, Mark Thomas1 and David Milford2
(1)
Department of Renal Medicine, Birmingham Heartlands Hospital, Birmingham, UK
(2)
Birmingham Children’s Hospital, Birmingham, UK
Abstract
In this chapter we explain:
Risk assessment using eGFR and proteinuria
Competing risks of dialysis and death
How a patient’s future may be predicted by their eGFR graph
How patients are prepared for end-stage kidney failure
Treatment options for someone with end-stage kidney failure
When dialysis should be started
Once you have made a diagnosis of kidney disease, the next step is to agree a treatment plan with the patient. We do not provide guidelines for the management of individual conditions in this book. However, there are some principles that apply generally to the planning of treatment for kidney disease.
Understanding Risk and Predicting the Future
eGFR and proteinuria are useful for assessing a patient’s prognosis. Patients with a low eGFR and proteinuria have an increased risk of end-stage kidney disease and of death, largely due to cardiovascular disease.
Figure 17.1 shows the relative risk of death for people in four age categories according to their eGFR and albuminuria. In each chart, the columns show someone’s risk of death compared to someone in the same age category with normal kidney function and no albuminuria. The reference category is eGFR = 75–89 ml/min/1.73 m2 with urine dipstick showing no proteinuria or an albumin:creatinine ratio <1.1 mg/mmol (<10 mg/g).
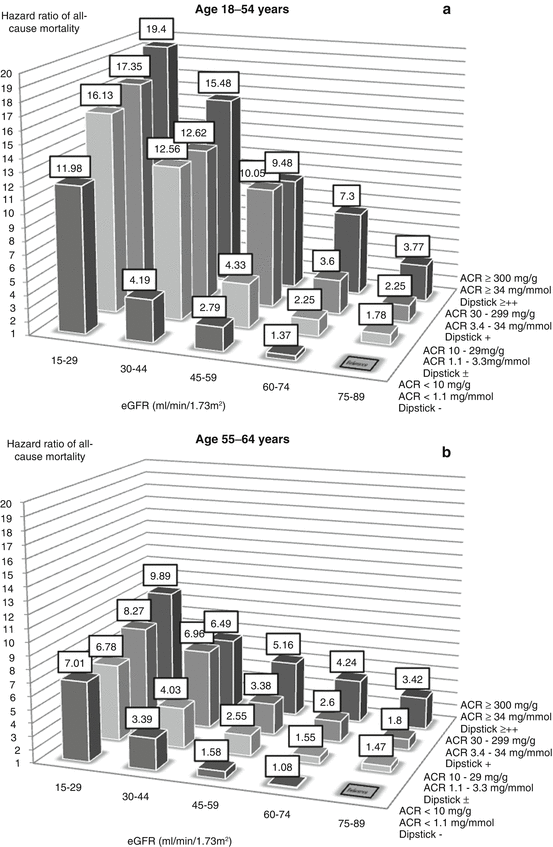
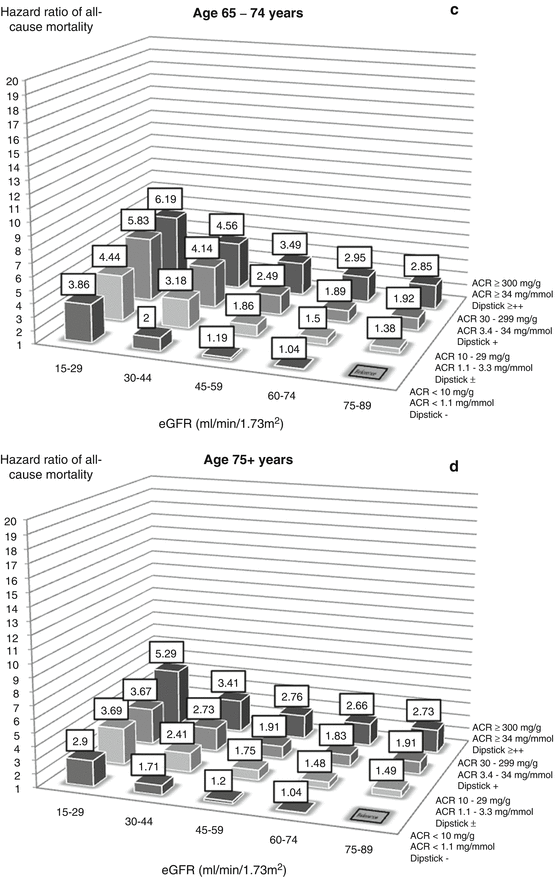


Fig. 17.1
Risk of mortality associated with eGFR and albuminuria by age category (a = 18–54 years, b = 55–64 years, c = 65–74 years, d = 75+ years) (Drawn using data from Hallan et al. [1])
One can draw a number of conclusions from these charts. Firstly, the lower someone’s eGFR and the greater their amount of albuminuria, the greater is their risk of death. eGFR and albuminuria each increases the risk in its own right. This applies to all age categories, implying that reduced eGFR and albuminuria are not inevitable features of ageing but represent markers of disease.
Secondly, the increase in relative risk is large, especially in the youngest age category. Someone aged 18–54 years with an eGFR of 15–29 and no albuminuria is 12 times more likely to die over the next 5.8 years than someone with an eGFR 75–89. Similarly, someone with a urine albumin:creatinine ratio >34 mg/mmol (ACR >300 mg/g, dipstick ++ or more) but normal eGFR is nearly four times more likely to die. Combining the two factors increases the risk nearly 20 times.
Thirdly, the relative risk of death associated with reduced eGFR and albuminuria gets smaller with age. However, as the underlying risk of death in the reference group increases with age, the effect of reduced eGFR and albuminuria on the number of people dying per year is greater in the older age groups.
What are the implications of these findings for clinical practice? It is important to remember that these are measurements of the risk of death associated with reduced eGFR and albuminuria. That does not mean that reduced kidney function or albuminuria in some way cause the deaths, or that increasing eGFR or reducing albuminuria will necessarily reduce the risk of death.
The effect of reduced eGFR on outcomes has been studied in kidney donors. When one of their kidneys is removed, the donors go from normal to CKD stage 2 or 3. Does this reduced eGFR increase their risk of future cardiovascular disease? The answer seems to be ‘no’. Comparing Canadian kidney donors with the healthiest segment of the general population there is no increase in the risk of major cardiovascular events over the next 10 years [2]. However, kidney donors do have twice the risk of developing high blood pressure or pre-eclampsia in pregnancy (11 % versus 5 %) [3].
Studies in animal models of more advanced CKD suggest a causal link between reduced kidney function and heart disease such as left ventricular hypertrophy. Kidney tubular epithelium is the main producer of a protein called Klotho that has anti-aging properties. It is released into the circulation like a hormone and its levels decline in CKD. One of its actions is to bind the uraemic toxin indoxyl sulfate which is cardiotoxic. In CKD, levels of Klotho are reduced and so too is its cardioprotective effect [4].
Knowing about your risk of dying is only useful if something can be done about it. The opportunity to alter the natural history of disease is more limited as people get older. Nonetheless, treatment to a target systolic blood pressure of 150/80 mmHg in people aged over 80 years who are otherwise well reduces the rate of death, stroke and heart failure within 1–2 years [5]. On the other hand, over-aggressive treatment of blood pressure in elderly people with other comorbidities increases the risk of falls and harm.
Finally, the wishes of the patient must be taken into account when deciding how much attention to give to these risk factors. An elderly person suffering from a number of long-term conditions may give a much higher priority to improving the quality of their life than its length.
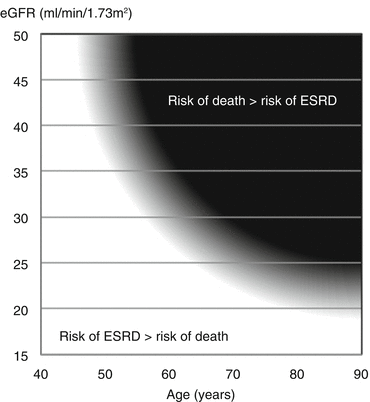
Competing Risks: Dialysis or Death?
The risk of developing end-stage kidney disease can be gauged from three factors: age, eGFR and the level of proteinuria. Although the type of disease causing the kidney failure does play a part, when considering people with the same pathological condition the prognosis is largely determined by these three factors.
Studies of populations of patients with CKD show that, for a given level of eGFR, the risk of dying before reaching the need to start dialysis increases steadily with age (Fig. 17.2).
Damage to the glomerular endothelial cells reflects damage to the endothelium of microvessels elsewhere in the body. This includes the vasa vasorum that provide the blood supply to the walls of large arteries. This may explain the link between albuminuria, uraemia and cardiovascular disease [8]. Measuring albuminuria or proteinuria is a useful way of measuring the health of someone’s blood vessels and with it their risk of renal failure and death.
Data from a community mass-screening programme in Japan showed how the risk of renal failure increased with the level of proteinuria [9]. Even small amounts were significant; having + proteinuria on dipstick testing increased the risk of end-stage renal failure by 1.9 times in men and 2.4 times in women.
For older patients, a validated risk score that incorporates nine clinical variables gives an assessment of the risk of death within 5 years before reaching end-stage kidney disease [10].
Risk analyses such as these are very helpful for identifying categories of people who may benefit from more intensive treatment. They are less useful for planning the treatment for individual patients. Unlike in most chronic medical conditions where we can offer only probabilities of what might happen in the future, with kidney disease we can often make a prediction for the individual patient.
Prognostication: “Be Prepared”
Many patients attend the kidney clinic anxious they may need to start dialysis. The eGFR graph can be very helpful for judging if that is true and, if so, when it is likely to happen (Patient 17.1).
Patient 17.1: Projection of the eGFR Graph to Predict the Need for Dialysis
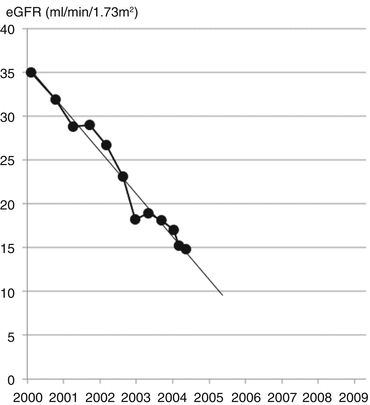
Mr. Arnold had been followed regularly in the clinic for his CKD. Over a 9-year period his eGFR had declined by 15 ml/min/1.73 m2 (Fig. 17.3).
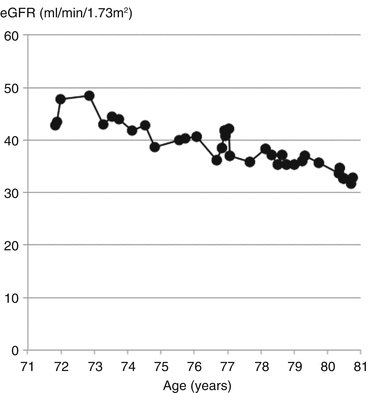

Fig. 17.3
Decline in eGFR by approximately 1.5 ml/min/1.73 m2/year
To predict the future, a trendline was projected on the chart (Fig. 17.4).
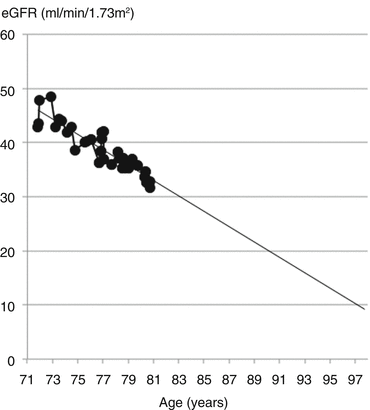

Fig. 17.4
Projected to reach ESRD at age 97 years
If Mr. Arnold’s loss of kidney function continued at the same rate, he would reach an eGFR of 10 ml/min/1.73 m2 at the age of 97 years. He was very relieved to know that he did not need to worry about having dialysis for the foreseeable future.
When the trendline on the eGFR graph indicates that someone is likely to need dialysis , care should be planned accordingly. We can learn how to do this from the example of another condition in which health needs are predictable – pregnancy.
Ante-natal classes are provided to help parents prepare for an event that will transform their lives. Like having your first baby, renal failure and dialysis can disrupt your sleep and leave you feeling exhausted. Like bringing up a child, dialysis limits your freedom and continues week-in, week-out for years.
Education and care of patients with advanced kidney failure is provided by a team of professionals, coordinated around the needs of the patient. They address issues that also feature in ante-natal classes, such as:
Choice of medical treatment
Symptom control and self-care
Psychological support
Involvement of carers and relatives
The decision about when to transfer care to this multidisciplinary team is determined by the time needed to prepare for dialysis or a kidney transplant. In pregnancy there are 9 months to prepare; in kidney failure a year is ideal, possibly longer if a transplant is the preferred option. Asking the ‘surprise’ question is helpful: “Would you be surprised if this patient needed to start dialysis within the next year?”
The eGFR graph helps answer this question. The rate of decline in eGFR varies between patients so there is no single value of eGFR when planning should start. Instead, projecting the eGFR graph forwards to 10 ml/min/1.73 m2 will give an estimate of the time remaining (Fig. 17.5).
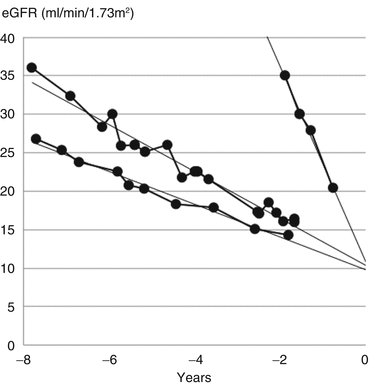

Fig. 17.5
Examples of differences in the rate of decline of GFR between three patients. Transfer to the multiprofessional team occurred at different levels of eGFR to allow at least a year for preparation for transplantation or dialysis
It is wise to have a lower limit of 15 ml/min/1.73 m2 for referral. When the remaining kidney function is this low, an unpredictable acute illness can easily precipitate the need for dialysis. When a transplant is planned as the first renal replacement treatment, it is usually carried out when eGFR falls below 15 ml/min/1.73 m2 to avoid the risk of unplanned dialysis.
The following story (Patient 17.2) illustrates the kinds of problems that may be addressed during the period of preparation.
Patient 17.2: Planning Dialysis
Mr. Fielding was a 72-year-old retired businessman who divided his time between the family home in Birmingham and a business in Wales. His type 2 diabetes was well treated with insulin but his blood pressure was not well controlled. He reported home systolic blood pressure readings between 165 and 170 mmHg despite taking four antihypertensive drugs.
His kidney function had steadily deteriorated through the late 1990s due to diabetic nephropathy. At a clinic visit in February 2003 his eGFR graph predicted that he would reach end-stage kidney failure in 2005 (Fig. 17.6).
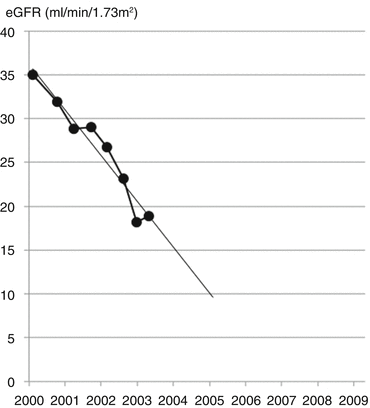

Fig. 17.6
Projected decline in eGFR using trendline
We talked at length about what type of dialysis he would wish to have, a kidney transplant being inappropriate because of angina. In the end he settled on peritoneal dialysis, which would suit his need to return to Wales at regular intervals to manage the business. As he had had no previous abdominal surgery, the peritoneal dialysis catheter could be inserted under a local anaesthetic nearer the time.
A year later, the graph showed that his eGFR had declined as predicted (Fig. 17.7).