This article discusses the diagnostic and therapeutic options in the management of urethral cancer recurrence in patients treated with urethral sparing cystectomy as well as those who had urethral preservation following primary urethral carcinoma.
Key points
- •
Urethral carcinoma is a rare disease and may present as primary cancer or be associated with disease of the bladder at the time of cystectomy.
- •
There are relatively more data on management of urethral recurrence following cystectomy for urothelial carcinoma of the bladder.
- •
Urethral metachronous relapses have been managed with both urethra preserving as well as urethrectomy.
- •
There are minimal data on management of relapse following primary urethral carcinoma. The management options are similar to those seen with primary urethral cancer.
- •
Multimodal therapies with chemotherapy and radiation should be considered in more advanced disease.
Introduction
Urethral carcinoma can be encountered in the settings of primary urethral cancer, synchronous presentation with other genitourinary (GU) malignancies, relapse following primary urethral cancer, or metachronous recurrence after treatment of other GU malignancies. Regardless of clinical situation and presentation, there are comparatively few data on treatment options for primary and recurrent urethral cancer. There are no prospective studies in the management of urethral carcinoma, and the current information is largely based on retrospective experience, often from single institutions.
This article will review some of the reported therapeutic strategies in the management of recurrent urethral carcinoma. It describes treatment options in recurrences encountered following cystectomy as well as relapse following treatment for primary urethral cancer.
Introduction
Urethral carcinoma can be encountered in the settings of primary urethral cancer, synchronous presentation with other genitourinary (GU) malignancies, relapse following primary urethral cancer, or metachronous recurrence after treatment of other GU malignancies. Regardless of clinical situation and presentation, there are comparatively few data on treatment options for primary and recurrent urethral cancer. There are no prospective studies in the management of urethral carcinoma, and the current information is largely based on retrospective experience, often from single institutions.
This article will review some of the reported therapeutic strategies in the management of recurrent urethral carcinoma. It describes treatment options in recurrences encountered following cystectomy as well as relapse following treatment for primary urethral cancer.
Urethral recurrence after cystectomy
Prophylactic urethrectomy at the time of radical cystectomy for urothelial carcinoma is often performed in patients who are considered to be at high risk for urethral recurrence. Certain contemporary indications for prophylactic urethrectomy in men include cystectomy for nonmuscle invasive bladder cancer (NMIBC), history of recurrent NMIBC, involvement of the prostatic urethra by urothelial cancer, or microscopic involvement of the urethra based on positive intraoperative urethral frozen sections. In female patients, cancer at the bladder neck or cancer involving the anterior vaginal wall is associated with an increased risk of urethral recurrence.
It is reported that following radical cystectomy, new urethral tumors occur in 1.5% to 6% of men and 6% to 11% of women. Some have suggested that the incidence of urothelial recurrence in the female urethra may be lower, possibly related to prominence of squamous mucosa in the female urethra. Furthermore, the incidence of urethral recurrence is reportedly also lower in both men and women treated with neobladder reconstruction (1%–4%). Most urethral recurrences occur within 24 months after surgery, with reported median time to relapse of 8 to 28 months. In a report that included 7 patients with neobladder substitution who had initial negative intraoperative frozen sections but positive urethral margins at final pathology, only 1 patient subsequently developed recurrence in the urethra. Similarly, of 136 patients with moderate-to-severe atypia or positive urethral margins, only 5 patients (3.7%) subsequently developed urethral recurrence.
Lower incidence of urethral recurrence in patients with neobladders may to some extent relate to selection bias, with lower-risk patients or those with negative bladder neck or prostatic urethral margins being selected for neobladder diversion. However, some authors have suggested that even after controlling for potential risk factors such as tumor grade and multifocality, carcinoma in situ, and pathologic stage, as well as prostatic involvement, patients with neobladder diversions may still have lower adjusted rates for urethral recurrence compared with cutaneous diversions. Others have suggested urine remaining in contact with the urothelium may be protective or that juxtaposition of the ilium to the urethra may provide a degree of protection, although none of these purposed mechanisms has been proven.
The detection and diagnosis of urethral recurrence following radical cystectomy can be secondary to symptomatic presentation or detection through routine surveillance with urethral wash cytology or endoscopic examination.
One reported technique for performing a urethral wash involves the insertion of a minimally lubricated 14 French (Fr) catheter into the proximal penile urethra, followed by irrigation of 100 mL of normal saline solution and collection of all extruded solution at the level of the urethral meatus. The catheter should be withdrawn gradually and removed while flushing continues ( Fig. 1 ).
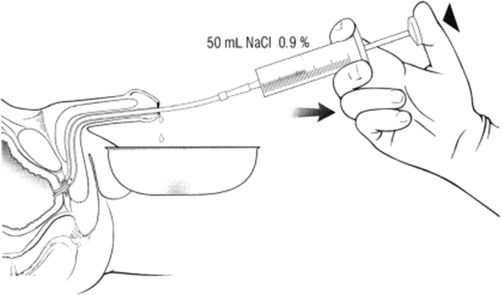
The value of routine surveillance, however, is questioned by some, as there is evidence that positive urethral wash cytology may not improve survival. In several case series, patients experienced similar survival rates, regardless of whether they received treatment following diagnosis based on symptoms (bleeding, urethral discharge, pain, or palpable mass) or based primarily on positive urethral cytology. It has also been suggested that while asymptomatic patients diagnosed by cytology might have a higher chance of harboring noninvasive disease, overall survival may not be significantly better than survival in symptomatic patients with invasive (pT1-pT4) cancer. On the contrary, others have demonstrated that noninvasive recurrences have a favorable prognosis when detected early, and there is evidence that patients who present with symptomatic urethral recurrences have significantly worse survival than those who present asymptomatically, most likely related to more advanced or potentially metastatic disease at presentation. An additional advantage of urethra wash cytology may be diagnosis at an earlier stage, permitting urethral preservation.
Management of urethral recurrence is dictated by disease stage, grade and location, patient comorbidities, the type of diversion, as well as the presence or absence of systemic disease.
Urethral preserving approaches, both with orthotopic and cutaneous diversions, have been described. In a series of 15 urethral recurrences in patients with previous cystectomy and orthotopic neobladder reconstruction, 10 patients were treated with intraurethral bacillus Calmette-Guérin (BCG). In this series, all but one of the patients were diagnosed through surveillance with urethral wash cytology.
The technique of intraurethral BCG administration involved modification of an existing 14 Fr Foley catheter where the catheter just distal to the Foley balloon was ligated to occlude the drainage holes. Instead, 5 new irrigation openings were cut in the lumen of the catheter over a 2 to 3 cm length, starting approximately 0.5 cm proximal to the balloon. This nonlubricated catheter was subsequently inserted into the bladder and the balloon inflated to 10 mLs of fluid. Thereafter100 mL of standard concentration of BCG was infused at 20 cm of water pressure over 75 minutes, with the patient changing position during urethral irrigation. Once the irrigation was completed, the catheter was removed, and another 25 mLs of standard concentration BCG was instilled into the urethra and penile clamp placed to facilitate topical contact of the BCG with the urethra for an additional 25 minutes. This was repeated again with another 25 mLs of BCG solution ( Fig. 2 ). This treatment approach was utilized for 6 weeks total. No systemic adverse effects were reported in patients treated with this technique. Five of 6 men with new urethral carcinoma in situ (CIS) responded to intraurethral BCG therapy, whereas none of the 4 patients with papillary disease responded to the treatment. Subsequently, patients with CIS who responded to BCG had significantly longer survival (85 months compared with 26 months). Investigators from a subsequent publication reported a 92% response rate using this technique in 13 patients with recurrence of urethral CIS.
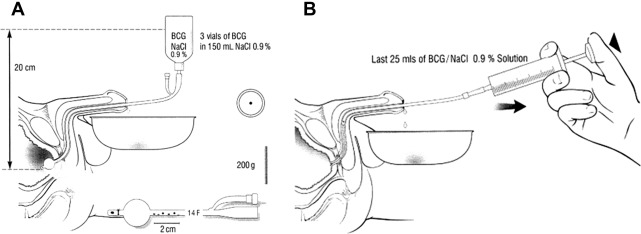
Several small series have described transurethral resection (TUR) and fulguration without BCG therapy in patients with clinical Ta and clinical T1 tumor recurrences, with reported satisfactory local response rates. However, in another report of 4 patients treated with TUR and no topical therapy, 3 patients eventually required urethrectomy. Overall, topical ablative therapies should only be considered in selected cases, where tumors or tumor volumes are considered small, adequately accessible, and with a good chance for complete excision or destruction.
Distal urethrectomy with perineal urethrostomy has also been described in patients with urethral recurrence after neobladder diversion. In a series of five such cases, 2 patients subsequently required total urethrectomy. Nevertheless, a subtotal urethrectomy remains an option in selected cases.
In situations where urethral preservation is not feasible, urethrectomy should be considered. In men with orthotopic neobladder substitutions, conversion to an ileal conduit may be necessary. Long-term outcomes in these patients will depend on local stage and likelihood of metastatic progression. In a relatively large series, 49 patients with recurrent urethral tumors following cystectomy were treated with urethrectomy. Of these patients, 57% eventually died of disease. At a median follow-up of 38 months, disease-specific survival rates were 85%, 65%, and 55% at 2, 3, and 5 years, respectively. The authors demonstrated that symptomatic presentations, and earlier recurrences were associated with worse survival outcomes.
Akkad and colleagues described 4 urethral recurrences following radical cystectomy in female patients. Two of the patients had orthotopic neobladders, and 2 patients had ileal conduit diversions. All were treated with urethrectomy, with conversion of neobladders to ileal conduits. Patients without metastatic disease had good long-term outcomes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




