Introduction
Erectile dysfunction (ED) affects up to 30 million men in the United States [1]. Seven percent of men aged 18–29 years report symptoms of ED with the prevalence increasing to more than 50% of men between 40 and 70 years of age [2,3]. As the male population in the developed world ages and worsens in overall health and fitness, the risks of diabetes, obesity and cardiovascular disease will dramatically increase the incidence and prevalence of erectile dysfunction [4]. Combining these trends with men’s expectations for quality of life throughout the lifespan, a significant burden will be placed on healthcare systems to diagnose and treat male sexual dysfunctions. Such expectations will increasingly come into conflict with fiscal and regulatory constraints.
Between 1994 and 2000, annual medical expenditures for ED in the United States increased substantially from $185 million to nearly $330 million [5]. The economic impact of ED has until recently excluded pharmaceutical costs, which now make up the majority of treatment-related ED costs. For example, the 2006 worldwide sales of the phosphodiesterase 5 enzyme (PDE5) inhibitors were over $3 billion: Viagra™ $1.6 billion, Cialis™ $971 million and Levitra™ $464 million. US sales represent approximately half of the global sales total (www.wikinvest.com/concept/Erectile_dysfunction_drug_market). It is certain that policy makers and third-party payers will seek evidence-based approaches to determine the feasibility and appropriateness of coverage for the treatment of ED. The stakes are not trivial: if all men in the United States sought treatment for ED, the yearly total costs, including pharmaceutical costs, could exceed $10 billion.
Release of nitric oxide from autonomic nerves and endothelial cells during sexual stimulation leads to relaxation of vascular and sinusoid smooth muscle with resultant increased inflow of blood into the cavernosal spaces [6]. Nitric oxide stimulates the formation of cyclic guanosine monophosphate (cGMP) by guanylate cyclase. cGMP, the key second messenger involved in the initiation of penile erection, is then hydrolyzed by cGMP-specific phosphodiesterase type 5 enzyme (PDE5) to terminate the erectile response.
Current pharmacological treatments target the NO-cGMP-PDE5 pathway to initiate or enhance penile erection. Treatment options for ED have expanded dramatically in the last 10 years to include psychological counseling; oral, topical, intraurethral, and intracavernosal vasoactive therapy; oral therapies with other or unknown mechanisms; hormone replacement; vacuum constriction devices; and surgery, including vascular bypass procedures and penile implants. The goal of treatment is to preserve or restore satisfactory penile erections with minimal adverse effects. Men have demonstrated a strong preference for oral treatments even if they have lower efficacy [7], suggesting that efforts to optimize treatment of ED should address patient/partner satisfaction and preference in addition to the more standard physiologic and clinical measures of improvement. Men with ED want a safe, well-tolerated, convenient treatment requiring little or no invasiveness that is reliably effective in all types of ED. Unfortunately, no single therapy is satisfactory for every patient [8].
The purpose of this chapter is to summarize and evaluate the key evidence which supports the major treatment modalities for male erectile dysfunction.
Definition and measurement of erectile dysfunction
An NIH consensus panel defined ED as the persistent “inability to achieve or maintain an erection sufficient for satisfactory sexual performance” [1]. The diagnosis of ED requires a detailed sexual and medical history, physical examination and laboratory tests. Comprehensive, validated scales to reproducibly quantify the presence and severity of ED have become useful adjuncts to the case history, but are not sufficient to diagnose ED correctly or treat it safely.
The International Index of Erectile Function (IIEF [9]) is an example of such a symptom-based definition; it has been fundamental to the development of objective primary endpoints for randomized clinical trials of pharmaceutical agents, and has replaced use of physiological measures of erectile function in phase II and III clinical trials. This 15-item questionnaire addresses several domains of sexual function including erectile function (EF) domain: questions 1–5 and 15, maximum score 30. Responses to the IIEF-EF domain allow categorization of ED as severe (0–10), moderate (11–16), mild to moderate (17–20), mild (21–25) or no erectile dysfunction (26–30). Trials usually also include global efficacy questions (GEQ) relating to improvement in erection and or intercourse success.
The ability to objectively measure response to ED treatments is foundational to the value of this chapter to clinicians and policy makers; while pharmaceutical trials have most successfully used these outcome measures, definitional questions limit the ability to analyze and compare results from intervention and prevention trials focused on radical prostatectomy-associated ED (10) and many studies of penile implants. Finally, the importance of partner perspective is well recognized in the clinical evaluation and treatment of ED; how these parameters are measured in clinical trials, and how the data should be interpreted, is less well defined.
Potentially relevant studies were identified by computerized search of Medline (1966 to February 2009), restricted to English-language human studies. Relevant text searches included: sildenafil, vardenafil, tadalafil, alprostadil, intracavernosal injection, intraurethral suppository, radical prostatectomy, penile rehabilitation or penile implant with studies restricted to randomized controlled trial, meta-analysis or controlled clinical trial article types. The reference lists of relevant articles were searched by hand to identify additional relevant articles. Studies were selected for inclusion if they focused on patients with erectile dysfunction and were randomized controlled trials, prospective or retrospective cohort studies. In the case of penile implant surgery, we included large case series of currently available devices approved by the Food and Drug Administration.
Estimates for efficacy and safety outcomes in patients treated with the various pharmacological and surgical treatments were tabulated. Levels of evidence quality were assessed for each study using the GRADE system [11] and included in the tables. Due to the widely divergent outcome measures, risks, and safety issues, we did not attempt to compare between broad categories of treatment.
Clinical question 15.1
What is the effectiveness and safety of PDE5 inhibitors in the treatment of erectile dysfunction?
The evidence
Randomized placebo-controlled trial data strongly and uniformly indicate a significant improvement in erectile function with the use of all three available oral phosphodiesterase 5 inhibitors (Table 15.1). Overall, the quality of evidence for all three agents is high. It should be noted that trials of vardenafil and tadalafil in general had higher baseline IIEF-EF domain scores compared to sildenafil; this is a known effect in studies of subsequent drugs introduced in patient populations already exposed to agents in the same drug class. Finally, results from clinical trials are not uniformly generalizable to clinical practice. Patients in clinical trials in general will have fewer and less severe co-morbid conditions, better control of diabetes, and absence of hypogonadism. Placebo run-ins, common in the PDE5 inhibitor trials, also may bias towards better responses.
Table 15.1 Efficacy and safety of oral phosphodiesterase 5 inhibitors for the treatment of erectile dysfunction
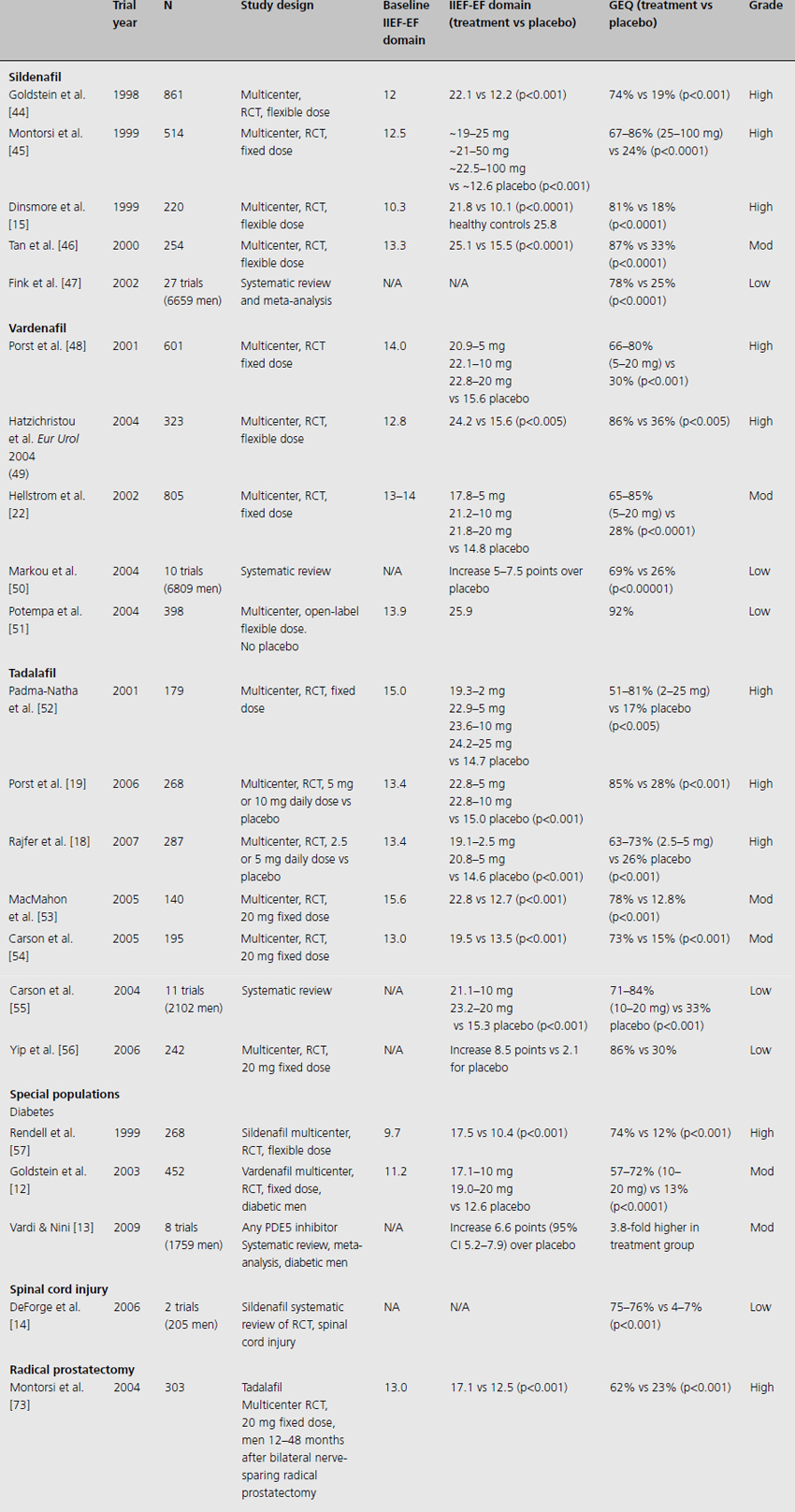
Sildenafil administered on demand at either fixed doses (ranging from 25 to 100 mg) or in flexible dosing leads to statistically significant increases in the IIEF-EF domain of 6.4 to 11.7 points (all studies p < 0.001). This difference is of clinical significance. Sildenafil demonstrates excellent drug efficacy in patients over a wide range of ages, with different causes of ED (organic, psychogenic and mixed causes) and in all severities of ED. Patients expected to be the most refractory to treatment, such as those with diabetes, spinal cord injury, post radical prostatectomy, and with other co-morbidities, were found to have significant improvement in erectile function in clinical trials [12–14]. All studies of sildenafil included in Table 15.1 report significant improvement in erections compared to placebo as evaluated by the GEQ “Did the treatment improve your erections?”. Overall, 67–87% of men receiving sildenafil reported improvement in their erections compared to 18–33% of men receiving placebo (all studies p < 0.001). There was a dose–response effect on both the difference in IIEF-EF score and patient-reported improvement in erections as measured by the GEQ with near normalization of erectile function for many patients [15].
Men administered vardenafil in a RCT had statistically and clinically significant increases in IIEF-EF score (3–8.6 points) versus placebo. They also reported significantly higher response to GEQ (65–85% improvement) compared to placebo (26–36%, all studies p < 0.005). Improvements were similarly dose responsive in patients taking 5–20 mg.
Tadalafil has been extensively studied in a variety of administration schedules. Due to the long 17.5-h half-life of this medication [16,17], efficacy has been demonstrated when taken on demand, three times per week and daily. Some men may find alternative dosing schedules attractive. Studies evaluating daily tadalafil use at various doses indicate that doses as low as 2.5 mg or 5 mg daily improve IIEF-EF scores by 6–8 points over placebo, with higher IIEF-EF scores and more patients reporting improved erections at the 5 mg dose [18]. However, no additional benefit was derived at 10 mg daily doses [19]. At fixed doses ranging from 5 to 25 mg, IIEF-EF scores were 6–10 points higher than placebo in a dose–response fashion and positive GEQ responses were 60–86% positive versus 13–33% for placebo.
Regardless of the oral PDE5 inhibitor studied, the majority of patients in flexible-dose trials concluded the trial at the highest allowed dosage. There was strong evidence across studies of improved response at higher doses, although side effects increased with increased dosages as well.
Additional comments
Mechanism of action
Sildenafil, vardenafil and tadalafil are competitive inhibitors of the PDE5. Although sildenafil and vardenafil share a closer molecular structure compared to tadalafil, each acts via inhibition of PDE5 to block cGMP hydrolysis in corporal tissues. This results in sustained increased levels of cGMP, consequent amplification of neurally mediated and endothelium-dependent NO release and smooth muscle relaxation, resulting in enhanced erectile responses.
Efficacy
Efficacy at the first attempt is an important aspect of ED therapy since this has a great effect on the patient’s sexual confidence and long-term compliance with the selected ED treatment. First-time response is a marker of continued response, and these medications tend to remain effective for men even after years of medication use. With repeat challenges and counseling, a percentage of initial nonresponders may be salvaged; treatment should be continued at increased doses up to three separate attempts before conceding failure of a medication [20–22]. With improvement in erectile function, men tend to note improvements in self-esteem, confidence and relationship satisfaction which correlate with improvement in IIEF-EF scores [23].
An area of some controversy is the impact of PDE5 inhibitors on the sexual function of men without ED. One small RCT [24] administered a fixed dose of sildenafil to 47 men without significant ED. Those receiving placebo showed no improvement in erectile function, whereas those given sildenafil demonstrated a statistically significant two-point increase in IIEF-EF domain scores. However, the small sample size, lack of dose–responsiveness data, and questionable clinical significance of the increase in EF domain scores all reduce the quality of evidence.
There have been no rigorous RCTs designed to evaluate comparative efficacy of the three available PDE5 inhibitors. A recent meta-analysis attempted to pool RCT data from fixed-dose studies involving sildenafil, vardenafil and tadalafil [25]. It concluded that at maximum doses, all three drugs improve IIEF-EF scores by 7–10 points compared to placebo treatment. Scores from the pooled studies indicated that sildenafil might be slightly more efficacious than vardenafil, but the differences between drugs were slight. Other randomized cross-over studies have demonstrated similar improvements in IIEF-EF score, similar sexual attempts during treatment, and similar safety profiles, but a 52–73% patient preference for tadalafil over sildenafil or vardenafil [26,27]. Patient preference appears to be primarily due to differences in dosing schedule and reduced performance pressure. These data were of moderate and low evidence due to methodological and study design issues, with one study comparing the highest recommended dose of tadalafil to an intermediate dose of sildenafil.
Safety
Overall, oral PDE-5 inhibitors are well tolerated, with few patients in any of the studies terminating treatment due to adverse effects. The side effects of these medications tend to be dose related. In general, men may report headaches (8–14%), flushing (4–6%), dyspepsia (5–18%), rhinitis (,5%), visual effects and blue vision (3%). Sudden hearing loss is rare, as is nonarteritic anterior ischemic optic neuropathy, which may occur in patients with underlying risk factors.
Absolute contraindications to all PDE5 inhibitors include concurrent nitrate use. Caution should also be exercised in patients taking alpha-adrenergic antagonists, CYP3A4 inhibitor drugs (phenytoin, ketoconazole, rifampin, HIV protease inhibitors, erythromycin, grapefruit juice), excessive alcohol intake, and in those with low baseline blood pressure, active coronary ischemia not on nitrates, and in those with congestive heart failure.
Implications for practice: recommendations
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








