Management of interstitial cystitis/bladder pain syndrome (IC/BPS) is individualized for each patient. All patients benefit from education and self-care advice. Patients with Hunner lesions usually respond well to fulguration or triamcinolone injection, which can be repeated when the symptoms and lesions recur. For patients without Hunner lesions, numerous treatment options are available. The tiers of the American Urological Association Guidelines present these options in an orderly progression, balancing the benefits, risks, and burdens. Along with specific IC/BPS treatments, it is also important to have resources for stress reduction, pain management, and treatment of comorbid conditions.
- •
Education and advice on self-care for all patients.
- •
Fulguration or triamcinolone injection for Hunner lesions.
- •
For patients without Hunner lesions, many options are available to balance benefits, risks, and burdens.
- •
Pain management and treatment of comorbid conditions as needed.
Introduction and definitions
Most experienced clinicians recognize the syndrome originally known as interstitial cystitis (IC). However, a formal clinical definition for IC has never been established. The National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) established criteria for IC, but these criteria were intended for enrollment of patients into research studies and were not intended for clinical use. In fact, the NIDDK criteria are so restrictive that they exclude approximately half of patients thought by experienced clinicians to have IC.
In addition to the lack of a clinical definition, the term “interstitial cystitis” also suffers from being scientifically inaccurate. The disease may not involve the bladder interstitium, and some patients lack bladder inflammation (cystitis). For all of these reasons, different organizations have proposed new definitions. The International Continence Society published the Painful Bladder Syndrome (PBS) definition in 2002 ; the European Society for the Study of IC published the Bladder Pain Syndrome definition in 2008, and the Society for Urodynamics and Female Urology published the IC/BPS definition in 2009. The differences between these definitions are summarized in Appendix 1 .
For research articles, it is important to specify one of these definitions to allow comparison of study outcomes. In clinical use, the importance of the name depends on the scenario. If a patient is applying for Social Security disability, the name IC should be used because it is a recognized diagnosis for that purpose. It may also be important to use the name IC if prescribing pentosan polysulfate (PPS) or dimethylsulfoxide (DMSO) because they are specifically indicated for IC. On the other hand, the name does not affect one’s decision to treat the bladder, after determining that the bladder is the source of pain.
American Urological Association Guidelines
In 2011 the American Urological Association (AUA) completed guidelines on the treatment of IC/BPS, based on a literature review from January 1, 1983 to July 22, 2009. The guidelines are published and are available online ( http://www.auanet.org/content/clinical-practice-guidelines/clinical-guidelines/main-reports/ic-bps/diagnosis_and_treatment_ic-bps.pdf ). For each treatment, a statement was made based on the available evidence. The different types of statements (eg, standard, recommendation) are summarized in Appendix 2 . The guidelines include general clinical principles, followed by 6 specific tiers of treatment.
The general clinical principles were defined as being widely agreed on by urologists or other clinicians, for which there may or may not be evidence in the medical literature. These principles are important for the care of IC/BPS patients and should be kept in mind throughout treatment; they are summarized in Appendix 3 . Among these, the authors especially emphasize to stop ineffective treatment after a clinically meaningful interval. Such action is easy to overlook in a busy practice, but is important for 2 reasons. First, it avoids the usual concerns with polypharmacy (expense, drug interactions, and so forth). Also, specific to IC/BPS, many of the usual medicines (and muscle relaxants for comorbid pelvic floor spasm) cause fatigue. If ineffective medicines are stopped, the patient can tolerate higher doses of potentially effective medicines.
Although not specifically discussed in the guidelines, clinicians who care for IC/BPS patients should be aware of the placebo and nocebo effects. The placebo effect refers to real physiologic changes that improve pain and other symptoms. In contrast to common belief, it is not necessary to give an inert substance to elicit the placebo response. In fact, this response can be additive to active drug treatment. Clinicians can elicit the placebo response by explaining the mechanism of symptoms and the mechanisms by which the treatment is expected to relieve the symptoms, thus increasing the patient’s expectation of success and giving the patient an increased sense of control. The nocebo effect also is physiologic and refers to the fact that anxiety increases pain perception, something that can be blocked chemically by diazepam or a cholecystokinin receptor antagonist. It follows that clinicians can decrease pain perception through behaviors that decrease anxiety. Not only should the clinician convey that he or she cares, but it is also important to have a reliable person in the office to return phone calls and treat flares promptly. Dedicated urology nurses are very helpful.
The 6 tiers of treatments are listed in Appendix 4 and are discussed in detail in the guidelines. Tier 1 involves education, including IC/BPS knowledge base, risks and burdens of available treatments, the likely need to try multiple treatments, and self-care practices.
It is important to explain clearly the elimination diet trial. The authors’ usual practice is to give the patient a list of foods that may possibly exacerbate symptoms. These lists can be found on the International Cystitis Association (ICA) Web site ( www.ichelp.org ) or in The Interstitial Cystitis Survival Guide . It is explained to the patient that these foods are possible bladder irritants, but that they may not all apply to that individual. It is recommended that the patient avoid all foods on the list for 1 week, after which individual foods can be tried one at a time to evaluate for symptom exacerbation. If a specific food is going to exacerbate symptoms, it will do so within 24 hours.
Stress is well known to exacerbate IC/BPS symptoms; therefore, stress management is an essential aspect of IC/BPS care. Stress management has 2 main components, the first of which is to decrease stress as much as is feasible: working a reduced schedule at work, obtaining help with household chores, psychological help for emotional difficulties, and so forth. However, because some degree of life stress is unavoidable, the second component is to decrease the numerous physiologic effects of stress, which may increase pain in IC/BPS and other pain disorders. Meditation, yoga, mindfulness training, and guided imagery are among methods that may be used to decrease the effects of stress on the body. Future research may reveal specific medical therapies that interrupt the pathways by which stress increases IC/BPS symptoms.
Examples of other self-care practices include: (1) altering the concentration and/or volume of urine, by either fluid restriction or additional hydration; (2) application of local heat or cold over the bladder or perineum; (3) over-the-counter products (eg, neutraceuticals, calcium glycerophosphates, pyridium); (4) bladder training with urge suppression; (5) avoidance of tight-fitting clothing; and (6) avoidance of constipation. Two excellent self-care resources are The Interstitial Cystitis Survival Guide and the ICA Web site www.ichelp.org .
The efficacy of education must not be underestimated. An interesting example comes from two placebo-controlled trials of amitriptyline. In the first trial, the mean decrease in International Cystitis Symptom Index/International Cystitis Problem Index (ICSI/ICPI) scores was 8.4 in the amitriptyline group and 3.5 in the placebo group. In the second trial, mean decrease in ICSI/ICPI scores was 10 in the amitriptyline group and 7.2 in the placebo group. A key difference was that all patients in the second trial received education. Thus, education plus placebo was almost as effective as amitriptyline alone, and much better than placebo alone.
Tier 2 includes several treatments. First, as a clinical principle, appropriate manual physical therapy techniques (eg, maneuvers that resolve pelvic, abdominal, and/or hip muscular trigger points, lengthen muscle contractures, and release painful scars and other connective tissue restrictions), should be offered if appropriately trained clinicians are available. It is important to emphasize that the goal of therapy is muscle or connective tissue relaxation. Pelvic-floor strengthening exercises (eg, Kegel exercises) should be avoided. Second, multimodal pain management approaches (eg, pharmacologic, stress management, manual therapy if available) should be initiated. Third, the guidelines list a variety of oral and intravesical medication options. In brief, these include amitriptyline, cimetidine, hydroxyzine, and PPS; and intravesical DMSO, heparin, and lidocaine.
Tier 3 includes cystoscopic treatments. It is important to recognize Hunner lesions because, if present, the AUA Guidelines recommend going directly to cystoscopic treatment instead of proceeding through the tiers sequentially. In most cases, Hunner lesions can be recognized without bladder distention. Descriptions of the appearance of Hunner lesions vary. For example, Peeker and Fall described “a reddened mucosal lesion with small vessels radiating toward a central pale scar, fibrin deposit or coagulum. This site ruptures with increasing bladder distention with petechial oozing of blood from the ulcer and mucosal margins.” Parsons described “velvet red patch that looks, for all practical purposes, like carcinoma in situ.” They are illustrated in Fig. 1 and elsewhere.
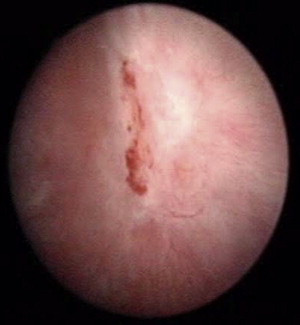
Direct treatment of the Hunner lesion can be fulguration (laser or cautery) or triamcinolone injection. With fulguration, the authors find it useful to first outline the ulcer with the laser or cautery, then fill it in. If one starts from the inside of the ulcer and works out, reactive erythema spreads outward and obscures the original boundaries of the lesion. For either treatment, initial success rates are high but the symptoms (and lesions) usually recur over time. If so, treatment can be repeated.
If Hunner lesions are not present, the Tier 3 option is bladder distention under full general or regional anesthesia, which should be done with low-pressure (60–80 cm water) and duration of less than 10 minutes. The purpose of bladder distention here is to improve symptoms. Distention currently has no role in diagnosis. Symptom relief usually lasts less than 6 months. Partial relief occurs in 50% to 60% of patients, but fewer than 20% achieve excellent improvement.
The evidence supporting Tiers 4 and 5 (neuromodulation, cyclosporine A, and botulinum toxin injection) for IC/BPS is limited by many factors including study quality, small sample sizes, and lack of durable follow-up. None of these therapies have been approved by the US Food and Drug Administration (FDA) for this indication. The guidelines state that these interventions are not for generalized use, but rather should be limited to practitioners with experience in managing IC/BPS and willingness to provide long-term care of these patients after intervention.
Tier 6 is substitution cystoplasty or urinary diversion, and should also be limited to experienced providers. Patients with end-stage, structurally small bladders, that is, capacity under anesthesia less than 400 mL, are most likely to have good outcomes.
American Urological Association Guidelines
In 2011 the American Urological Association (AUA) completed guidelines on the treatment of IC/BPS, based on a literature review from January 1, 1983 to July 22, 2009. The guidelines are published and are available online ( http://www.auanet.org/content/clinical-practice-guidelines/clinical-guidelines/main-reports/ic-bps/diagnosis_and_treatment_ic-bps.pdf ). For each treatment, a statement was made based on the available evidence. The different types of statements (eg, standard, recommendation) are summarized in Appendix 2 . The guidelines include general clinical principles, followed by 6 specific tiers of treatment.
The general clinical principles were defined as being widely agreed on by urologists or other clinicians, for which there may or may not be evidence in the medical literature. These principles are important for the care of IC/BPS patients and should be kept in mind throughout treatment; they are summarized in Appendix 3 . Among these, the authors especially emphasize to stop ineffective treatment after a clinically meaningful interval. Such action is easy to overlook in a busy practice, but is important for 2 reasons. First, it avoids the usual concerns with polypharmacy (expense, drug interactions, and so forth). Also, specific to IC/BPS, many of the usual medicines (and muscle relaxants for comorbid pelvic floor spasm) cause fatigue. If ineffective medicines are stopped, the patient can tolerate higher doses of potentially effective medicines.
Although not specifically discussed in the guidelines, clinicians who care for IC/BPS patients should be aware of the placebo and nocebo effects. The placebo effect refers to real physiologic changes that improve pain and other symptoms. In contrast to common belief, it is not necessary to give an inert substance to elicit the placebo response. In fact, this response can be additive to active drug treatment. Clinicians can elicit the placebo response by explaining the mechanism of symptoms and the mechanisms by which the treatment is expected to relieve the symptoms, thus increasing the patient’s expectation of success and giving the patient an increased sense of control. The nocebo effect also is physiologic and refers to the fact that anxiety increases pain perception, something that can be blocked chemically by diazepam or a cholecystokinin receptor antagonist. It follows that clinicians can decrease pain perception through behaviors that decrease anxiety. Not only should the clinician convey that he or she cares, but it is also important to have a reliable person in the office to return phone calls and treat flares promptly. Dedicated urology nurses are very helpful.
The 6 tiers of treatments are listed in Appendix 4 and are discussed in detail in the guidelines. Tier 1 involves education, including IC/BPS knowledge base, risks and burdens of available treatments, the likely need to try multiple treatments, and self-care practices.
It is important to explain clearly the elimination diet trial. The authors’ usual practice is to give the patient a list of foods that may possibly exacerbate symptoms. These lists can be found on the International Cystitis Association (ICA) Web site ( www.ichelp.org ) or in The Interstitial Cystitis Survival Guide . It is explained to the patient that these foods are possible bladder irritants, but that they may not all apply to that individual. It is recommended that the patient avoid all foods on the list for 1 week, after which individual foods can be tried one at a time to evaluate for symptom exacerbation. If a specific food is going to exacerbate symptoms, it will do so within 24 hours.
Stress is well known to exacerbate IC/BPS symptoms; therefore, stress management is an essential aspect of IC/BPS care. Stress management has 2 main components, the first of which is to decrease stress as much as is feasible: working a reduced schedule at work, obtaining help with household chores, psychological help for emotional difficulties, and so forth. However, because some degree of life stress is unavoidable, the second component is to decrease the numerous physiologic effects of stress, which may increase pain in IC/BPS and other pain disorders. Meditation, yoga, mindfulness training, and guided imagery are among methods that may be used to decrease the effects of stress on the body. Future research may reveal specific medical therapies that interrupt the pathways by which stress increases IC/BPS symptoms.
Examples of other self-care practices include: (1) altering the concentration and/or volume of urine, by either fluid restriction or additional hydration; (2) application of local heat or cold over the bladder or perineum; (3) over-the-counter products (eg, neutraceuticals, calcium glycerophosphates, pyridium); (4) bladder training with urge suppression; (5) avoidance of tight-fitting clothing; and (6) avoidance of constipation. Two excellent self-care resources are The Interstitial Cystitis Survival Guide and the ICA Web site www.ichelp.org .
The efficacy of education must not be underestimated. An interesting example comes from two placebo-controlled trials of amitriptyline. In the first trial, the mean decrease in International Cystitis Symptom Index/International Cystitis Problem Index (ICSI/ICPI) scores was 8.4 in the amitriptyline group and 3.5 in the placebo group. In the second trial, mean decrease in ICSI/ICPI scores was 10 in the amitriptyline group and 7.2 in the placebo group. A key difference was that all patients in the second trial received education. Thus, education plus placebo was almost as effective as amitriptyline alone, and much better than placebo alone.
Tier 2 includes several treatments. First, as a clinical principle, appropriate manual physical therapy techniques (eg, maneuvers that resolve pelvic, abdominal, and/or hip muscular trigger points, lengthen muscle contractures, and release painful scars and other connective tissue restrictions), should be offered if appropriately trained clinicians are available. It is important to emphasize that the goal of therapy is muscle or connective tissue relaxation. Pelvic-floor strengthening exercises (eg, Kegel exercises) should be avoided. Second, multimodal pain management approaches (eg, pharmacologic, stress management, manual therapy if available) should be initiated. Third, the guidelines list a variety of oral and intravesical medication options. In brief, these include amitriptyline, cimetidine, hydroxyzine, and PPS; and intravesical DMSO, heparin, and lidocaine.
Tier 3 includes cystoscopic treatments. It is important to recognize Hunner lesions because, if present, the AUA Guidelines recommend going directly to cystoscopic treatment instead of proceeding through the tiers sequentially. In most cases, Hunner lesions can be recognized without bladder distention. Descriptions of the appearance of Hunner lesions vary. For example, Peeker and Fall described “a reddened mucosal lesion with small vessels radiating toward a central pale scar, fibrin deposit or coagulum. This site ruptures with increasing bladder distention with petechial oozing of blood from the ulcer and mucosal margins.” Parsons described “velvet red patch that looks, for all practical purposes, like carcinoma in situ.” They are illustrated in Fig. 1 and elsewhere.






