Epithelial tumors
Hepatocellular:
Hepatoblastoma
Epithelial
Pure fetal with low mitotic activity
Fetal, mitotically active
Pleomorphic, poorly differentiated
Embryonal
Small cell undifferentiated
INI-1 negative
INI-1 positive
Mixed epithelial (any or all of the above)
Cholangioblastic
Epithelial macrotrabecular pattern
Mixed epithelial and mesenchymal
With teratoid features
Without teratoid features
Hepatocellular carcinoma (HCC)
Classic HCC
Fibrolamellar HCC
Hepatocellular neoplasm, NOSa
Mesenchymal:
Embryonal sarcoma
Rhabdomyosarcoma
Vascular tumors
Epithelioid hemangioendothelioma
Angiosarcoma
Germ cell tumors:
Teratoma
Yolk sac tumor
Tumor of uncertain differentiation:
Malignant rhabdoid tumor
INI1 negative (INI1 mutation)
INI1 positive
Calcifying nested-stromal epithelial tumor
Desmoplastic small round cell tumor
Peripheral primitive neuroectodermal tumor
14.1 Hepatoblastoma
14.1.1 Definition
Hepatoblastomas are malignant epithelial tumors that show a spectrum of hepatocellular differentiation, recapitulating normal embryonal and fetal liver, with or without an associated mesenchymal component.
14.1.2 Clinical Features
Hepatoblastoma represents the most common pediatric malignant liver tumor, with approximately two-thirds of the cases presenting before age 2 and 90 % prior to age 5. Hepatoblastomas occasionally arise in older children and very rarely in adults, but a diagnosis of hepatoblastoma in older children and adults should be approached cautiously, as some of these on second review are not hepatoblastomas, but represent a mixture of various tumors, ranging from poorly differentiated hepatocellular carcinomas with sarcomatoid areas to metastatic sarcomas with epithelioid areas.
Boys are diagnosed with hepatoblastomas at least twice as often as girls. Risk factors are poorly understood, but include prematurity and low birth weight, especially birth weight less than 1,500 g. About 15 % of hepatoblastomas have associated syndromes and malformations, with the most common being familial adenomatous polyposis [1], and Beckwith–Wiedemann syndrome [2]. Other associations include trisomy 18, other trisomies [3], and the Aicardi syndrome, which is caused by X chromosome defects [4].
Presenting signs and symptoms include palpable abdominal masses, abdominal pain, failure to thrive, vomiting, diarrhea, and jaundice. Rarely, patients may present with virilization and precocious puberty due to tumor production of human chorionic gonadotropin [5]. Many other paraneoplastic syndromes have been reported, but they are rare and do not help in making the diagnosis of hepatoblastoma.
Elevated serum levels of alpha-fetoprotein (AFP) are seen in nearly all hepatoblastomas and represent a useful tool for the diagnosis and monitoring of metastatic or recurrent disease (Table 14.2). However, elevated serum AFP levels are not specific for hepatoblastoma and can be seen occasionally in mesenchymal hamartomas and infantile hemangiomas [6–10]. In addition, about 2–4 % of hepatoblastomas have AFP levels that are normal or only mildly elevated (less than 100 ng/mL) [11]. Hepatoblastomas that lack significant serum AFP elevations have a worse prognosis and most demonstrate a small cell undifferentiated morphology.
Table 14.2
Normal serum alpha-fetoprotein levels by pediatric age
Age | Reference range (ng/mL) |
|---|---|
Premature birth | 1,932–391,072 |
Term birth | 16–69,720 |
4–12 weeks | 32–651 |
3 months to 6 months | 4–275 |
6 months to 12 months | 3–148 |
1 year to 3 years | 3–21 |
3 years to 18 years | <5 |
Chronic liver disease is not a predisposing factor for hepatoblastomas and the background liver is normal and nonfibrotic. As a caveat, sections taken at the tumor–nontumor interface can show nonspecific localized inflammation and fibrosis. In addition, many cases are now treated by chemotherapy prior to surgical resection, and sections of the background liver can show chemotherapy-related changes.
14.1.3 Prognosis
Several clinical and pathologic features have been found to be of prognostic significance in hepatoblastoma. AFP levels <100 ng/mL (often seen in pure small cell undifferentiated histology) or >1,000,000 ng/mL carry a worse prognosis. In addition, unfavorable features include multifocal disease, tumors involving both lobes of the liver, vascular invasion, and distant metastases. Among the histologic subtypes, the presence of small cell undifferentiated histology represents an unfavorable prognostic factor, shown by multiple studies to be associated with aggressive behavior and decreased survival (although this appears to apply mainly to cases showing lack of INI-1 expression). Hepatoblastomas that are otherwise classified as very low risk or low risk (Children’s Oncology Group stages I and II; see Table 14.3) are upgraded to intermediate risk if any component of small cell undifferentiated histology is present. Pure fetal histology with low mitotic activity (particularly stage I tumors) is associated with an excellent prognosis [12–15].
Table 14.3
Children’s Oncology Group (COG) staging system
Stage I: The tumor is completely resected with negative margins |
Stage II: The tumor is removed with complete gross resection, but with microscopically positive margins |
Stage III: Only a biopsy is performed OR resected with grossly positive margins OR there is intraoperative tumor spill OR lymph node metastasis is present |
Stage IV: Distant metastasis present |
14.1.4 Gross Findings
The macroscopic appearances of hepatoblastomas are highly variable, depending on the subtype and the presence of heterologous elements. Typically, hepatoblastomas present as a single lobulated mass with varying degrees of necrosis and hemorrhage, in a background of noncirrhotic liver (Fig. 14.1). Following neoadjuvant therapy, extensive tumor necrosis is frequent and the residual tumor is often composed of mesenchymal elements, which seem to be relatively therapy resistant. Due to the frequent presence of different hepatoblastoma subtypes within the same tumor, extensive sampling is crucial for optimal evaluation. At least one section should be taken per centimeter of the tumor’s maximum diameter.
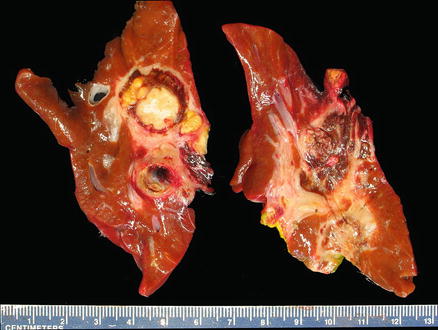

Fig. 14.1
Hepatoblastoma, gross. This resected hepatoblastoma shows a heterogeneous, multinodular mass with extensive necrosis
14.1.5 Microscopic Findings
Hepatoblastoma is an embryonal tumor arising from hepatocellular precursor cells and, as such, often recapitulates the various stages of hepatic development. Hepatoblastomas are purely epithelial in about 60–70 % of cases and have mixed epithelial and mesenchymal components in the remaining cases. By definition, an epithelial component is necessary to diagnose hepatoblastoma.
There are six key histologic patterns for the epithelial component of hepatoblastomas: small cell undifferentiated, embryonal, pleomorphic, cholangioblastic, fetal, and macrotrabecular. To date, no clear correlation between age at diagnosis and tumor pattern has been established. The small cell undifferentiated subtype shows the least resemblance to hepatocytes, while the fetal type and macrotrabecular type show the greatest resemblance to hepatocytes, with the others falling in between. In most studies, the fetal type makes up about 50–60 % of pure epithelial cases, the embryonal about 30 % (commonly admixed with fetal type epithelium), with each of the remaining epithelial types contributing 5 % or less (also commonly admixed with other types).
Depending on the treatment protocols of the institution, many patients with hepatoblastomas are treated with chemotherapy prior to resection. In all cases, there should be pretreatment biopsies to confirm the diagnosis. In the resected, posttreatment specimen, there can be extensive necrosis; the percentage of tumor necrosis should be provided in the final pathology report. Posttreatment changes can also include increased ossification and an increase in the mesenchymal component compared to pretreatment histology. Squamous nests can also be observed.
14.1.5.1 Small Cell Undifferentiated
A small cell undifferentiated component is present in some hepatoblastomas as small clusters of cells and can rarely be the sole pattern seen in the tumor (mostly in infants). The small cell undifferentiated pattern is composed of small basophilic blastemal-type cells characterized by a very high nuclear-to-cytoplasmic ratio, scant cytoplasm, relatively fine nuclear chromatin, and inconspicuous nucleoli (Fig. 14.2). The tumor cells grow in large solid sheets or with slightly nested patterns. The tumor cells are often poorly cohesive and mitotic activity is high. The small cell undifferentiated epithelium can be intimately admixed with other epithelial types and may be confused with “blastemal” or plump spindle cell areas.
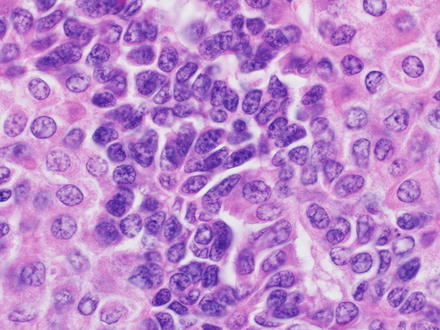

Fig. 14.2
Hepatoblastoma, small cell undifferentiated
Tumors that are composed largely of the small cell undifferentiated pattern need to be carefully examined for histological features of rhabdoid tumor, which can benefit from different chemotherapy. Immunostaining for INI-1, discussed further below, is critical in this distinction. Otherwise typical hepatoblastomas with small clusters of the small cell undifferentiated pattern tend to have retained INI-1 staining in all of the components and do not have tumor cells with typical rhabdoid morphology.
Epithelial hepatoblastomas with fetal or embryonal histology should be searched carefully for small clusters of the small cell undifferentiated pattern, as this increases their risk for aggressive behavior. However, recognizing small clusters of the small cell undifferentiated pattern can be challenging in practice, as they have histological overlap with the embryonal pattern. One helpful approach is to compare the cytology with published images or known study sets. Cytology findings can also be helpful, as the tumor cells in the small cell undifferentiated pattern have round or oval nuclei, finely dispersed chromatin, and inconspicuous nuclei. In contrast, the embryonal pattern tends to have more angulated and basophilic nuclei and can have somewhat more prominent nucleoli. The embryonal pattern also commonly has slightly bigger cells with more cytoplasm. Brisk mitotic activity can be seen in either morphology but is often more striking in the embryonal pattern. Finally, extensive sampling is very helpful in resected specimens, as most cases with foci of the small cell undifferentiated pattern will also have more obvious areas of the embryonal and fetal patterns, which are useful for comparison. There are currently no validated immunostains that can help in this distinction, but an INI-1 stain should be performed to ensure there is retained INI-1 nuclear staining.
14.1.5.2 Embryonal Pattern
The embryonal pattern has recognizable hepatocellular differentiation but consists of atypical, small, basophilic epithelial cells, with high nuclear-to-cytoplasmic ratios, and angulated nuclei (Fig. 14.3). The tumor cells grow in a predominantly solid pattern but there can be frequent formation of rosettes or pseudoglands (Fig. 14.4). This pattern essentially always coexists with the fetal pattern and can often be recognized at low power as distinct basophilic areas. Extramedullary hematopoiesis may be seen in association with either fetal or embryonal patterns (Fig. 14.5).
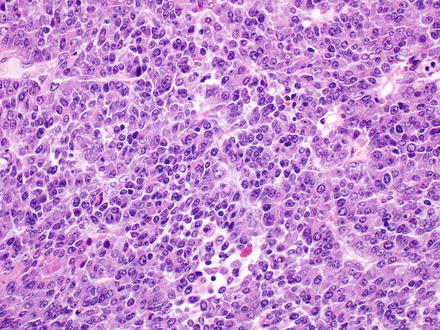
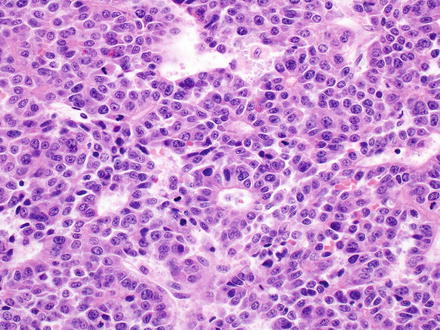
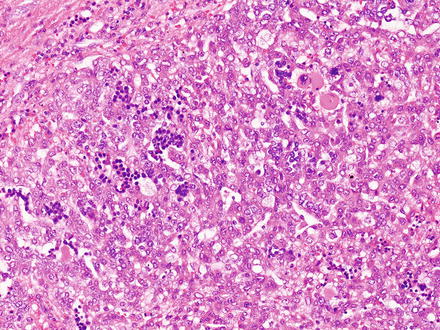

Fig. 14.3
Hepatoblastoma, embryonal. The embryonal pattern of hepatoblastoma shows significantly more pronounced cytological atypia compared to the fetal pattern, with higher nuclear-to-cytoplasmic ratio, larger, more irregular nuclei, and numerous mitoses

Fig. 14.4
Hepatoblastoma, embryonal. The embryonal pattern can show gland-like structures

Fig. 14.5
Hepatoblastoma, embryonal with extramedullary hematopoiesis. Extramedullary hematopoiesis is common in hepatoblastomas and is commonly seen in both fetal and embryonal areas. This image shows extramedullary hematopoiesis, including megakaryocytes in the upper right corner, within an area of embryonal morphology
14.1.5.3 Fetal Pattern
The fetal pattern (Fig. 14.6) represents the most common histologic component of hepatoblastomas and is present in most cases. This pattern is formed by a proliferation of generally bland-appearing hepatocytes. The fetal type hepatocytes have moderate amounts of eosinophilic cytoplasm and can also have varying degrees of glycogen accumulation that causes the classic “light and dark pattern” at low power examination (Fig. 14.7). A PAS stain further accentuates this pattern but is not needed for diagnosis. Also of note, this “light and dark pattern,” while distinctive, is not specific for hepatoblastoma and can sometimes be seen in hepatocellular carcinomas. The tumor cells grow in solid sheets or as thin trabeculae (2–3 cells thick), closely resembling fetal liver. They have finely dispersed chromatin and inconspicuous nucleoli. Mitotic activity is variable. For hepatoblastomas with pure fetal morphology and mitotic counts of less than 2 per high-power field, complete surgical resection is curative and additional chemotherapy is not necessary. The diagnosis of pure fetal hepatoblastoma with low mitotic counts requires complete resection and extensive sampling of the tumor. If there are more than two mitoses in ten high-power fields, the prognosis is worse and the hepatoblastoma is classified as the “crowded fetal type” or the more preferred designation of “Hepatoblastoma, fetal type, mitotically active” (Fig. 14.8).
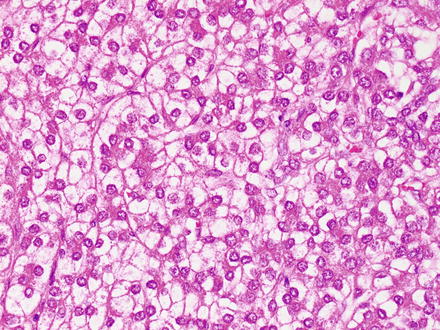
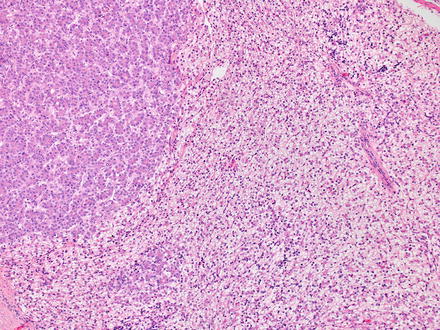
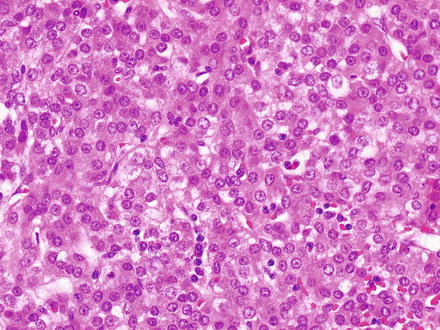

Fig. 14.6
Hepatoblastoma, fetal. The fetal pattern of hepatoblastoma, characterized by a proliferation of relatively bland hepatocytes with uniform nuclei, abundant eosinophilic to clear cytoplasm, and low mitotic activity, resembling the fetal liver

Fig. 14.7
Hepatoblastoma, fetal. Alternating areas of light and dark fetal cells are seen

Fig. 14.8
Hepatoblastoma, crowded fetal. In the so-called mitotically active or crowded fetal hepatoblastoma, there is slightly higher nuclear-to-cytoplasmic ratio than in the typical fetal pattern but the cytologic atypia is not as pronounced as in the embryonal pattern. Mitoses are typically increased (>2 mitoses/10 HPF) in this pattern (not shown)
14.1.5.4 Pleomorphic Pattern
The pleomorphic pattern is uncommon. The epithelium can be either fetal or embryonal type but has significantly increased atypical nuclear features (Fig. 14.9). The atypia includes moderate to marked nuclear pleomorphism, coarser chromatin than seen in the usual fetal or embryonal epithelium, and prominent nucleoli. Some cases can be difficult to reliably distinguish from hepatocellular carcinoma, especially if the sample is small and the age or clinical findings are not typical for hepatoblastoma.
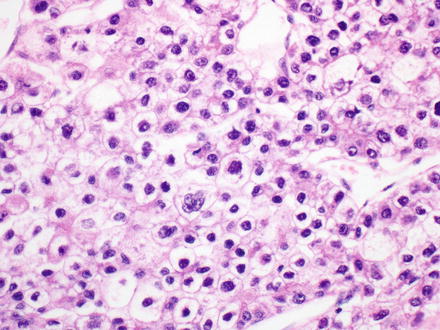

Fig. 14.9
Hepatoblastoma, pleomorphic pattern. Increased nuclear atypia is seen
14.1.5.5 Macrotrabecular Pattern
The macrotrabecular pattern (Fig. 14.10) is seen in a minority of cases (approximately 5 % of hepatoblastomas). The tumor shows a distinctive growth pattern with very thick trabecular architecture (>10 cells thick). The cytological features are otherwise identical to other epithelial subtypes, most commonly fetal or pleomorphic. A CD34 immunostain shows a distinctive pattern, outlining the thickened trabeculae (Fig. 14.11). Of note, hepatoblastomas with only a macrotrabecular pattern are essentially indistinguishable from adult hepatocellular carcinomas (see also Chap. 7), which can also have a similar growth pattern, high serum AFP levels, and similar cytology. Thus, a diagnosis of the macrotrabecular pattern of hepatoblastoma is made most confidently when sections show other areas of more typical hepatoblastoma. If only the macrotrabecular pattern is seen on a biopsy, then histology itself does not reliably separate these two possibilities. If other findings such as age or underlying liver disease are not informative, then the best approach is to convey the complexity of the case and provide a differential.
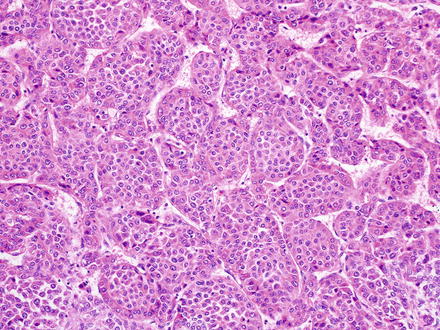
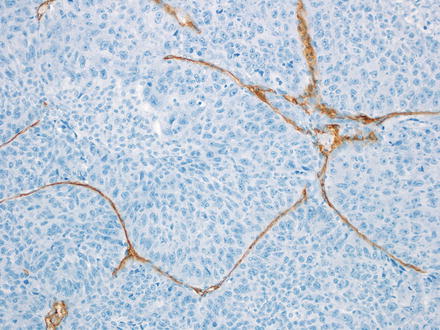

Fig. 14.10
Hepatoblastoma, macrotrabecular. The fetal type tumor cells are growing in thick tracabeculae

Fig. 14.11
Hepatoblastoma, macrotrabecular. A CD34 immunostain highlights the thick trabeculae
14.1.5.6 Cholangioblastic Pattern
The cholangioblastic pattern is also seen in a small subset of hepatoblastomas, in which small ductular structures are present within the tumor, either mixed within or surrounding areas showing a hepatocellular component. The ductular component is positive for keratins but negative for hepatocellular markers. The ductular component can be very difficult to distinguish from a benign bile ductular reaction on H&E stains. In these cases, aberrant beta-catenin nuclear expression indicates that the cholangiolar structures are neoplastic. Cholangiolar-type epithelium and structures can also be seen posttreatment, but in this setting there can be striking cytological atypia (Fig. 14.12).
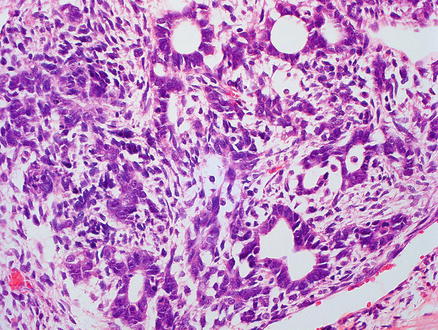

Fig. 14.12
Hepatoblastoma, cholangioblastic. Cholangioblastic hepatoblastoma, characterized by glandular/ductal structures
While an epithelial component is a required diagnostic feature of hepatoblastoma, a mesenchymal/stromal component is present in 20–40 % of cases and may include osteoid material (Fig. 14.13), skeletal muscle, cartilage, or a nondescript spindle cell (“blastemal”) component (Fig. 14.14); tumors containing such elements are termed “mixed” hepatoblastomas. Heterologous elements, including endoderm (squamous epithelium, glandular/mucinous epithelium, etc.) and neuroectoderm derivates (neural cells, glial elements, melanin-containing cells, etc.) are present in a minority of cases (approximately 10 %) and characterize a “teratoid” component. These basic patterns serve as the “backbone” for the currently recommended subclassification of hepatoblastomas (see also Table 14.1) [16].
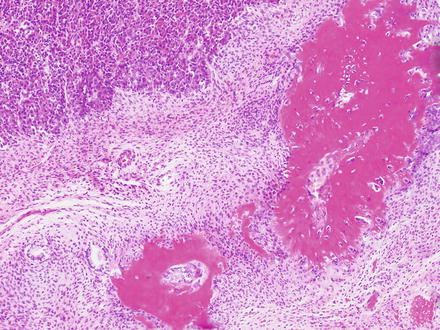
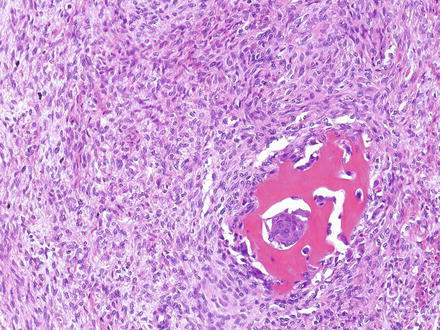

Fig. 14.13
Hepatoblastoma, with osteoid. Mixed epithelial–mesenchymal hepatoblastoma showing an embryonal epithelial component (upper left) and mesenchymal component including abundant osteoid (lower right)

Fig. 14.14
Hepatoblastoma, blastemal. Typical appearance of a cellular mesenchymal area (blastema) of a hepatoblastoma. A focus containing osteoid is also shown
1.
Pure fetal hepatoblastoma with low mitotic activity (also referred to as well-differentiated fetal hepatoblastoma)
2.
Pure fetal hepatoblastoma, mitotically active (>2 mitoses/10 high-power fields) (also referred to as “crowded fetal”)
3.
Embryonal
4.
Pleomorphic, poorly differentiated
5.
Small cell undifferentiated
6.
Cholangioblastic
7.
Epithelial macrotrabecular
8.
Mixed epithelial
9.
Mixed epithelial and mesenchymal
14.1.6 Immunohistochemical Features
Markers of hepatocellular differentiation (hepPar-1 and arginase-1) and glutamine synthetase are typically positive in some of the hepatoblastoma patterns (fetal and embryonal), variable in pleomorphic hepatoblastomas, and can be more weak or patchy in the remaining patterns. A fine granular pattern of glypican-3 expression has been reported to be typical of the well-differentiated fetal pattern, in contrast to the coarse cytoplasmic expression seen in the other patterns. However, this is often difficult to appreciate in practice, and the morphology is the best way to make the distinction between the different epithelial types. Glypican-3 can be weak to absent in the small cell undifferentiated components (Fig. 14.15). Cytokeratin 7 and 19 are expressed in cholangioblastic hepatoblastomas, usually in the absence of expression of hepatocellular markers. Finally, aberrant loss of INI-1 expression can be seen in pure small cell undifferentiated hepatoblastomas, in contrast with strong nuclear expression in all other patterns. In some cases, INI staining in the small cell undifferentiated components is largely retained, but there is loss of staining in a small minority of tumor cells (Fig. 14.16). The significance of this finding is unclear.
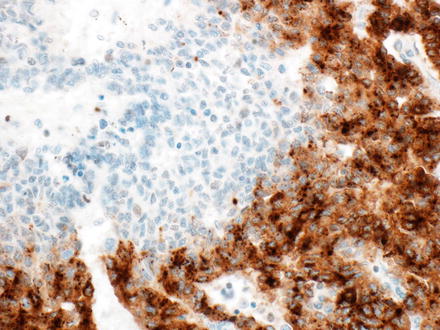
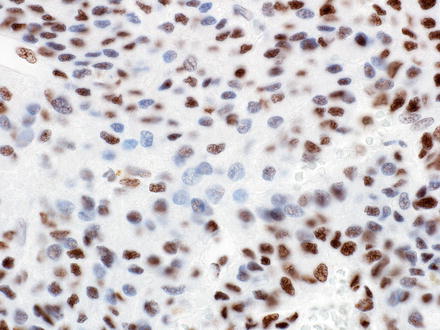

Fig. 14.15
Hepatoblastoma, glypican-3. There is strong staining in the embryonal and fetal components, but weak to absent staining in the small cell undifferentiated component

Fig. 14.16
Hepatoblastoma, INI-1. Nuclear staining is largely preserved in the small cell undifferentiated component in this case, but occasional tumor cells are negative
Beta-catenin, a marker of Wnt pathway activation, can be positive (nuclear staining) in all patterns, although some cases may show only focal expression and some cases are negative. Studies looking specifically at staining patterns in the different hepatoblastoma subtypes are limited, but embryonal hepatoblastomas appear to have a higher rate of nuclear staining (almost all) compared to mixed or fetal types of hepatoblastomas (approximately 50–70 %) (Figs 14.17 and 14.18). Some hepatoblastomas may be negative for nuclear accumulation but show diffuse cytoplasmic expression, which is also abnormal, in contrast to the strictly membranous pattern of expression in nonneoplastic hepatocytes and cholangiocytes.
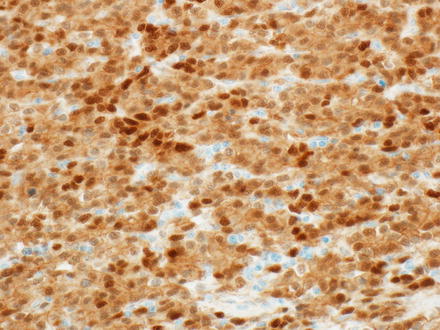
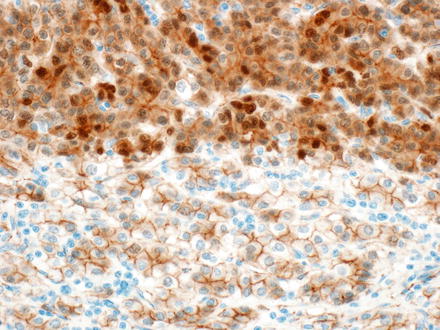

Fig. 14.17
Hepatoblastoma, beta-catenin. Strong nuclear staining is seen

Fig. 14.18
Hepatoblastoma, beta-catenin. Nuclear staining is weak to absent in the fetal component (bottom), but strongly positive in the embryonal component (top)
Glutamine synthetase is a downstream target of beta-catenin. Most but not all hepatoblastomas are positive for glutamine synthetase. The epithelium is positive but the mesenchymal components are negative. Interestingly, one study found that embryonal type epithelium showed diffuse staining for glutamine synthetase, while fetal type epithelium, at least in some cases, did not show diffuse staining, but instead the positivity was limited to a narrow band of strongly staining tumor cells adjacent to the tumor stroma [17].
14.1.7 Molecular Findings
Kariotypic abnormalities are present in approximately 50 % of hepatoblastomas [18]. Among the numerical aberrations, the most frequent are trisomy 20, 2, and 8. Structural defects most commonly involve chromosome 1q, with various deletions and translocations.
Multiple signaling pathways have been described in the carcinogenesis of hepatoblastomas. Approximately 70 % of hepatoblastomas harbor beta-catenin (CTNNB1 gene) mutations, which prevents its degradation and leads to nuclear accumulation, with subsequent activation of numerous downstream target genes related to cellular proliferation, antiapoptosis, and angiogenesis, such as c-myc, cyclin D1, MMP-7, FRA-1, urokinase plasminogen activator receptor, and VEGF receptor [19–23]. Beta-catenin mutations can result from either single base pair mutations or deletions. At this point, no strong correlation has been identified between the mutation status and the histological subtype of hepatoblastomas and mutational analysis currently does not play a role in diagnosis, subtyping, or prognosis of hepatoblastomas.
Interestingly, some hepatoblastomas show nuclear accumulation of beta-catenin in the absence of beta-catenin mutations. This is believed to occur due to abnormalities in several other genes and pathways that also play a role in the degradation of beta-catenin, such as APC and Axin. Dysregulation of the Notch signaling pathway (related to cell renewal, differentiation, and angiogenesis) is also well documented in hepatoblastomas and is most commonly seen in the pure fetal subtype. Finally, the several signaling molecules of the Hedgehog pathway are overexpressed in hepatoblastomas [24].
14.1.8 Differential Diagnosis
The differential diagnosis of hepatoblastomas largely depends on the pattern(s) present in each case. Conventional hepatocellular carcinoma represents the main differential diagnosis for the fetal, pleomorphic, and embryonal patterns (especially those classified as macrotrabecular), when these occur in the absence of a mesenchymal and/or teratoid component. Clinically, hepatocellular carcinomas are typically diagnosed in older children, in contrast to hepatoblastomas, which primarily occur before age 5. Histologically, the distinction between epithelial hepatoblastomas and conventional hepatocellular carcinomas may be difficult and relies on the recognition of various typical patterns of hepatoblastoma, as described above, or characteristic patterns of hepatocellular carcinomas. Nuclear expression of beta catenin, although present in a large majority of hepatoblastomas, is also seen in about 30 % of hepatocellular carcinomas and therefore must be interpreted with caution.
The cholangioblastic pattern of hepatoblastoma may pose diagnostic challenges and must be distinguished from pseudoacinar structures often seen in embryonal hepatoblastoma. The pseudoacinar structures in hepatoblastomas are typically positive for glypican-3, in contrast to the cholangioblastic pattern. Pediatric intrahepatic cholangiocarcinoma can also enter the differential diagnosis, but this typically shows prominent background desmoplasia, in contrast to cholangioblastic hepatoblastoma. Finally, benign areas of ductular reaction can be separated from the cholangioblastic pattern by beta catenin immunostain, as the ductular reaction is negative for nuclear expression.
Small cell undifferentiated hepatoblastomas may occur in association with other hepatoblastoma patterns and can show strong nuclear beta-catenin expression, which can be helpful in the differential diagnosis with other malignancies that also show a small round cell tumor-type morphology. The relatively recent molecular-genetic characterization of small cell undifferentiated hepatoblastomas and rhabdoid tumors (see further details under Sect. 14.2) has uncovered numerous overlapping features between these two entities and the distinction between hepatoblastomas showing pure small cell undifferentiated histology from rhabdoid tumors ultimately depends on the identification of rhabdoid morphology in neoplastic cells.
As with hepatocellular carcinomas (Chap. 7), hepatoblastomas must be distinguished from metastatic epithelial neoplasms that may mimic hepatocellular tumors, including adrenocortical carcinomas, germ cell tumors (particularly hepatoid yolk sac tumors), neuroendocrine tumors, and renal cell carcinomas.
14.2 Malignant Rhabdoid Tumor
14.2.1 Definition
Malignant rhabdoid tumors of the liver are extremely rare, highly aggressive neoplasms of unclear differentiation, showing rhabdoid cytological features.
14.2.2 Clinical Features
Malignant rhabdoid tumors are most commonly found in the central nervous system and kidneys but are also rarely found as primary liver tumors. This is an important tumor to recognize in view of its treatment and prognostic implications. Among 34 hepatic cases reported in literature [25], the mean age of presentation was 8 months, but cases are also seen in older children and teenaged youths. Common clinical findings include a palpable mass, hepatomegaly, as well as systemic symptoms (fever, lethargy, anorexia). Anemia and thrombocytosis are also common, while serum AFP levels are normal in most patients. Spontaneous rupture of the tumor has been reported by several authors [26–28] and appears to be more common than in other neoplasms in this age group. About two-thirds of individuals have metastatic disease at the time of presentation [25]. This tumor is associated with a very poor prognosis and is generally managed with aggressive chemotherapy regimens combined with surgical resection.
14.2.3 Gross Findings
14.2.4 Microscopic Findings
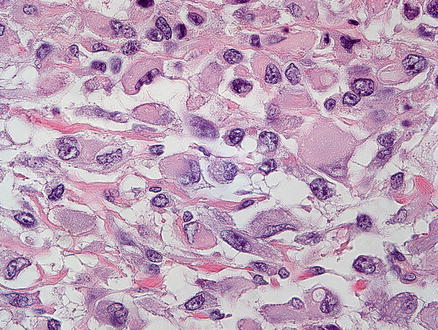
Malignant rhabdoid tumors are poorly differentiated. The tumor cells are round to polygonal, slightly discohesive, and relatively uniform epithelioid cells (Fig. 14.19). They have large nuclei, prominent nucleoli, and vesicular chromatin. In typical cases, the tumor nuclei are displaced by large eosinophilic paranuclear inclusions (composed of intermediate filaments), imparting a rhabdoid appearance. In some areas, the tumor cells can also have a more spindled morphology. The tumor has a predominantly solid growth pattern but can sometimes have a trabecular or alveolar pattern. Tumor necrosis is frequent and can elicit a dense histiocytic response at the edge of the necrotic areas.