, Adele Taibbi1 and Massimo Midiri1
(1)
Department of Radiology, University Hospital, Palermo, Italy
3.1 Primary Malignant Tumor
3.1.1 Intrahepatic Cholangiocarcinoma
The intrahepatic cholangiocarcinoma (iCC) can present as single or multiple lesions tending to coalescence [1]. There are no wide study populations in literature about cholangiocarcinomas evaluated by means of CEUS, but according to reported data, the iCC presents inhomogeneous, mainly peripheral, contrast enhancement in the arterial phase with substantially hypovascular appearance in the extended portal-venous phase. The CEUS findings of ICC relate to the degree of neoplastic cell proliferation at pathological examination. Hyperenhancing areas in the tumor always indicated increased density of cancer cells. On the other side, fibrosis is more extended where hypovascular areas are detected [2]. Moreover, CEUS can definitely better delineate margins and evaluate more accurately the size and the possible involvement of the adjacent structures [3].
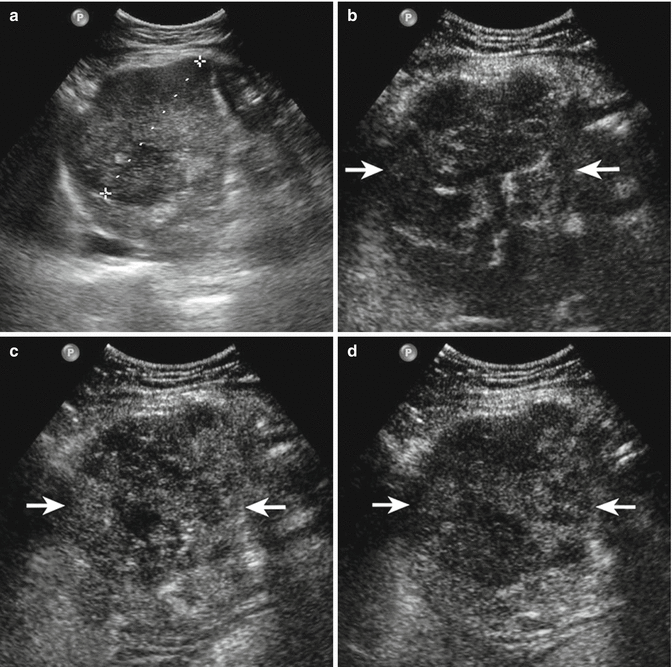
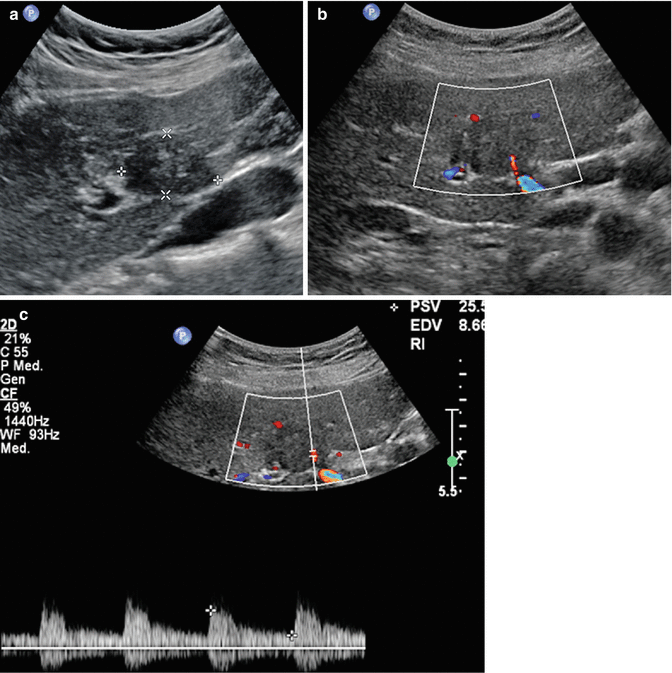
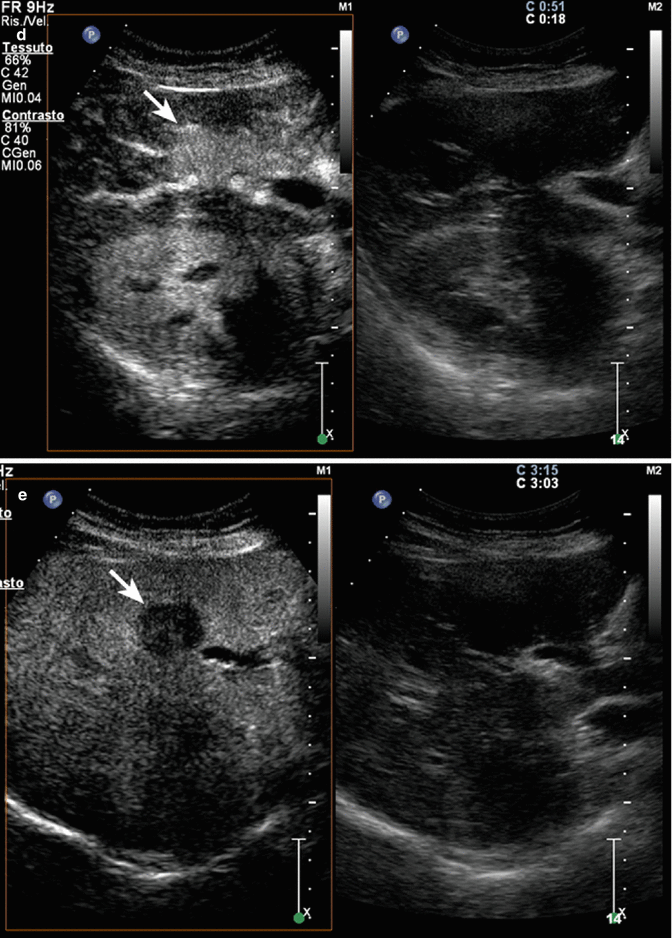

Fig. 3.1
Intrahepatic cholangiocarcinoma in a 62-year- old man. (a) Intercostal right baseline image reveals a large tumoral mass sized 8 cm in VI–VII hepatic segment (calipers). (b) At CEUS during the arterial phase, the lesion shows moderate and inhomogeneous enhancement (arrows). The lesion appears as hypoenhancing mass in comparison with adjacent parenchyma in the por-tal-venous (c) and late (d) phases (arrows)


Fig. 3.2
Intrahepatic clear cell cholangiocarcinoma in a 65-year-old woman. (a) Baseline image reveals an inhomogeneous hypoechoic lesion sized 3.5 cm in V hepatic segment (calipers) with some arterial intralesional vessel at color-Doppler (b) and pulsed-Doppler (c) evaluation. (d) At CEUS during the arterial phase, the lesion is highly hypervascular with marked washout in the late phase (e) (arrows)
3.2 Cirrhotic Patients
Hepatocellular carcinoma (HCC) is the most common primary malignant liver neoplasm, more frequent in patients with HCV- and HBV-related cirrhosis, alcoholic cirrhosis, or hemochromatosis.
In the last 20 years, the incidence has risen sharply, so HCC represents the fifth most common cancer in the world and the third leading cause of death for neoplasm [4]. Except for the limited percentage of “de novo” HCC arising in noncirrhotic patients, the genesis of HCC is a “multistep” process including regenerating nodules and low- and high-grade dysplastic nodules up to overt HCC. During carcinogenesis process, liver vascularization becomes irregular and mostly arterial, with progressive reduced portal supply [5, 6]. Therefore, at CEUS, HCC occurs, in its typical form, as inhomogeneously hypervascular in the arterial phase, with more or less rapid washout in the extended portal-venous phase with hypoechoic appearance (“washout” sign) sometimes associated with a peripheral hypervascular halo (pseudocapsule) in the extended portal venous phase. However, according to the degree of differentiation, HCC may present different aspects. In fact, well-differentiated HCC, although hypervascular in the arterial phase, cannot be hypoechoic in the extended portal-venous phase causing misunderstanding at CEUS [7]. Moreover, CEUS allows the differentiation between HCC from regenerative and low-grade dysplastic nodules since these latter are not hypervascular in the arterial phase and are mainly indistinguishable from the remaining liver parenchyma in the extended portal-venous phase [8].
Being a real-time imaging modality, CEUS can be considered as problem-solving technique when CT or MR are not able to definitively characterize a nodule in cirrhotic patients allowing a better delineation of contrast enhancement behavior especially in the arterial phase when CT or MRI fails to show it because of incorrect arterial-phase timing [9, 10].
Nevertheless, CEUS has important limitations in surveillance programs of these patients because the short duration of the arterial phase does not allow to detect possible new hypervascular foci in all liver segments except with multiple contrast agent injections, but this is not feasible during daily clinical practice [11].
3.2.1 Regenerative Nodule
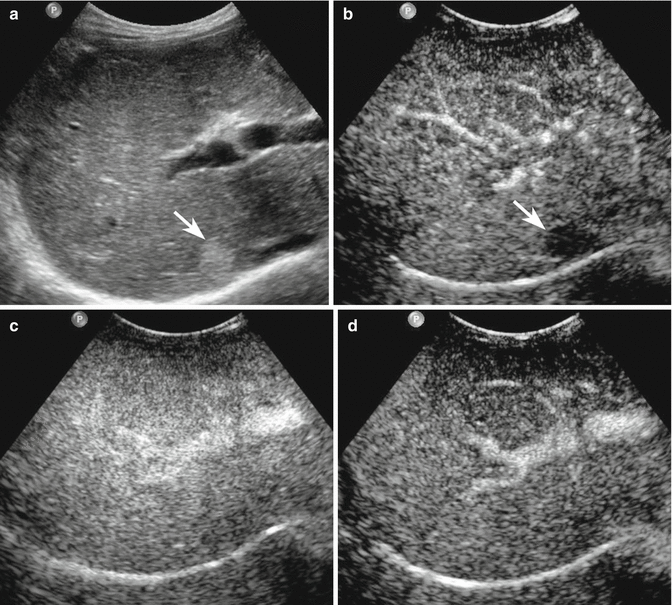
Fig. 3.3
Regenerative nodule in a 65-year-old man with liver cirrhosis. (a) Subcostal right baseline image reveals a well-defined hyperechoic lesion sized 2.1 cm in the VI–VII segment (arrow). (b) At CEUS during the arterial phase, the lesion shows no uptake of contrast agent (arrow). (c, d) The lesion appears isoechoic with respect to the surrounding liver parenchyma in the images acquired during the remaining vascular phases, revealing the same blood supply of the adjacent liver parenchyma
3.2.2 Dysplastic Nodule
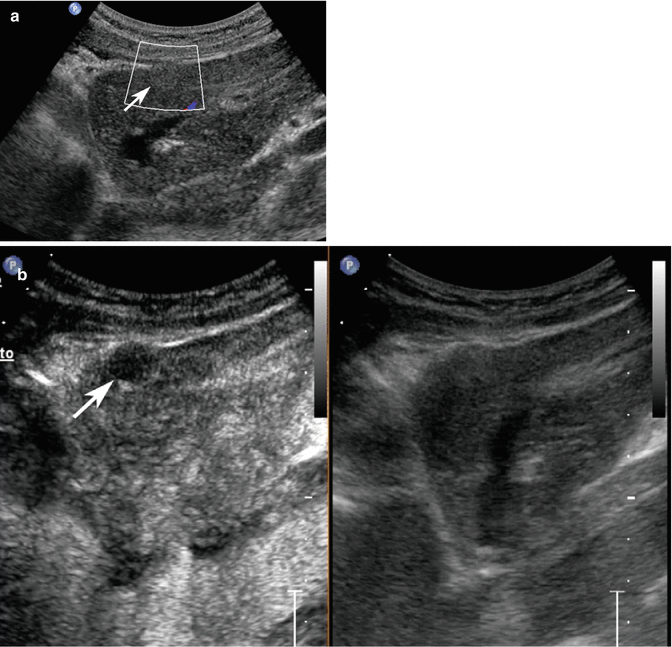
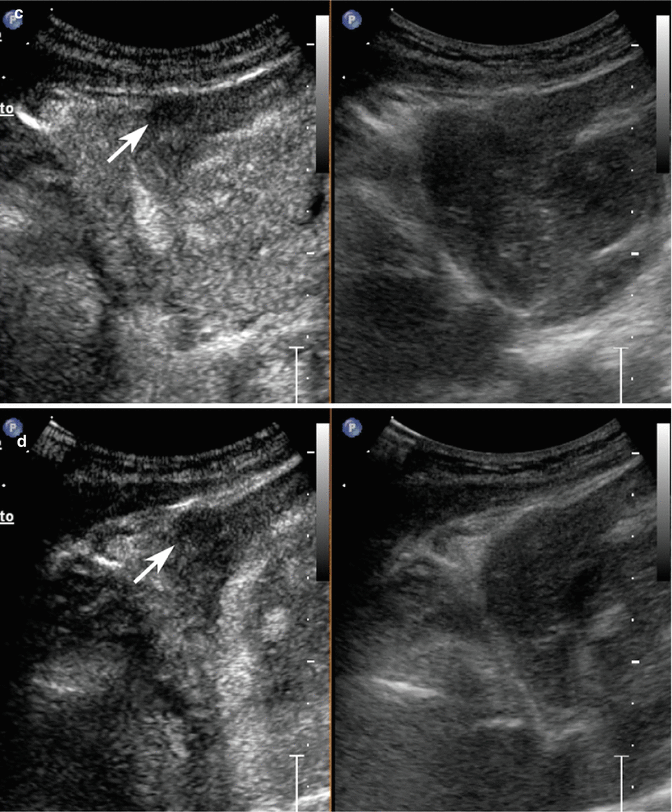
Fig. 3.4
Dysplastic nodule in a 58-year-old woman with HCV-related chronic hepatitis. (a) Sagittal subcostal baseline image reveals a moderately homogeneous partially exophytic hyperechoic nodule sized 1 cm in the left lobe without evident vascular signal at color-Doppler study (arrow). (b–d) At CEUS, the lesion presents hypoechoic aspect throughout the vascular study (arrows)
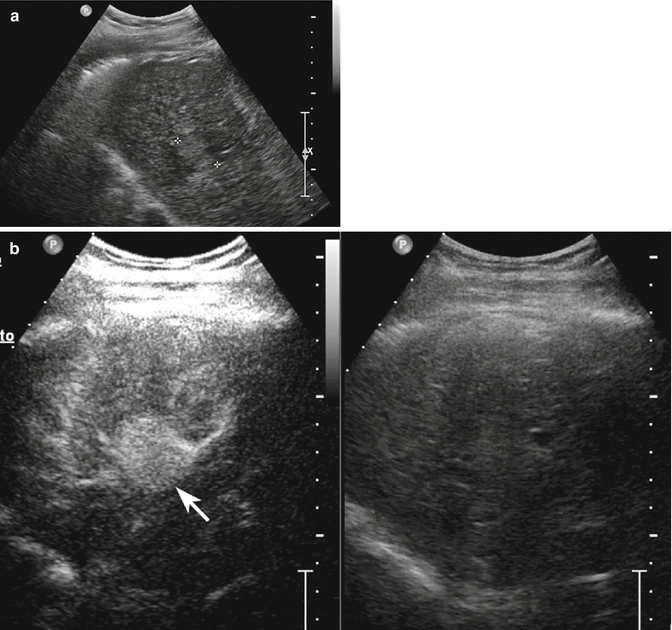
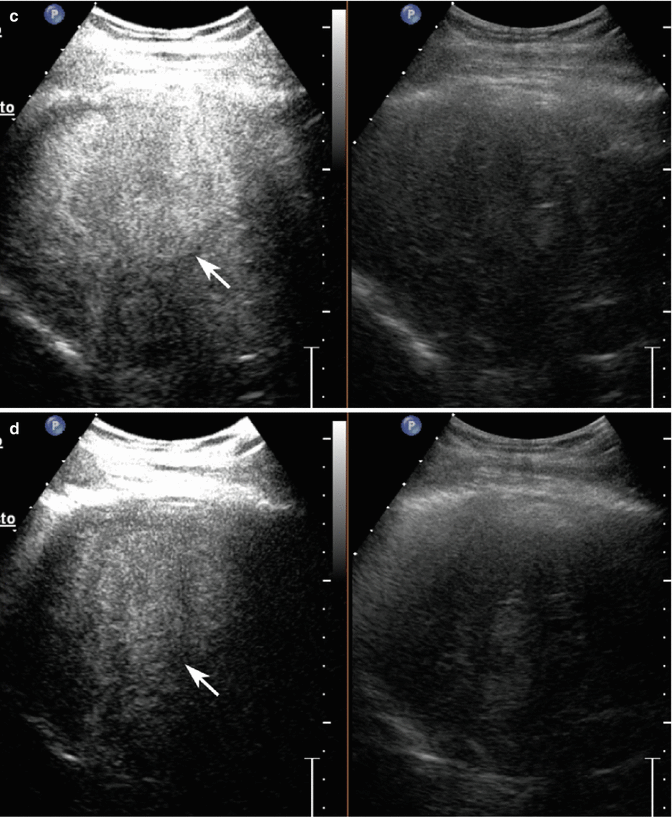
Fig. 3.5
Dysplastic nodule/early HCC in a 65-year-old-man with HCV-related chronic hepatitis. (a) Intercostal baseline image reveals an isoechoic lesion surrounded by a tiny hypoechoic rim sized 3 cm in the segment VIII (calipers). (b) At CEUS, the lesion presents a markedly hypervascular aspect in the arterial phase (arrow) becoming isovascular with respect to the surrounding liver parenchyma in the portal-venous (c) and late (d) phases (arrows)
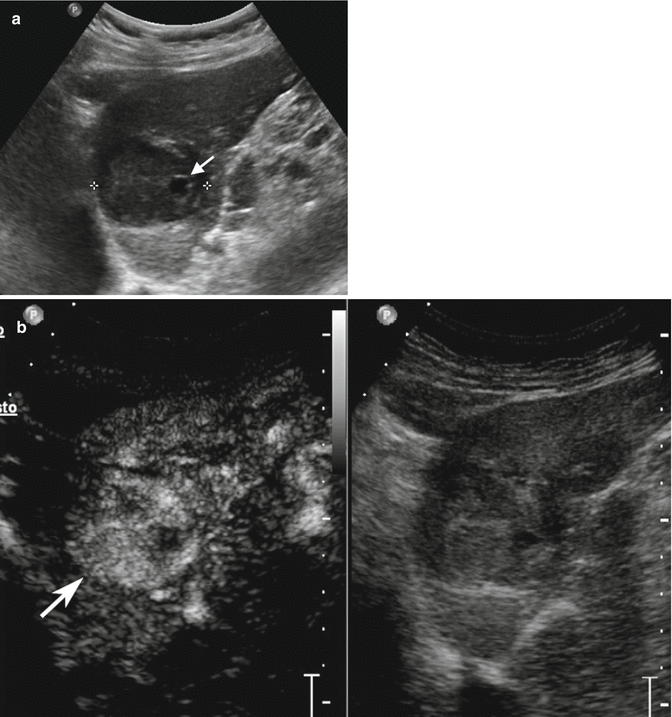
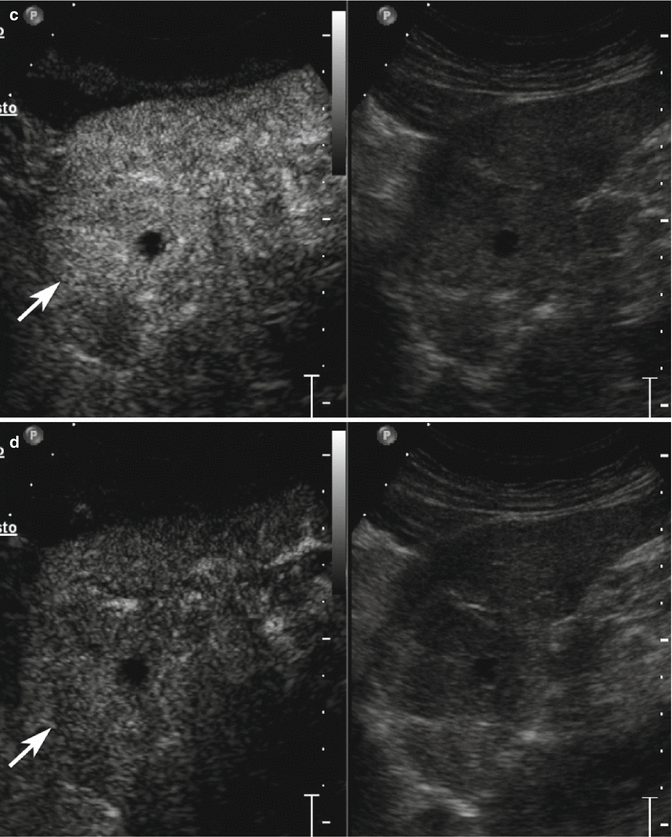
Fig. 3.6
Displastic nodule/early HCC in a 78-year-old man with HCV-related chronic hepatitis. (a) Sagittal subcostal baseline image reveals an inhomogeneous hypoechoic lesion sized 4.1 cm in the left lobe (calipers) with small fluid area in the context (arrow). (b) At CEUS, the lesion presents markedly hypervascular aspect in the arterial phase becoming isovascular with respect to the surrounding liver parenchyma in the portal-venous (c) and late (d) phases (arrows)
3.2.3 HCC
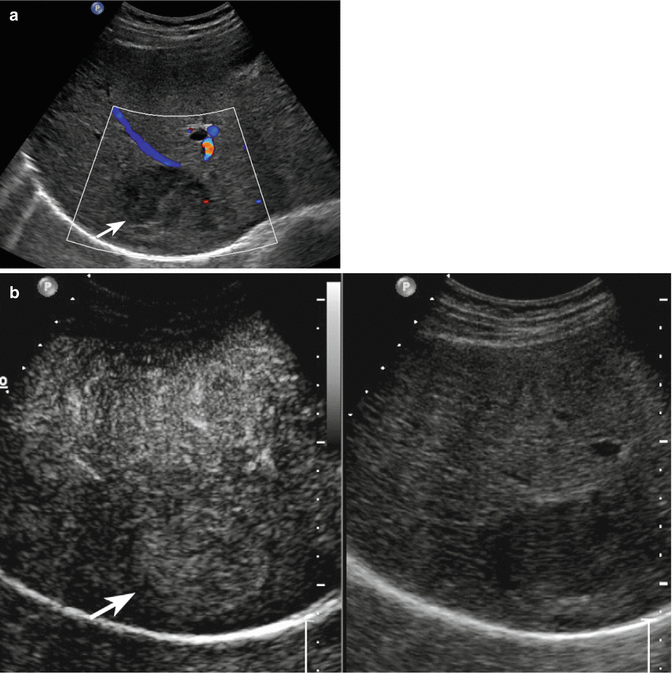
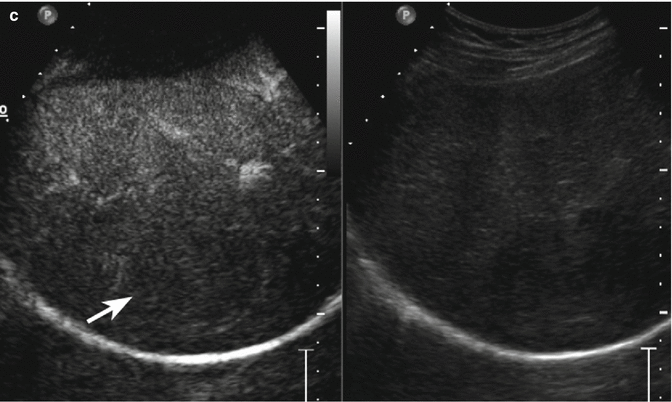
Fig. 3.7
HCC in a 78-year-old man with HCV-related chronic hepatitis. (a) Oblique ascending right subcostal baseline image reveals an inhomogeneous hypoechoic lesion sized 4.5 cm in the VII hepatic segment with tiny peripheral vascular signal at color-Doppler evaluation (arrow). (b) At CEUS, the lesion presents moderately hypervascular aspect in the arterial phase followed by washout in the extended portal-venous phase (c) (arrows)
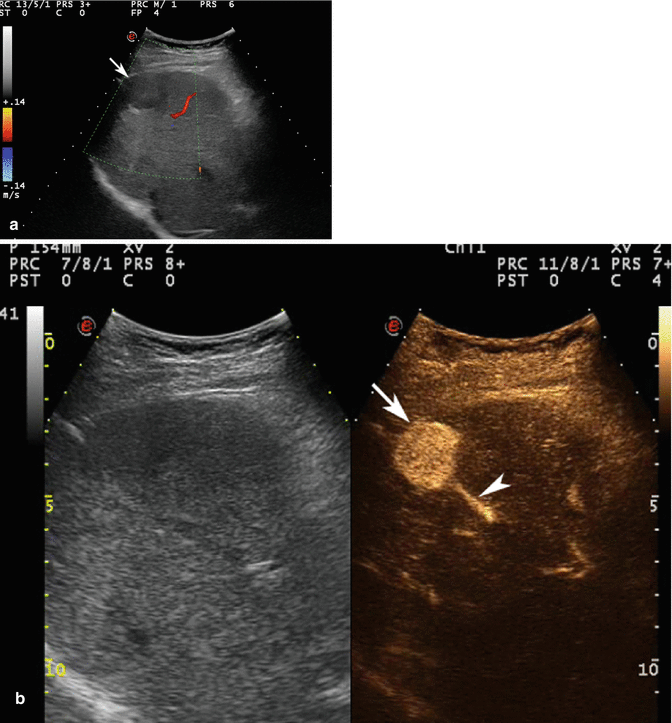
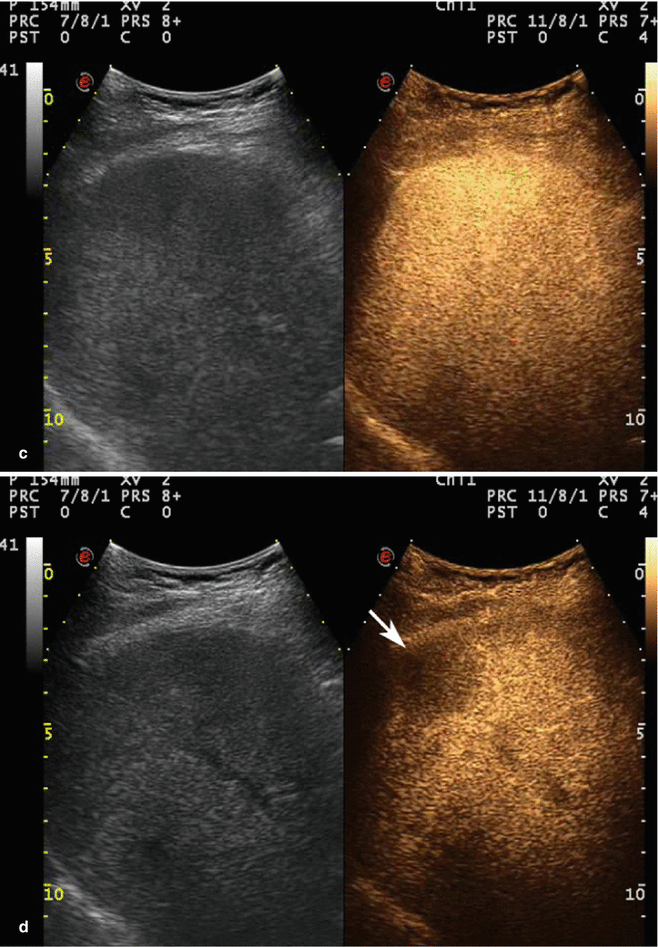
Fig. 3.8




HCC in a 64-year-old woman with HCV-related chronic hepatitis. (a) Intercostal right baseline US image reveals a homogeneous markedly hypoechoic lesion sized 3.2 cm in the VIII hepatic segment without vascular signal at color-Doppler evaluation (arrow). (b) At CEUS, the lesion shows a strong contrast enhancement (arrow) with evident feeding vessel (arrowhead) in the arterial phase. The lesion is not evident in the portal-venous phase (c) but shows a clear-cut washout in the late phase (d) (arrow)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







