Fig. 11.1
Epithelioid hemangioendothelioma, gross. A liver explant showing multiple tumor nodules ranging from a few millimeters to several centimeters
11.1.4 Microscopic Findings
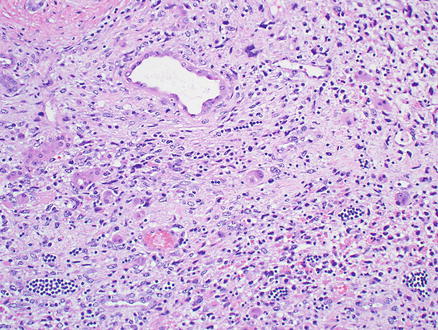
Microscopically, epithelioid hemangioendothelioma is characterized by individual and small groups of epithelioid cells scattered in a mucopolysaccharide-rich stroma, but some tumor cells can also form capillary lumina (Fig. 11.2). The tumor cells, especially at the peripheral of the lesion, tend to grow along hepatic sinusoids, terminal hepatic veins, and portal vein branches. The underlying hepatic acinar architecture is preserved (Fig. 11.3), but the liver cell plates become gradually atrophic, and eventually disappear due to replacement by tumor. At the center of the lesion, there is typical dense, often hyalinized fibrous tissue mimicking scar tissue. The tumor produces abundant mucopolysaccharide-rich stromal matrix, but in older lesions, there is progressive fibrosis and sometimes calcifications. Intravenous growth can sometimes be in the form of solid plugs, polypoid lesions, or tuft-like projections (Fig. 11.4). The epithelioid tumor cells have abundant cytoplasm and often contain cytoplasmic vacuoles, representing intracellular vascular lumina and sometimes containing red blood cells (Fig. 11.5). The cells containing vascular lumina can mimic signet ring cells. Besides epithelioid tumor cells, “dendritic” cells can also be seen in the myxoid stroma. Those cells have spindle or irregular shapes with multiple interdigitating processes (Fig. 11.6). Irregular intracellular lumina can also be seen in these cells.
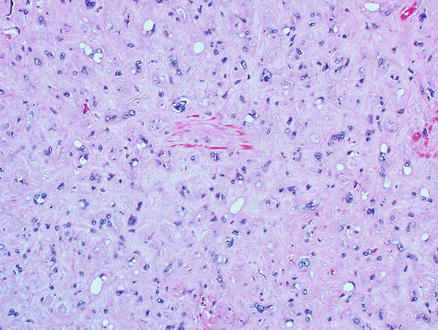
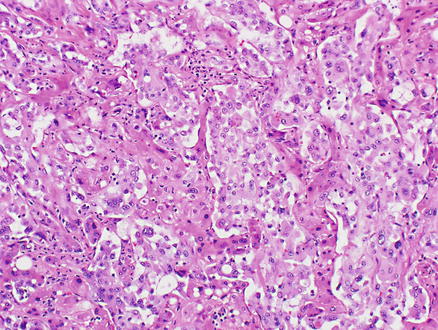
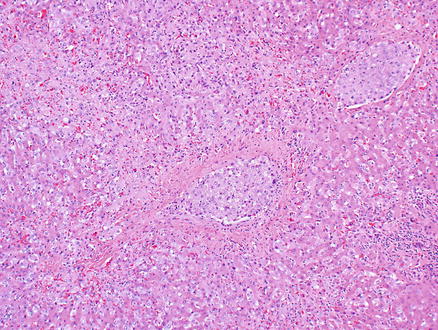
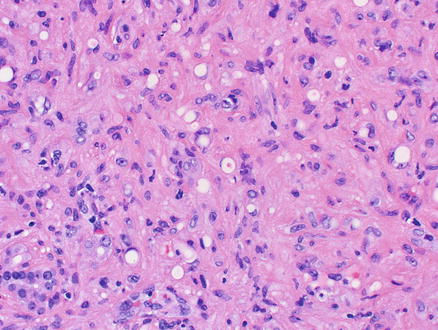
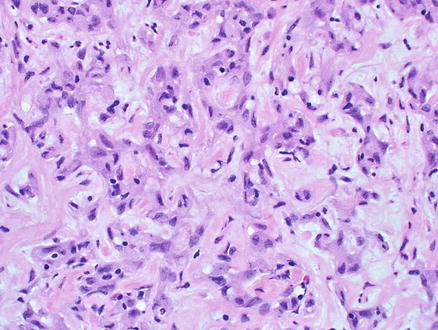

Fig. 11.2
Epithelioid hemangioendothelioma. Individual and small groups of epithelioid cells in a mucopolysaccharide-rich stroma

Fig. 11.3
Epithelioid hemangioendothelioma. Tumor cells grow along hepatic sinusoids but the underlying acinar architecture is preserved

Fig. 11.4
Epithelioid hemangioendothelioma. Intravenous growth of tumor forms tumor plugs

Fig. 11.5
Epithelioid hemangioendothelioma. Tumor cells with cytoplasmic vacuoles, representing intracellular vascular lumina, and containing red blood cells

Fig. 11.6
Epithelioid hemangioendothelioma. “Dendritic” tumor cells in the myxoid stroma. Those cells have spindle or stellate shapes with multiple interdigitating processes
11.1.5 Immunohistochemical Features
Immunostains for endothelial markers, such as Factor VIII, CD34, CD31, ERG, or Fli-1, are variably positive (Fig. 11.7), with approximate rates of positive staining: VIII (99 %), CD34 (94 %), and CD31 (86 %) [3]. In many cases, the use of more than one marker is helpful to confirm the diagnosis. Overall, vascular markers stain the epithelioid areas better than the dendritic areas. ERG is also a very sensitive marker for endothelial differentiation and was positive in 42/43 of epithelioid hemangioendotheliomas [8]. However, ERG is not entirely specific and stains about 40 % of prostate adenocarcinomas as well as some meningiomas, and rare Ewing sarcomas and mesotheliomas [8, 9]. However, when ERG results are combined with morphology and with other stains, it is very helpful for identifying vascular differentiation.
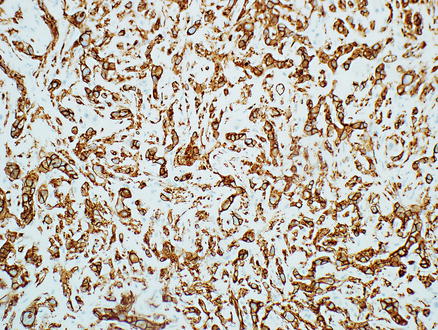

Fig. 11.7
Epithelioid hemangioendothelioma. Tumor cells express the endothelial marker CD34
Smooth muscle actin is positive in about 25 % of cases and cytokeratin AE1/3 and CK7 in 5–15 % of cases. Mucicarmine is always negative. Interestingly, immunostaining for CD10 is positive in most epithelioid hemangioendotheliomas [10], which can sometimes be confusing if the biopsy is small and the distinctive H&E findings not well represented.
11.1.6 Molecular Findings
Translocations appear to be important driver mutations in epithelioid hemangioendotheliomas. A translocation at t(1;3)(p36.3;q25) leads to a fusion transcript involving WWTR1–CAMTA1 and can be detected by FISH or RT-PCR and was found in all of 17 cases in one study [11].
11.1.7 Differential Diagnosis
The differential diagnosis includes other benign and malignant vascular tumors as well as epithelial tumors. Angiosarcoma has a much more destructive growth than epithelioid hemangioendothelioma. Although the cells grow along the sinusoids, they tend to disrupt hepatocellular plates and form larger vascular channels. The cells are often spindle with more severe cytological atypia than epithelioid hemangioendothelioma. Intracellular lumina are usually not present and complex tufting into the neoplastic vascular spaces is more frequent. Metastatic adenocarcinoma or primary cholangiocarcinoma can mimic epithelioid hemangioendotheliomas, especially when the tumor has low cellularity and dense, hyalinized fibrous stroma. The epithelial cells in cholangiocarcinoma, however, often form tubules or glands and often produce mucin. Immunostains for both epithelial and endothelial markers (such as Factor VIII, CD31, CD34, ERG, and Fli-1) can separate adenocarcinoma from epithelioid hemangioendothelioma. Sometimes epithelioid hemangioendotheliomas can be mistakenly diagnosed as non-neoplastic lesions, such as parenchymal collapse, scar, cirrhosis, or veno-occlusive disease. Careful microscopic examination to identify the characteristic neoplastic epithelioid cells and “dendritic” cells in a myxoid and fibrotic background should prevent misdiagnoses.
11.2 Angiosarcoma
11.2.1 Definition
Hepatic angiosarcoma is a high-grade malignant tumor composed of endothelial cells. It is a destructive vasoformative malignant neoplasm most frequently composed of spindled cells.
11.2.2 Clinical Features
Angiosarcoma is the third most common primary liver malignancy, but it only accounts for 2–3 % of all primary liver malignancies [12, 13], with hepatocellular carcinoma and cholangiocarcinoma the most frequent. Most angiosarcomas occur in men who are older than 50 years, but it can rarely be seen in children [14]. Angiosarcoma can be associated with chronic exposure to Thorotrast, androgen steroids, vinyl chloride, arsenicals, radium, possibly copper, and with chronic idiopathic hemochromatosis [13, 15]. Many cases, however, occur in the absence of risk factors [16]. The clinical presentation of hepatic angiosarcoma is nonspecific and includes abdominal pain, weakness, and weight. Patients often have hepatomegaly, ascites, and jaundice at presentation [13]. Catastrophic intra-abdominal bleeding occurs in about one-fourth of all cases [13]. Tumors can be complicated by acute liver failure [17]. The survival of hepatic angiosarcoma is very poor [13, 17], which is attributable to its rapid progress, high recurrence rate, and resistance to traditional chemotherapy and radiotherapy. The median survival rate is 5–6 months without treatment [13]. Even after treatment, only 3 % of patients are reported to live longer than 2 years [13, 15].
11.2.3 Gross Findings
Grossly, hepatic angiosarcomas can involve the either lobe or the entire liver and can also involve the spleen. The masses are gray-white with ill-defined borders and alternating areas of hemorrhage and necrosis (Fig. 11.8). Blood-filled cavities can be seen in some areas. Thorotrast-associated tumors often have subcapsular hepatic and splenic deposits of yellow chalky material.
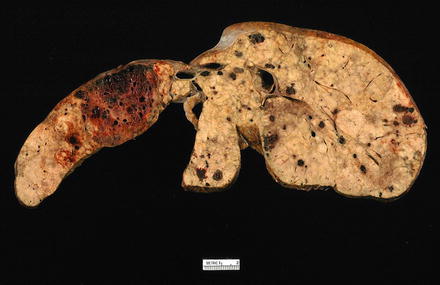

Fig. 11.8
Angiosarcoma, gross. Angiosarcoma involves entire liver with numerous gray-white and dark red masses with alternating areas of hemorrhage and blood-filled cavities with sponge appearance
11.2.4 Microscopic Findings
The background liver can show chronic inflammatory disease or fatty disease and be either cirrhotic or non-cirrhotic. Histologically, angiosarcomas can have several different growth patterns. One pattern is that of a clearly vascular tumor with irregular blood vessels. A second major pattern is that of a solid epithelioid tumor that can mimic a poorly differentiated carcinoma (Fig. 11.9). A third major pattern is of a spindle cell tumor with abundant extracellular matrix that mimics other sarcomas (Fig. 11.10). In a fourth pattern, the solid areas in some angiosarcomas can undergo necrosis and cavitation, leaving a cavity filled with blood, fibrin, and necrotic debris that has only small rim of viable malignant cells. Finally, an important but subtle growth pattern is growth along the sinusoids, replacing the normal benign sinusoidal endothelial cells but leaving the hepatic plates relatively intact (Fig. 11.11). This pattern can be very diagnostically challenging, especially on biopsy. With fully resected specimens, a sinusoidal growth pattern is almost always evident in some part of the tumor.
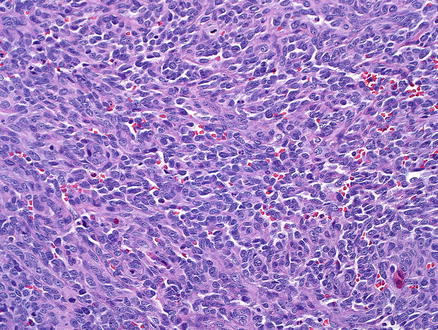
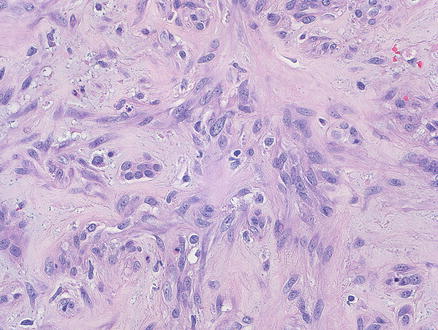
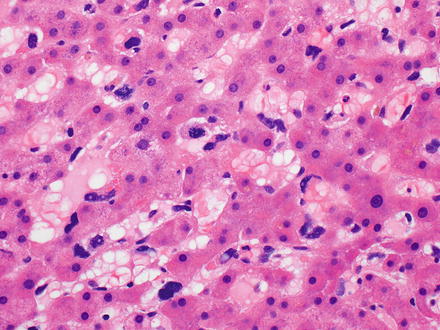

Fig. 11.9
Angiosarcoma. The tumor is growing in solids sheets, mimicking a poorly differentiated carcinoma

Fig. 11.10
Angiosarcoma. This tumor was submitted for consultation as a possible cholangiocarcinoma

Fig. 11.11
Angiosarcoma. This subtle growth pattern can be easily missed on biopsy specimens
Microscopically, tumor cells grow along the hepatic sinusoids between the hepatocellular cords can show solid and pseudopapillary patterns (Fig. 11.12). The sinusoidal growth of tumor cells is often associated with progressive destruction of liver plates, with the eventual formation of larger vascular channels and blood cavities. The pseudopapillary pattern is mainly identified along the lining in blood-filled cavities. The tumor cells typically form irregular infiltrative and anastomosing vascular channels (Fig. 11.13) and can be spindled or epithelioid. The spindle tumor cells have poorly defined cell borders, and are usually markedly pleomorphic with hyperchromatic nuclei, but sometimes may be only mildly atypical (Fig. 11.14). The solid epithelioid pattern of growth has cells with abundant cytoplasm and prominent nucleoli that can mimic carcinoma. Intracellular lumina are not a feature of angiosarcoma (Figs. 11.9 and 11.15). Mitotic activity is easily identified. Vascular invasion of portal or hepatic vein branches leads to progressive obstruction of their lumens, which can account for the hemorrhage, infarction, and necrosis in the tumor (Fig. 11.16). Extramedullary hematopoiesis can be seen in most cases.
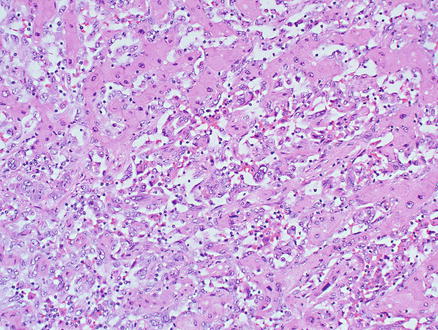
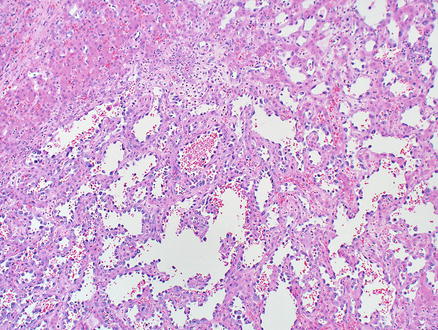
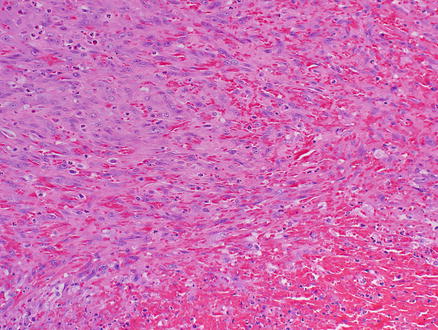
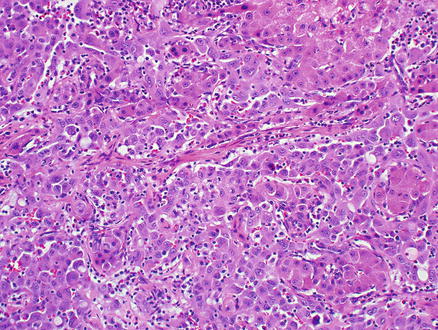
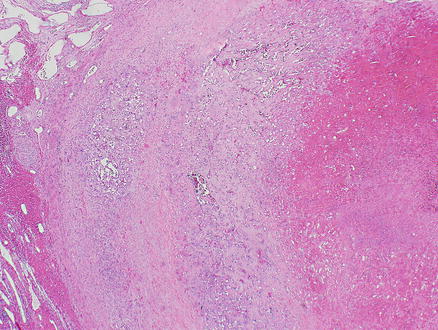

Fig. 11.12
Angiosarcoma. Tumor cells grow along hepatocellular sinusoids

Fig. 11.13
Angiosarcoma. Tumor cells form irregular infiltrative and anastomosing vascular channels

Fig. 11.14
Angiosarcoma. Spindle tumor cells have eosinophilic cytoplasm, poorly defined cell borders, and atypical hyperchromatic nuclei

Fig. 11.15
Angiosarcoma. Epithelioid neoplastic cells grow along sinusoids with abundant cytoplasm and prominent nucleoli. No intracellular lumina are present

Fig. 11.16
Angiosarcoma. Vascular invasion of large portal or hepatic vein branches leading to progressive obstruction of the lumen, hemorrhage, infarction, and necrosis in the tumor
Hepatic angiosarcoma in children may have Kaposiform areas consisting of spindle cells, sometimes containing PAS-positive intracytoplasmic globules. Thorotrast-associated cases may have granules of Thorotrast that are brown-gray and refractile but not birefringent, either free or within macrophages. A precursor lesion consisting of endothelial hypertrophy and hyperplasia has been described in cases related to Thorotrast, vinyl chloride, and arsenic exposure [18, 19].
11.2.5 Immunohistochemical Features
Immunostains for endothelial markers, such as Factor VIII, CD31, CD34, ERG, Fli-1, and Ulex europaeus lectin type 1, are variably positive (Figs. 11.17 and 11.18). ERG and FLI-1 are more recently available stains and are very helpful for identifying angiosarcomas. One study found 100 % of hepatic angiosarcomas are positive for ERG [20]. Overall, the frequency of positivity in angiosarcomas for the traditional markers of endothelial differentiation are as follows: 40–90 % of cases are positive for Factor VIII, 60–90 % are positive for CD34, and 30–100 % are positive for CD31 [20–23]. The wide variation in reported frequencies may in part be because these studies include angiosarcomas from different organs, but they also underscore the value of using a combination of stains for identifying an endothelial phenotype. The neoplastic cells are also positive for vimentin and can sometimes express keratin stains, which can lead to the mistaken diagnosis of carcinoma. Cytokeratin AE1/3, Cam5.2, or pan-keratin positivity can be seen in the epithelioid areas of angiosarcomas in about 30–50 % of cases [22, 23]. Rare angiosarcomas also express synaptophysin and/or chromogranin, mimicking neuroendocrine tumors [24].
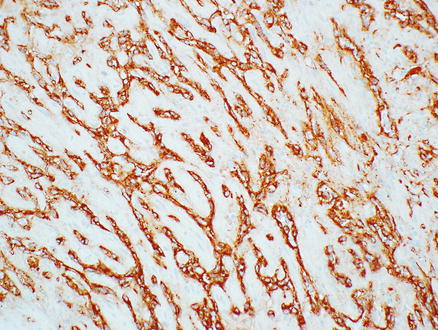
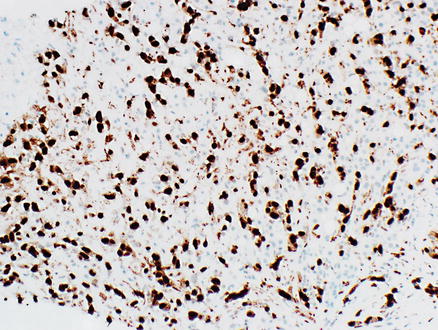

Fig. 11.17
Angiosarcoma. Tumor cells express the endothelial marker Factor VIII

Fig. 11.18
Angiosarcoma. Tumor cells express the endothelial marker ERG
11.2.6 Differential Diagnosis
Primary angiosarcoma cannot be differentiated from metastatic angiosarcoma histologically; this differentiation requires correlation with the patient’s clinical findings. Epithelioid hemangioendothelioma consists of individual and small groups of epithelioid cells in a mucopolysaccharide-rich stroma, and some tumor cells have capillary lumina. The tumor cells also grow along hepatic sinusoids, but the underlying acinar architecture is predominantly preserved. In contrast, angiosarcoma often has significantly more destructive growth and more striking cytologic atypia.
Distinguishing Kaposi’s sarcoma from a well-differentiated angiosarcoma can be challenging, but Kaposi’s sarcoma is overall more bland cytologically and is usually centered in portal tracts, and immunoreactivity of HHV-8 in Kaposi’s sarcoma is a key feature. Sinusoidal growth and formation of vascular structures can help differentiate angiosarcoma from other high-grade sarcomas (primary or metastatic) in resection specimens. In biopsy specimens, this differentiation can be difficult and usually requires immunohistochemical workup to demonstrate an endothelial phenotype. Angiosarcoma can sometimes have epithelioid morphology and express keratin stains, which can be mistaken for carcinoma. The sinusoidal growth pattern, the presence of areas of spindled cell growth, and expression of endothelial markers help sort out this differential diagnosis.
11.3 Kaposi’s Sarcoma
11.3.1 Definition
Kaposi’s sarcoma is a locally aggressive endothelial tumor that is uniformly associated with human herpes virus (HHV-8) infection.
11.3.2 Clinical Features
Four different forms of Kaposi’s sarcoma are recognized and all can involve liver: (1) the “classic” form typically affects elderly individuals (>60 years), mainly in the Eastern Europe and the Mediterranean region; (2) the “African endemic” form affects young adults of equatorial Africa and is characterized by localized nodular lesions; (3) the “epidemic” form of Kaposi’s sarcoma occurs in patients with acquired immunodeficiency syndrome (AIDS); (4) the “iatrogenic” form is caused by immunosuppressive drugs administered after organ transplant and has aggressive behavior with a tendency to spread. Hepatic Kaposi’s sarcoma is rarely detected in living patients, but autopsies show liver involvement in 35 % of patients with Kaposi’s sarcoma. Abdominal computed tomography or ultrasonography with contrast agents can reveal the presence of lesions in the hepatic capsule, hilum, and large portal areas, with or without invasion of the liver parenchyma [25]. In most cases, antiretroviral therapy alone is effective in controlling this neoplastic process.
11.3.3 Gross Findings
Kaposi’s sarcoma in the liver has been described as irregular, blue-black, or red-brown lesions involving large portal areas. Confluence of larger foci can resemble hemangiomas.
11.3.4 Microscopic Findings
Microscopically, Kaposi’s sarcoma is usually centered in portals tracts, but the tumor can infiltrate into the adjacent liver parenchyma. The lesion consists of a vascular proliferation composed of spindle cells with large plump nuclei admixed with collagen fibers, extravasated red blood cells, and hemosiderin-laden macrophages (Figs. 11.19 and 11.20). Slit-like spaces are a common finding of Kaposi’s sarcoma. PAS-positive hyaline globules, which may represent destroyed red blood cells, are frequently found in neoplastic cells.
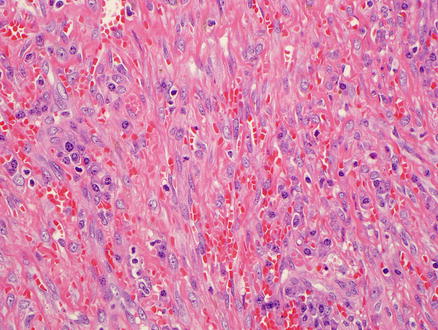
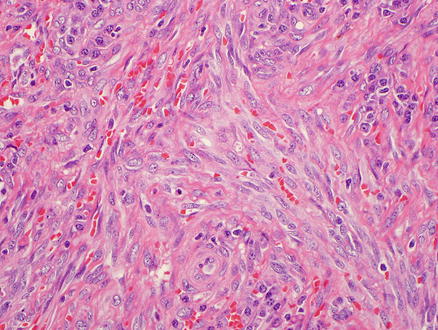

Fig. 11.19
Kaposi’s sarcoma. Vascular proliferation and spindle cells with large, plump nuclei admixed with collagen fibers and extravasated red blood cells

Fig. 11.20
Kaposi’s sarcoma. Higher power view of the spindle cells and extravasated red blood cells
11.3.5 Immunohistochemical Features
The neoplastic cells are positive for pan-endothelial markers including CD31, CD34, FLI-1, and ERG, but Factor VIII immunostain is usually negative. In addition, lymphatic markers such as D2–40 and podoplanin are also positive. Almost all cases display strong nuclear immunoreactivity for HHV-8 in both the spindle cells and in the cells lining the neoplastic vascular spaces, regardless their subtypes (Fig. 11.21).
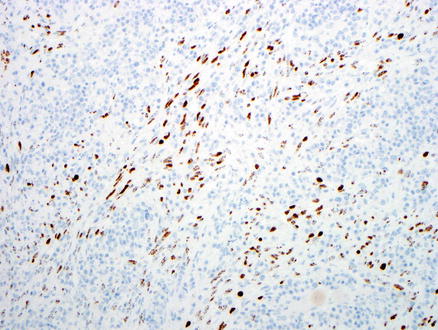

Fig. 11.21
Kaposi’s sarcoma. Tumor cells express HHV-8
11.3.6 Differential Diagnosis
An accurate clinical history is very important for establishing the diagnosis. An early lesion may display only portal-based irregular, infiltrative growth of endothelial cells. In some cases, the tumor may be subtle and easy to miss. In other cases, the tumor may be obvious, but difficult to distinguish from angiosarcoma. Immunoreactivity of HHV-8 is a very helpful feature since Kaposi’s sarcoma is universally positive for this antigen, whereas other vascular tumors are negative, even those from HIV-infected patients. Advanced Kaposi’s sarcoma with high cellularity may also be confused with other spindle cell lesions such as metastatic GIST or fibrosarcoma. Features that distinguish Kaposi’s sarcoma from these tumors include the presence of ectatic vessels and inflammatory cells at the periphery of the lesion, the presence of more curvilinear fascicles, the presence of hyaline globules, and immunoreactivity to endothelial markers and HHV-8.
11.4 Embryonal Sarcoma
11.4.1 Definition
Embryonal sarcoma is a rare primitive malignant mesenchymal neoplasm unique to liver [26]. This tumor has also been called undifferentiated sarcoma, primary sarcoma of liver, mesenchymal sarcoma, or malignant mesenchymoma, but embryonal sarcoma is the preferred term.
11.4.2 Clinical Features
Embryonal sarcoma is predominantly a disease of children and is therefore further discussed in Chap. 14. More than half the patients are between the ages of 6 and 10 years, but rare cases occur in adults [26–29]. The tumor usually grows very rapidly with areas of cystic degeneration and necrosis. The patients often present with abdominal swelling, with or without a palpable mass and pain. The diagnosis can be strongly suspected in a child with a large, rapidly growing cystic liver mass. The prognosis was very poor in the past, with a median survival of less than 1 year, but in recent years, the survival has significantly improved, with some patients living 5 or more years after combination treatments of surgery and chemotherapy [28, 30].
11.4.3 Gross Findings
Most embryonal sarcomas are located in the right lobe of the liver. They are often solitary, well demarcated, and their size range from 10 to 30 cm. The cut surface is variegated with solid, cystic, and gelatinous areas alternating with hemorrhagic and necrotic foci (Fig. 11.22) [31].
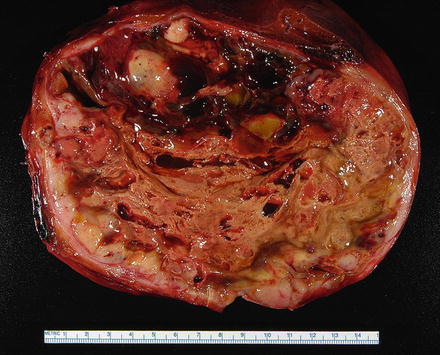

Fig. 11.22
Embryonal sarcoma, gross. A solitary, large, and well-demarcated mass. The cut surface is variegated with solid, cystic, and gelatinous areas alternating with hemorrhagic and necrotic foci
11.4.4 Microscopic Findings
Microscopically, the tumor may have a fibrous pseudocapsule separating it from the adjacent liver parenchyma. Entrapped hepatocytes and bile ducts can be identified at peripheral areas of the tumor and can be cystically dilated (Fig. 11.23). The tumor shows variable cellularity. The neoplastic cells are stellate or spindle shaped with ill-defined outlines and frequent mitotic activity (Fig. 11.24). The nuclei show marked anisonucleosis with stippled chromatin and inconspicuous nucleoli. The cells may be compactly or loosely arranged in a myxoid or fibrotic matrix, and numerous thin-walled veins can be often seen (Fig. 11.25). A characteristic feature is the presence of multiple, variably sized eosinophilic globules in the cytoplasm of tumor cells (Fig. 11.26). The globules are PAS positive and diastase resistant. Bizarre tumor cells and/or multinucleated giant tumor cells are often seen. Extramedullary hematopoiesis is common. Tumors in adults may have partial smooth muscle differentiation.