cephalad to caudad direction [33–42].
Once the sternum is open, two Army-Navy retractors are used to elevate the transected right and left portions of the sternum while electrocautery is utilized to secure hemostasis. Electrocautery must be used directly under the border of the sternum at a depth of no more than 0.5 cm in order to avoid iatrogenic injury to the internal mammary arteries. A Finochietto retractor is then placed and gradually opened. At times, it is important to place several laparotomy packs and folded surgical towels to buttress and support this retractor inferiorly. The thymus in young patients is identified in the upper portion of this incision and should be swept in both cephalad and lateral directions utilizing either sponge sticks or Kittner dissectors. The fibrous tissues of the pericardium are then identified and grasped between two Allis clamps and are then incised from a cephalad to caudad direction in a linear fashion to expose the heart. We prefer utilizing radial incisions in the inferior borders of the pericardium in order to provide wider exposure for visualization of the heart. The pericardium is then secured with stay sutures of 0 Silk to the flanges of the Finochietto sternal retractor. The heart is then identified and attention is then directed toward locating the space of Gibbon which defines the entrance of the suprahepatic cava through the diaphragm into the right atrium. Frequently this space is fused. This space should not be dissected as it is easy to injure the very delicate intrapericardial portion of the IVC. Enough space exists for the placement of a Crafoord-DeBakey aortic clamp at the IVC should this be necessary [33–42].
Median Sternotomy
Median sternotomy was frequently used in the past when trauma surgeons utilized Schrock’s atrio-caval shunt. In our opinion, median sternotomy should now be infrequently used and only for very selective cases. Similarly, we as well as the vast majority of trauma surgeons have abandoned the use of the atrio-caval shunt. Nevertheless, the trauma surgeon must be facile in performing either a thoracotomy or median sternotomy in selected cases of grade IV and V hepatic injuries.
Injury Grading
Since the advent of the AAST Liver Injury Scale (Table 5.1), the injury grading system for the liver has undergone several modifications. Consistent hepatic injury grading has proven to be a great advancement in the management of hepatic injuries (Fig. 5.5), as it allows the trauma surgeon to select the appropriate surgical technique from their vast armamentarium to manage injuries appropriately stratified to injury grade, thus reserving the most complex surgical techniques for the most complex injuries. Similarly, establishing uniformity in the description of these injuries has advanced research as means of comparing outcomes for these injuries [1–4, 7].
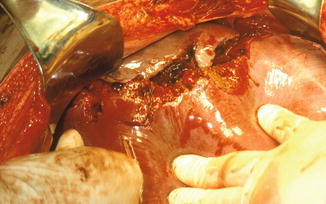
Table 5.1
The American Association for the Surgery of Trauma (AAST) liver injury scale (1994 revision)
Gradea | Type of injury | Description of injury |
|---|---|---|
I | Hematoma | Subcapsular, <10 % surface area |
Laceration | Capsular tear, <1 cm parenchymal depth | |
II | Hematoma | Subcapsular, 10–50 % surface area |
Intraparenchymal <10 cm in diameter | ||
Laceration | Capsular tear 1–3 parenchymal depth, <10 cm in length | |
III | Hematoma | Subcapsular, >50 % surface area of ruptured subcapsular or parenchymal hematoma; intraparenchymal hematoma |
>10 cm or expanding | ||
Laceration | >3 cm parenchymal depth | |
IV | Laceration | Parenchymal disruption involving 25–75 % hepatic lobe or 1–3 Couinaud’s segments |
V | Laceration | Parenchymal disruption involving >75 % of hepatic lobe or >3 Couinaud’s segments within a single lobe |
Vascular | Juxtahepatic venous injuries, i.e., retrohepatic vena cava/central major hepatic veins | |
VI | Vascular | Hepatic avulsion |

Fig. 5.5
Case of a hepatic complex injury AAST-OIS grades IV–V
Maneuvers to Control Hemorrhage
The Pringle Maneuver and Initial Decision Making
Since this maneuver was described in 1908 by J. Hogarth Pringle [38], it has proven to be a significant and valuable adjunct in the surgical armamentarium for the management of hepatic injuries (Fig. 5.6). Although originally described in animals, specifically in rabbits, its use was extended to patients. This maneuver was utilized by Madding and Kennedy [44] in their classical paper from their experiences based in the European theater of combat during World War II and subsequently emphasized by the same authors in their classical 1965 monograph Trauma to the Liver [45].