Symptoms
Storage versus voiding versus post-micturition
Duration
Severity: i.e. incontinence episodes
Degree of bother
Which symptom is most bothersome
Any treatment previously trialled
Impact on quality of life
Any precipitating factors
Drug history
Diuretics, herbal formulations, illicit drug use (especially ketamine)
Co-morbidities
Diabetes mellitus/insipidus
Previous surgery: penile, prostatic or rectal (e.g. for inflammatory bowel disease)
Previous trauma
Neurological disorders: Parkinson’s, multiple sclerosis, cerebrovascular accident, spinal cord injury, disc prolapse, spina bifida
Cardiorespiratory disease: heart failure, sleep apnoea
Renal disease
Table 9.2
Examination
Examination | Abdomen Urinary retention Surgical scars External genitalia Phimosis Meatal stenosis Balanitis xerotica obliterans Penile cancer Digital rectal examination Anal tone, sensation Prostate: size, irregularity, tenderness, bogginess Rectal mass Perineal/lower limbs: motor and sensory function |
Symptom Questionnaires
There are several available questionnaires which are validated in a variety of languages. They are usually sensitive to changes in symptoms and therefore can be used to monitor responses to treatment. The most widely used questionnaire is the International Prostate Symptom Score (IPSS). This eight-item questionnaire has seven symptom and one quality of life (QoL) questions. The symptom questions assess four voiding and three storage symptoms for the previous month (see Fig. 9.1). The response options range from ‘not at all’ (0 points) to ‘almost always’ (5 points). The minimum score is 0 and the maximum score 35. The symptom severity is determined based on the basis of the total symptom scores as:
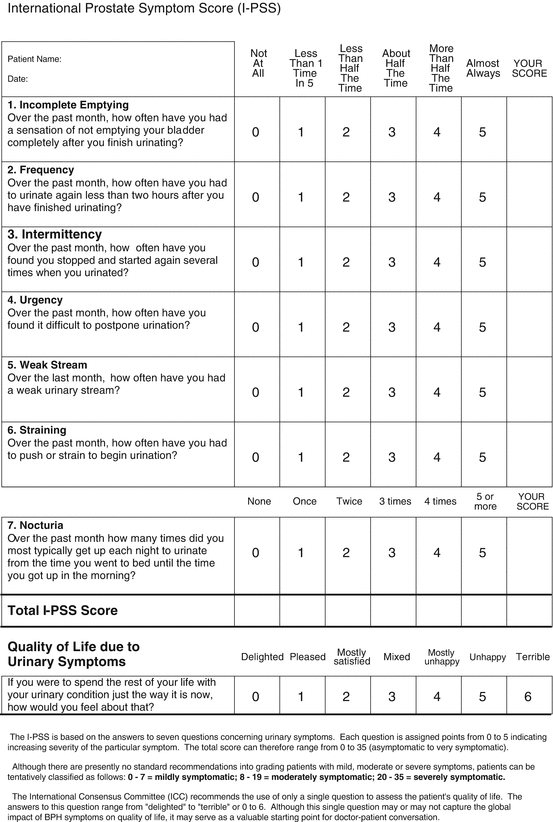

Fig. 9.1
International Prostate Symptom Score (IPSS)
Asymptomatic: 0 points
Mildly symptomatic: 1–7 points
Moderately symptomatic: 8–19 points
Severely symptomatic: 20–35 points
The QoL scores range from 0 to 6. The main limitation of the IPSS is the lack of assessment of incontinence. This means that LUTS severity may be underestimated.
International Consultation on Incontinence Questionnaire (ICIQ-MLUTS)
This validated questionnaire resulted from the ICS-BPH study. This 11-item questionnaire assesses a large spectrum of LUTS and bother scores for individual symptoms.
Bladder Diary
These are useful adjuncts in providing information about patients’ drinking and voiding habits. A bladder diary records volumes voided and their times, incontinence episodes, pad usage, fluid intake and degree of urgency. Information about fluid intake allows counselling regarding fluid reduction at specific times of the day and avoidance of stimulants. The diary may also be used to diagnose nocturnal polyuria (nocturnal urine production >20 % in young individuals and >33 % in elderly of the total 24-h urine production) or 24-h polyuria (24-h urine production >40 ml/kg bodyweight). Frequent small-volume voids may indicate OAB. The time between voids may be utilised in counselling in bladder training techniques. Lastly, the maximum volume voided may be useful when performing invasive urodynamics in guiding volumes to which the patient’s bladder can be filled. This also provides information about bladder capacity.
Bright et al. have recently illustrated that the validated ICIQ-bladder diary is as reliable when completed over 3 days versus 4 days. They recommend the 3-day version.
Urinalysis
This is an inexpensive tool to exclude underlying pathologies such as diabetes, UTI, renal disease or urogenital malignancy. The European Association of Urology (EAU) expert panel recommends its use, although there is no strong evidence for its use in LUTS.
Prostate-Specific Antigen (PSA)
The serum PSA concentration may be used as a surrogate for prostate volume. A prostate volume >30 mL is associated with 3 times greater risk of acute urinary retention (AUR) and BPO-related surgery. The PSA thresholds for volumes greater than 30 mL are:
1.3 ng/mL for ages 50–59 years
1.5 ng/mL for ages 60–69 years
Recent studies have also used PSA to predict the likelihood of BPO; a PSA >4 ng/mL was shown to have an 89 % chance of being associated with BPO. The EAU currently recommends that the PSA should only be measured if it will change the patient’s management or in those at risk of disease progression.
Renal Function Measurement
There is a very low risk of renal impairment in men with LUTS, less than 1 % in the Medical Therapy of Prostatic Symptoms Study (MTOPS). However, renal impairment is associated with an increased risk of complications following TURP.
The EAU recommends renal function should be checked if:
Renal insufficiency is suspected.
Hydronephrosis is seen on imaging.
Surgical therapy is being considered for LUTS.
Post-void Residual (PVR) Measurement
This can be measured by transabdominal ultrasound, bladder scan or catheterisation. It can be calculated by height × width × length × 0.7.
There are a variety of available formulae for calculating PVR, usually varying in the multiplication factor used in the formula. A high PVR was found to be associated with an increased risk of symptom progression in both MTOPS and ALTRESS. A significant PVR may be associated with BOO/BPO and/or DUA. EAU recommendation: PVR measurement should be part of routine assessment.
Uroflowmetry
This is a non-invasive urodynamic test which produces a visual representative of the strength of urinary flow. It evaluates the function of the lower urinary tract. It is a quick test which may be easily performed and interpreted in the office.
The key parameters of the test are:
Maximum urinary flow rate (Q max)
Voided volume (VV)
PVR
Flow pattern
Ideally, uroflowmetry should be performed twice to maximise the reliability of the results, especially when flow parameters are pathological, and the voided volume should exceed 150 mL. The flow pattern may provide suggestions regarding a diagnosis:
Bladder outlet obstruction: reduced Q max, prolonged tail
Urethral stricture: reduced Q max with plateau flat trace
Detrusor underactivity: reduced Q max, intermittent, fluctuating flow
A normal flow trace is bell shaped with Q max attained with 3–10 s. Q max may be affected by age, voided volume, bladder contractility and urethral resistance. The values give an indication of probability of BOO/BPO (Table 9.3). Q max >15 mL/s can exclude BOO/BPO in 97 % of patients and is associated with a poorer outcome after TURP; therefore, Q max >15 mL/s is one of the indications for performing invasive urodynamics prior to prostate surgery in symptomatic patients.
Table 9.3
Risk factors for bladder outlet obstruction
Q max (mL/s) | % Obstructed |
|---|---|
≥15 | 3 |
<15 | 59 |
≥10 | 28 |
<10 | 69 |
Invasive Urodynamics (UDS)
This invasive test involves the insertion of intravesical and rectal catheters which allows for simultaneous bladder filling, vesical and rectal pressure measurements and the calculation of detrusor pressure. The test consists of filling and voiding phases. It allows for the diagnosis of bladder detrusor overactivity (DO) during filling and BOO and/or DUA during voiding which may significantly affect management. BOO and DUA may be determined using the bladder outflow obstruction index (BOOI, previously known as the Abrams-Griffiths number) and bladder contractility index (BCI), respectively (Fig. 9.2). BOO is characterised by increased detrusor pressure and reduced flow, while DUA is characterised by reduced detrusor pressure and flow during the voiding phase. The importance of establishing a UDS diagnosis is that in men with ‘clinical’ BOO, 57–61 % may have DO, 29 % BOO and 11 % DUA. The prevalence of DUA in men with LUTS ranges between 11 and 40 %. A study by Cannon et al. revealed that there are no UDS or symptomatic gains from TURP in men shown to have DUA. Lastly, the other advantage of invasive UDS prior to surgery is in determining the presence of preoperative DO which, after bladder outlet surgery, may result in DO incontinence. This allows for better patient counselling prior to surgery (Table 9.4).
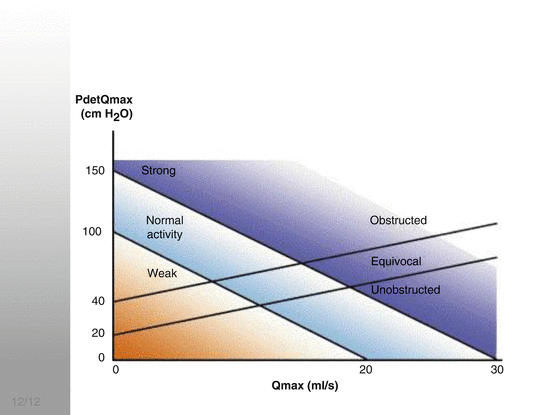

Fig. 9.2
Composite Bladder outlet obstruction index (BOOI) and Bladder contractility index (BCI) nomograms. (Hashim Hashim et al. Eur Urol 2007;4:1186–94)
Table 9.4
EAU recommendations for invasive urodynamics
Voided volume on uroflowmetry ≥150 ml |
|---|
Q max ≥ 15 ml/s |
Age <50 years or >80 years |
PVR >300 mL |
Suspicion of neurogenic bladder dysfunction |
Bilateral hydronephrosis |
Previous radical pelvic surgery |
Previous failed invasive treatment |
Others
Ultrasound: only if large PVR or history of urolithiasis (EAU 2014) (Tables 9.5 and 9.6)
Table 9.5
Predictors for progression of LUTS/BPO
Predictor
Risk increase
Age >70 years
8
PSA >1.4 ng/mL
3
IPSS >7
3
Q max <12 mL/s
4
Prostate volume >30 mL
3
PVR >50 mL
3
Prostatic inflammation
Failure to respond to medical treatment
Table 9.6
Complications of LUTS/BPO
Complication
Risk
Symptom progression
17–40 %
Acute urinary retention
1–2 %/year
UTI
0.1–12 %
Bladder stone
0.3–3.4 %
Renal impairment
<2.5 %
Urinary incontinence
<1 %
Haematuria
10 %
Conservative Treatment of Male LUTS
The origin of LUTS in adult men, as described above, is multifactorial and shifted away from the prostate (BPH, BPE or BPO). Although the prostate may be responsible for LUTS in some men, different organs or structures can also cause or contribute to LUTS in others, for example, the urinary bladder, pelvic floor, central or peripheral nervous system and even the kidney in case of nocturia due to nocturnal polyuria. It is sometimes difficult or even impossible to detect the primary origin of LUTS in individual patients. Therefore, symptomatic therapy of LUTS with conservative treatment modalities, regardless the exact cause, should always be the first and an essential part of the treatment in all patients without absolute indications for surgery. Conservative treatment modalities consist of:
Education: explanation of the anatomy of the lower urinary tract, physiology of urine storage and voiding and pathologies which can lead to LUTS, instructions concerning drinking volumes as well as fluid types and explanation of the correlation between fluid volume intake and voiding frequency (www.patients.uroweb.org/library-bpe/).
Reassurance: explanation that not (bladder or prostate) cancer is the cause for LUTS.
Lifestyle advice: is diverse, should be applied according to the predominant complaint and includes:
Reduction of the fluid volume in cases of excessive fluid intake, emptying the bladder before going to bed or when urinary frequency is most inconvenient (e.g. during travels or going out in public)
Avoidance of, or moderation in, caffeinated and/or alcoholic beverages which can have a diuretic or irritant effect on the bladder
Distraction techniques (e.g. penile squeeze, breathing exercises, perineal pressure or mental tricks) to take the mind off the bladder or toilet and to control bladder storage symptoms, i.e. the brain controlling the bladder and not the other way round
Bladder retraining to encourage men to hold on when they have urgency in order to increase bladder capacity and increase the time between voids
Providing assistance in cases of cognitive dysfunction or impairment of dexterity and mobility
Reviewing medications (for other indications than LUTS) and optimising the time of administration or replacement in case of urinary adverse events (e.g. diuretics)
Treatment of constipation
Use of relaxed or double-voiding techniques, pelvic floor muscle exercises with or without biofeedback support
Urethral milking or use of absorbents to cope with post-micturition dribble
Periodic monitoring: regular follow-up examinations of the patient with re-evaluation of LUTS, the prostate and pathologies which have been identified and quantified during initial workup, offer to return to the clinic in case of symptom deterioration.
Conservative treatment has shown to significantly reduce LUTS to a greater extent than standard care when three self-management sessions were offered. Approximately 64 % of men do well with conservative treatment over a period of 5 years. Conservative treatment has level 1b evidence (randomised-controlled trials, RCTs), and the EAU guidelines on male LUTS recommend this treatment approach for patients with mild (to moderate) symptoms in the absence of complicated LUTS, absolute indications for surgical treatment or parameters of disease progression. Physicians should always offer conservative treatment prior or concurrent to drug treatment (grade of recommendation A).
Drug Treatment of Male LUTS
If symptom relief has been insufficient with conservative treatment modalities alone and the patient still suffers of LUTS, drug treatment can be added. There are currently five different drug classes available which have been licensed for the treatment of male LUTS and can be used either alone or in combination:
α-Adrenoceptor antagonists (α-blockers)
5α-Reductase inhibitors (5ARI)
Muscarinic receptor antagonists (antimuscarinics)
Phosphodiesterase type 5 inhibitors (PDE5i)
Arginine-vasopressin analogues (desmopressin)
Approved drug classes, drugs within the different classes, the key pharmacokinetic features and recommended daily doses are listed in Table 9.7. This chapter focuses only on the drugs which have been analysed by the NICE, EAU and AUA guidelines.
Table 9.7
Drug classes, drugs within the classes, key pharmacokinetic properties and standard doses of drugs licensed for the treating of male LUTS (license text added next to the drug class) [Oelke M et al. Eur Urol. 2013;64:118–40]
Drug (class) | t max [h] | t½ [h] | Recommended daily dose |
|---|---|---|---|
α 1 -Adrenoceptor antagonists (for treating signs or symptoms of BPH) | |||
Alfuzosin IR | 1.5 | 4–6 | 3 × 2.5 mg |
Alfuzosin SR | 3 | 8 | 2 × 5 mg |
Alfuzosin XL | 9 | 11 | 1 × 10 mg |
Doxazosin IR | 2–3 | 20 | 1 × 2–8 mg |
Doxazosin GITS | 8–12 | 20 | 1 × 4–8 mg |
Silodosin | 2.5 | 11–18 | 1 × 4–8 mg |
Tamsulosin MR | 6 | 10–13 | 1 × 0.4 mg |
Tamsulosin OCAS | 4–6 | 14–15 | 1 × 0.4 mg |
Terazosin | 1–2 | 8–14 | 1 × 5–10 mg |
5α-Reductase inhibitors (for treating benign prostatic enlargement due to BPH) | |||
Dutasteride | 1–3 | 3–5 weeks | 1 × 0.5 mg |
Finasteride | 2 | 6–8 | 1 × 5 mg |
Antimuscarinic drugs (for treating OAB/storage symptoms) | |||
Darifenacin | 7 | 12 | 1 × 7.5–15 mg |
Fesoterodine | 5 | 7 | 1 × 4–8 mg |
Oxybutynin IR | 0.5–1 | 2–4 | 3–4 × 2.5–5 mg |
Oxybutynin ER | 5 | 16 | 2–3 × 5 mg |
Propiverine | 2.5 | 13 | 2–3 × 15 mg |
Propiverine ER | 10 | 20 | 1 × 30 mg |
Solifenacin | 3–8 | 45–68 | 1 × 5–10 mg |
Tolterodine IR | 1–3 | 2–10 | 2 × 1–2 mg |
Tolterodine ER | 4 | 6–10 | 1 × 4 mg |
Trospium IR | 5 | 18 | 2 × 20 mg |
Trospium ER | 5 | 36 | 1 × 60 mg |
Β3 agonist (for treating OAB/storage symptoms) | |||
Mirabegron | 3.5 | 50 | 1 × 50 mg |
Antidiuretic (for treating nocturnal polyuria) | |||
Desmopressin tbl. | 1–2 | 3 | 1 × 0.1–0.4 mg orally before sleeping |
Desmopressin oral lyophilisate (MELT) | 0.5–2 | 2.8 | 1 × 60–240 μg* sublingually before sleeping |
Phosphodiesterase 5 inhibitors (for treating signs or symptoms of benign prostatic hyperplasia with or without erectile dysfunction) | |||
Tadalafil | 2 (0.5–12) | 17.5 | 1 × 5 mg |
In some European countries, phytotherapy is also popular and has a market share as high as 50 % of the national male LUTS market. However, plant extracts are a heterogeneous group of drugs, not universally available, and can contain different concentrations of active ingredients when comparing the extract of different producers and even different brands of the same producer. Additionally, it has yet not been clarified which active ingredient is responsible for LUTS improvement. Phytotherapy was excluded from the NICE, EAU and AUA guidelines due to methodological reasons but may be a viable treatment option for some men with mild to moderate LUTS who refuse using chemical drugs. Therefore, phytotherapy could be used to support conservative treatment.
The prescription of one drug of the above-mentioned five chemical drug classes depends on baseline values determined during systematic assessment of the patient and is largely dependent on the type of symptom (storage, voiding, storage + voiding or nocturnal polyuria), concomitant erectile dysfunction, prostate volume and the patient’s willingness to use the drug long term. The flow diagram (Fig. 9.3) illustrates the evidence-based drug treatment of male LUTS with key results of the initial diagnostic tests, as described in the EAU guidelines on male LUTS in the year 2013.
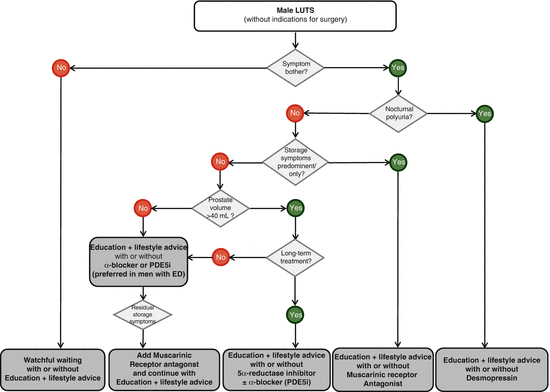

Fig. 9.3
Treatment algorithm of male LUTS with conservative treatment options and drugs, EAU guidelines on male LUTS, including BPO. PDE5i phosphodiesterase type 5 inhibitor, ED erectile dysfunction [Oelke M et al. Eur Urol. 2013;64:118–40]
α-Adrenoceptor Antagonists (α-Blockers)
The urinary bladder, bladder neck and prostate contain α1-adrenoceptors in high density which can increase, after noradrenaline stimulation, smooth muscle tone. The effect on smooth muscle cells in the bladder outlet is primarily mediated by α1A-adrenoceptors. In contrast, α1B– and α1D-adrenoceptors are mainly located in blood vessels, central nervous system and in the urinary bladder proximal to the bladder outlet and may also contribute to LUTS. α-Blockers act by reversible inhibition of these α1-adrenoceptors, thereby reducing the tone of smooth muscle cells of the prostate and bladder neck and, eventually, reducing BPO and LUTS. However, the effect on BPO is only modest and does not fully explain the effects of α-blockers; other factors may therefore be responsible for LUTS reduction, such as central nervous effects.
α-Blockers are available in different formulations (Table 9.7) and have become the most popular drug class for the treatment of male LUTS during the past 25 years. Primary candidates for α-blocker therapy are men with bothersome moderate-to-severe voiding or voiding + storage LUTS. Reasons for this first-line treatment choice are obvious:




α-Blockers can be administered in the majority of symptomatic men because the majority suffer of voiding or voiding + storage symptoms.
They are efficacious after a few days.
They significantly and substantially reduce storage and voiding LUTS within weeks (IPSS decrease by 30–40 % on average after placebo run-in and up to 50 % in open-label trials).< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








