Fig. 8.1
Bismuth and Corlette classification of malignant biliary strictures
Targeted unilateral endoscopic drainage using a single stent of MRCP-selected ducts has been associated with a reduced risk of post-ERCP cholangitis [23]. However, draining more than 50 % of the liver parenchyma is associated with higher effectiveness and longer survival [26]. Incomplete biliary drainage , especially if opacified ducts have been left undrained, often leads to cholangitis. To minimize risk of cholangitis, MRCP should be performed to map out the biliary tree before ERCP, a wire can be used rather than contrast to access the intrahepatic ducts, as much bile as possible should be aspirated to decompress the biliary system before contrast injection, and overinjection should be avoided. Some authors have also suggested air cholangiography in lieu of contrast to visualize the biliary system during planned placement of unilateral metal stent [42]. This technique involves aspirating at least 20 cc of bile first and then injecting 10–15 cc of air into the bile duct. Antibiotics should be administered in every patient to minimize the risk of cholangitis before and after ERCP typically for 5–7 days.
The intrahepatic bile ducts in complex malignant hilar strictures generally are drained initially with multiple plastic stents (Fig. 8.2a and b). Negotiation of a complex hilar stricture requires a highly skilled endoscopist, adequate accessories, time, and patience. In some cases, pneumatic or mechanical dilation of the neoplastic stricture can facilitate the placement of multiple stents. The length of the stents in malignant hilar strictures is usually between 12 and 15 cm. During bilateral stent placement whether with plastic or metal stents, it is always better first to drain the left biliary tree due to its anatomy.

Fig. 8.2
a Type III Bismuth and Corlette malignant hilar stricture with three guidewires in place. b Placement of three 8.5 French plastic stents
Multiple SEMS are usually placed in patients previously treated with plastic stents or percutaneous biliary drainage, and are rarely the initial treatment (especially if more than two SEMS are required due to the small caliber of the CBD below the stricture). Previously placed plastic stents are exchanged to SEMS only in symptomatic patients with clear signs of stent occlusion, and of course always accounting for the patients’ general condition and expected survival. Recently it has been described that SEMS can be used as a bridge to surgery in hilar cholangiocarcinoma without major complications [43].
Technique of Inserting and Removing Self-Expandable Metal Stents
After deep cannulation of the bile ducts, a biliary sphincterotomy may be performed. A recent randomized trial of patients with unresectable pancreatic cancer undergoing partially covered SEMS placement with or without biliary sphincterotomy found no difference in adverse events between the two groups, implying that sphincterotomy is not essential before SEMS insertion [44]. In distal biliary strictures, after gaining access to the bile duct, the guidewire is passed through the stricture. Dilation of the stricture before SEMS placement is rarely necessary because the radial force of the nitinol stents dilates the stricture gradually over 24–72 h. The SEMS is delivered over the guidewire and deployed under endoscopic and fluoroscopic vision. Because the stents have a tendency to move forward into the bile duct during deployment, gentle traction in the opposite direction should be applied by the endoscopist on the stent delivery system to maintain position. Some SEMS deploying systems allows contrast injection through the catheter in order to check immediately the correct position and the patency of the stent.
There are numerous types of SEMS available commercially with variations in length, expanded diameter, presence or absence of covering, design and material of the mesh, ability to reconstrain the stent during deployment, presence of stent shortening following deployment, and size of the delivery system. The endoscopists should be familiar with the stents in their endoscopy unit. Stents that cannot be recaptured during deployment must be carefully and slowly deployed under constant endoscopic and fluoroscopic visualization. For stents that shorten, when possible the stent is deployed with the stricture positioned in the middle of the stent.
Multiple SEMS placement is performed for palliation of complex malignant hilar strictures. This procedure is quite different from single SEMS placement. After selective opacification and cannulation of the intrahepatic ducts above the complex stricture, two to four guidewires are placed through the stricture deeply into the intrahepatic ducts that should be drained. Every metal stent is released under endoscopic and fluoroscopic control and “side-by-side” over the previously placed guidewires. It is advisable to release the stents trans-papillary in order to facilitate future re-interventions for SEMS occlusion (Fig. 8.3). Another reported technique of deploying bilateral hilar metal stents is the stent-within-stent technique using large cell width SEMS [45]. This may be useful for patients with small extrahepatic bile duct diameter which would make deploying multiple SEMS adjacent to each other difficult. Guidewire access to typically the left biliary system is procured followed by SEMS placement into that duct. Wire access to this system is maintained while removing the stent delivery system and advancing an ERCP catheter over the wire. After pulling the wire back into the catheter, it is advanced into the other intrahepatic system by passing the wire in between the interstices of the first stent. The interstices must be dilated using a 4–8-mm hydrostatic balloon dilator or a Soehendra stent retriever. Then the second stent is advanced through the interstices of the first stent into position.
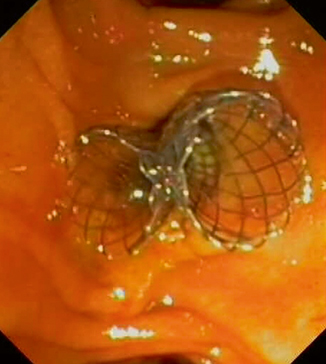
Fig. 8.3
Endoscopic view of two self-expandable metal stents
The number of stents that should be placed is usually chosen according to the complexity of the stricture. It is ideal although not always possible to place two SEMS for complete drainage of Bismuth type II stricture, three stents for type III, and three to four stents for type IV. In addition, SEMS for palliative treatment of malignant hilar strictures always must be uncovered in order to avoid occluding the side branches of the bile duct [26].
Multiple SEMS in complex malignant hilar strictures do not impede light delivery for photodynamic therapy but adjustments of the light dose are required [26]. SEMS in malignant hilar strictures can clog due to sludge and tissue ingrowth and/or overgrowth. This can be managed by extracting the sludge and debris with a balloon or, in cases of ingrowth, by the placement of additional SEMS or plastic stents inside the metal one.
Removing covered SEMS is usually straightforward and accomplished by grasping the retrieval loop at the end of the stent with rat tooth forceps, snaring the end of the stent, or using the stent-in-stent technique. This involves placing a second longer covered SEMS within the first stent and removing both about 2 weeks later with the hope that the second stent causes pressure necrosis of the ingrown/overgrown tissue [46]. Other typically more labor-intensive techniques of removing uncovered SEMS have been reported in various case reports and series including the use of endoscopic scissors to cut the distal end of the stent and sequentially remove each wire of the stent, and grasping the proximal end of the stent with rat tooth forceps and then pulling back to invaginate the stent [47, 48.
Case Continued
The patient underwent surgical consultation. Because of very high bilirubin levels, the surgeon preferred to delay surgery. The patient underwent endoscopic preoperative biliary drainage. On ERCP (Fig. 8.4) there was a malignant-appearing stricture located in the distal CBD with dilation of the proximal biliary three (Type I Bismuth and Corlette). Biliary forceps biopsy and brushing were performed at the level of the stricture, and one 10 Fr, 5-cm-long plastic stent was placed because the surgeon planned to take the patient to surgery soon. If surgery were to be delayed, a SEMS would have been indicated. The bilirubin level decreased to twice the normal range after 2 days. Biliary histology confirmed cholangiocarcinoma, while cytology was not diagnostic. The patient underwent pancreaticoduodenectomy (Whipple procedure) 1 week after ERCP and on 12 months follow-up was free of disease.
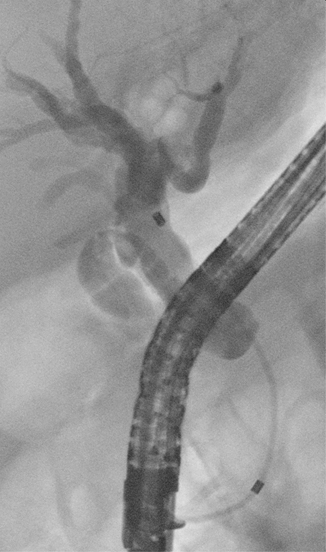
Fig. 8.4
Type I Bismuth and Corlette malignant biliary stricture with dilation of the biliary tree above the stricture
Case Study 2
Initial Presentation
A 35-year-old male presented with itching and dark urine. A laparoscopic cholecystectomy had been performed 6 months before. Liver function tests were all elevated and US showed dilation of the intrahepatic bile ducts. Since post-cholecystectomy bile duct injury was suspected, the patient underwent MRCP. On MRCP a type III Bismuth and Lazorthes [49], postoperative biliary stricture (POBS) was diagnosed. The stricture was located at the level of the main biliary confluence, which was not transected. Metal clips placed during cholecystectomy likely caused the stricture.
How Are Bile Duct Injuries and Postoperative Biliary Strictures Classified?
Injury of the bile ducts may occur during any surgical procedure involving the biliary tract. The main cause of bile duct injuries is laparoscopic cholecystectomy, and it is six times higher compared with open cholecystectomy [50, 51]. A classification of postoperative biliary injuries has been proposed by Bergman et al. in 1996 (Table 8.1) [52].
Type A | Cystic duct leak or leakage from aberrant or peripheral hepatic radicles (minor lesions) |
Type B | Major bile duct leak with or without concomitant biliary stricture (major lesions) |
Type C | Bile duct strictures without bile leakage (major lesions) |
Type D | Complete transection of the duct with or without excision of some portion of the biliary tree (major lesions) |
Postoperative biliary strictures (POBS) usually develop as a consequence of an injury to the bile ducts. Surgical repair with hepatico-jejunistomy is the traditional treatment of POBS. Bismuth et al in 1978 proposed a classification of postoperative biliary stricture that is based on the location of the stricture (Table 8.2) [49]. Endoscopy with placement of multiple biliary stents has become the preferred treatment for POBS [52–54].
Table 8.2
Bismuth and Lazorthes classification of postoperative biliary stricture
Type I | ≥ 2 cm distal to the hepatic bifurcation |
Type II | 2 cm distal to the hepatic bifurcation |
Type III | At the level of the hepatic bifurcation |
Type IV | Involves the right or left hepatic duct |
Type V | Extends into the left or right intrahepatic branch ducts |
Overview of Management of Bile Duct Injuries
With some exceptions, the endoscopic treatment of postoperative biliary injuries and strictures is possible only when the continuity of the bile ducts is maintained and the ducts have not been completely transected. Surgery is usually indicated for complete transection of the CBD (Bergman type D) (Table 8.1, Fig. 8.5) and refractory strictures. The endoscopic treatment of major biliary injuries and strictures mainly consists in the placement of one or more biliary plastic stents (Fig. 8.6) bypassing the site of injury (Video 8.1). Minor bile leaks may be treated by short plastic stent placement to overcome the pressure gradient at the biliary sphincter and allow the bile to flow through the stent into the duodenum rather than out the leak. During initial ERCP, cholangiogram should be performed carefully to examine for choledocholithiasis which is present in about 25 % of bile leaks. The stent is usually removed after 1–3 months. After stent removal an occlusion cholangiogram should be performed to assess for complete healing of the bile leak, ongoing choledocholithiasis, and the presence of strictures. Refractory bile leaks may be managed by prolonged plastic stent placement, combination of biliary sphincterotomy with stent, or covered SEMS [55, 56]. If a biliary stricture is identified, the patient should undergo placement of multiple biliary stents to dilate the stricture.

Fig. 8.5
Occlusive cholangiography showing complete transection of the common bile duct with multiple metal clips placed during laparoscopic cholecystectomy (Bergman type D lesion of the common bile duct)
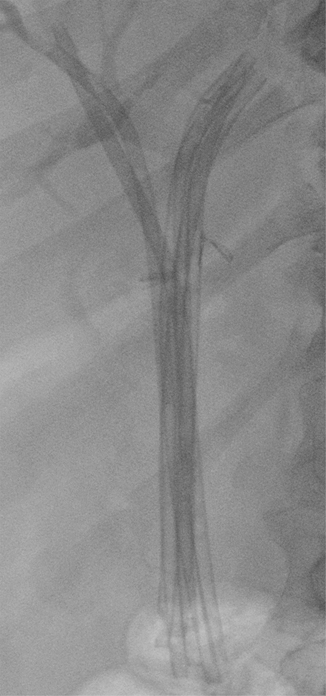
Fig. 8.6
Plain X-ray after placement of six plastic stents for postoperative biliary stricture. Metal clips placed near the hilum during laparoscopic cholecystectomy are also visible. This is the same patient in Fig. 8.7a
How to Manage Postoperative Biliary Strictures and Other Benign Biliary Strictures
POBS occur frequently after a bile duct injury as a result of fibrotic scarring and generally are diagnosed a few months later. In patients with suspected POBS, an MRCP should be performed to assess the type and morphology of the stricture. CT scan can be useful to evaluate the liver parenchyma for atrophy, especially with long-standing strictures.
Endoscopic treatment of POBS is quite different from the treatment of malignant biliary strictures (Video 8.1). Negotiation of the stricture with a guidewire is usually much more difficult in POBS than in a malignant stricture . This is because POBS are usually short, asymmetric, angulated, and rich with fibrotic tissue. It is preferable to use hydrophilic guidewires (0.035, 0.021, or 0.018 in. in diameter) with a straight or curved (J-shaped) tip. Proper technique for torquing guidewires requires skill, good fluoroscopy, and patience. Torquing guidewires must be done very gently to avoid bile duct perforation and additional injury.
In order to pass the stricture, the direction of the catheter and the wire must be in the same axis as the stricture. Orientation of the guidewire can be achieved by pulling an inflated stone extraction balloon below the stricture. Furthermore, there are different types of rotatable catheters that can be useful in some cases.
Once the stricture has been negotiated with the guidewire, placement of one or two large bore stents (usually 10 French) can suffice as initial treatment, especially in a type I Bismuth and Lazorthes POBS. In complex POBS, especially type III, IV, and V, placement of more stents may be necessary to avoid cholangitis due to undrained opacified ducts (Video 8.1). In these cases thinner stents are often initially placed.
Balloon dilation alone as treatment is immediately effective, but inadequate because of the high restenosis rate (up to 47 %) [57–59]. Dilating the stricture is frequently required before stent placement. It is recommended to choose the size of the balloon according to the size of the duct below the stricture in order to avoid perforation . Balloon dilation is usually performed only during the first stenting procedure and should be avoided during subsequent procedures because the forceful tissue disruption provoked by balloons may cause traumatic damage of the bile ducts , leading to the development of additional fibrosis at the level of the stricture.
Currently, one of the most accepted protocols to manage POBS consists of the insertion of as many 10 French plastic stents as possible side-by-side through the stricture. The stents are usually exchanged every 3–5 months. The number of the stents is increased at every ERCP. The treatment is usually continued for 12 months, or until the complete disappearance of any narrowing of the bile duct at cholangiography. [53].
Multiple biliary plastic stent placement is the treatment of choice for POBS, with excellent long-term results [26, 53, 54]. In our center, after mean 7-year follow-up , POBSs recurred in 11 % of treated patients, which were retreated with stenting. Following another 7 years on average, no further recurrences were noted. Benign biliary strictures from other etiologies, including chronic pancreatitis and post-liver transplant (Chap. 9), also respond well to this technique of multiple plastic stenting. Anastomotic strictures following liver transplant resolved in 97 % of patients treated for over 12 months with plastic stenting, and dropped significantly to 78 % in patients stented for less than 12 months [60]. Non-anastomotic strictures do not respond as well with 50–75 % resolution [61]. Chronic pancreatitis-related biliary strictures are also more challenging to treat with small series reporting overall 44–92 % success with this technique [57, 62, 63].
Is There a Role for Fully Covered SEMS in Postoperative Biliary Strictures and Other Benign Biliary Strictures?
Fully covered SEMS may have a role in the management of POBS although they are not FDA approved for use in benign biliary strictures . Many reports have been published in the literature, but usually have included patients with strictures caused by many different diseases (e.g., chronic pancreatitis, liver transplantation, primary sclerosing cholangitis, stones) and with only short follow-up following SEMS removal. Recently a large multicenter study of 187 patients with benign biliary strictures (68 % chronic pancreatitis) treated with covered SEMS followed them for 5 years after stent removal [64]. Covered SEMS were left in for a median 28 months in chronic pancreatitis, 13 months in POBS, and 9 months in liver transplant-related strictures. All stents were removed using rat-tooth forceps to grasp the retrieval loop on the end of the SEMS or snare to grab the distal end of the SEMS with only 3 patients requiring the stent-in-stent technique . Stent migration occurred in 29 %. Strictures had resolved in 76 % of patients at the time of stent removal, and over 5 years, 15 % recurred. Some cases of “de novo strictures” that occurred at the level of the proximal end of the SEMS have been observed. Complications related to the stent and stent removal occurred in 27 % of patients with cholangitis and abdominal pain being most common. Interestingly there was a trend toward increased cholecystitis when the stent covered the cystic duct orifice compared to when it did not (7 % versus 0 %, p = 0.074). While these results are encouraging, the use of fully covered SEMS for benign biliary strictures should be limited to clinical trials or in situations where plastic stenting has failed [26].
Case Continued
The patient underwent ERCP that confirmed a type III Bismuth and Lazorthes stricture and during the first treatment only two plastic stents were placed (Fig. 8.7a and b). The patient underwent three more ERCPs every 3 months over a period of 1 year with multiple plastic stents exchanged (Fig. 8.7c) until complete resolution of the stricture (Fig. 8.7d).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








