Magnetic Resonance Imaging of the Gastrointestinal Tract
Multiplanar capability, lack of ionizing radiation, and potential for superior tissue characterization represent distinct advantages of magnetic resonance imaging (MRI) over other imaging modalities for evaluation of the gastrointestinal tract. However, respiratory motion, peristalsis, susceptibility artifact from air, and the presence of stool have impeded progress in the development of gastrointestinal MRI applications. With the advent of newer imaging sequences and an increasing array of intravenous and enteric contrast agents, a renewed interest in gastrointestinal applications of MRI has emerged in recent years, although many of the MRI techniques discussed herein are still undergoing clinical testing and optimization.
Technique
The two most commonly used sequences for imaging the gastrointestinal tract are HASTE and gadolinium-enhanced, fat-suppressed, T1-weighted gradient echo. Both of these sequences are relatively rapid, allowing imaging of the entire region of interest during a single breath-hold. Half-Fourier single-shot techniques are highly sensitive for intraluminal fluid and are resistant to the susceptibility effects of intraluminal air, although signal-to-noise issues limit the spatial resolution attainable with HASTE sequences (Fig. 2.59). Fat-suppressed, contrast-enhanced techniques allow visualization of enhancing gastrointestinal mucosa and inflammatory tissues but are plagued by the susceptibility artifact created by intraluminal air. This latter limitation is becoming less problematic as sequences with shorter echo times become available. Alternative rapid steady-state sequences such as trueFISP or balanced FFE are being used by some MR practitioners to image fluid-filled bowel. These sequences provide sharp, motion-free images of the bowel during a single breath-hold.
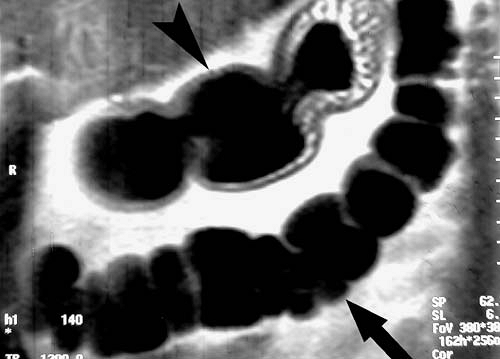 FIG. 2.59. HASTE image of normal bowel. Air-distended transverse colon (arrow) and stomach (arrowhead) are clearly demonstrated on this coronal HASTE image. |
Various oral contrast agents have been investigated for bowel imaging, including man-made and naturally occurring (e.g., blueberry juice) paramagnetic substances, barium, perfluorocarbon, polyethylene glycol, methylcellulose, and particulate iron oxides. Aside from obvious safety and patient acceptance issues, desirable attributes of an oral contrast agent include lack of significant intestinal absorption, uniform distribution, and a dilution-resistant effect on intraluminal signal intensity. A substance that improves bowel distention is also desirable in certain clinical settings. Agents that are bright on T2-weighted images and dark on T1-weighted images may be preferable, because they act as positive intraluminal contrast agents on T2-weighted images while improving conspicuity of the bowel wall and inflammatory processes on fat-suppressed gadolinium-enhanced T1-weighted images.
Despite the wide variety of MRI techniques and contrast agents reported in the literature, gastrointestinal MRI can be relatively simple. Water makes an excellent contrast agent for evaluation of the stomach and proximal small bowel, especially when combined with the motion-resistant HASTE sequence (Fig. 2.60). Water can also be administered rectally for colonic distension before imaging. Oral 2% barium sulfate makes a readily available, inexpensive small bowel contrast medium that is particularly effective when combined with intravenous gadolinium and fat-suppressed T1-weighted gradient echo imaging (1). For the evaluation of inflammatory and neoplastic processes, we prefer the combination of fat-suppressed T2-weighted images and gadolinium-enhanced, fat-suppressed T1-weighted images. Fat-suppressed T2-weighted images are superior for the detection of abnormal fluid collections and edema, whereas the latter images provide the best evaluation of the bowel wall. Fat-suppressed T1-weighted imaging may be performed serially after the administration of intravenous contrast using either a two-dimensional (2D) or three-dimensional (3D) spoiled gradient echo sequence (SPGR, FFE, FLASH) during breath-holding. Delayed imaging performed 3 to 5 minutes after contrast administration using a high spatial
resolution T1-weighted sequence is also suggested. Intravenous glucagon can be used to minimize bowel motion during image acquisition. We suggest injecting 1 mg in a split dose of 0.5 mg per injection just before HASTE and gadolinium-enhanced imaging.
resolution T1-weighted sequence is also suggested. Intravenous glucagon can be used to minimize bowel motion during image acquisition. We suggest injecting 1 mg in a split dose of 0.5 mg per injection just before HASTE and gadolinium-enhanced imaging.
Clinical Applications
Inflammatory Bowel Diseases
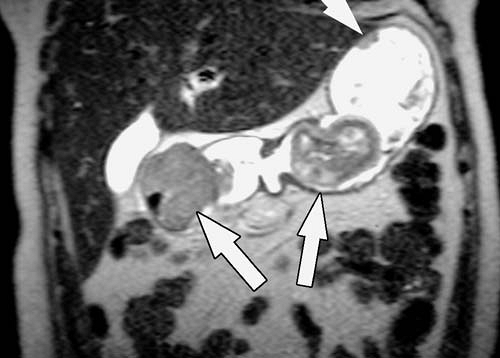
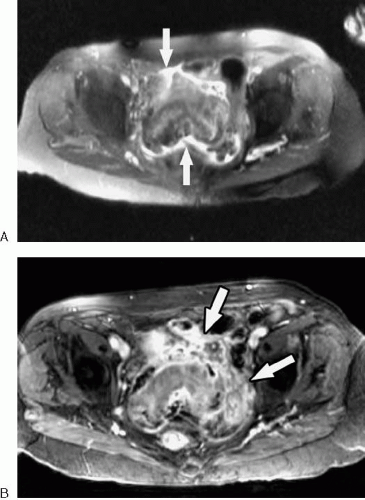
Inflammatory processes of the bowel (Figs. 2.61, 2.62) are characterized by wall edema, thickening, and enhancement, features that are easily demonstrated with available MRI techniques. Despite the potential of MRI for imaging a wide variety of inflammatory bowel processes, much interest has centered on the assessment of Crohn disease. In patients with Crohn disease, transmural inflammation of the bowel wall develops, resulting in wall thickening, surrounding inflammation, and, in some cases, fistula and abscess formation. Contrast-enhanced, fat-suppressed gradient echo images performed after ingestion of dilute oral barium can accurately depict the site and degree of bowel wall thickening. The intensity of bowel wall enhancement provides a qualitative measure of the activity of inflammation, which can help guide therapy (2,3). T2-weighted and contrast-enhanced T1-weighted images are used together to assess extraluminal inflammation, fistulae, and abscesses. The presence of intestinal strictures (which require adequate bowel distention to demonstrate) and fibrofatty proliferation do not, by themselves, imply active inflammation.
Several other techniques have been investigated for the evaluation of patients with Crohn disease. These include MR enteroclysis with a nasojejunal tube using a variety of intraluminal contrast agents. The sensitivity and specificity of
MR enteroclysis for diagnosing inflammatory bowel disease and demonstrating fistulae and abscesses may exceed that of conventional enteroclysis (4).
MR enteroclysis for diagnosing inflammatory bowel disease and demonstrating fistulae and abscesses may exceed that of conventional enteroclysis (4).
Bowel Obstruction
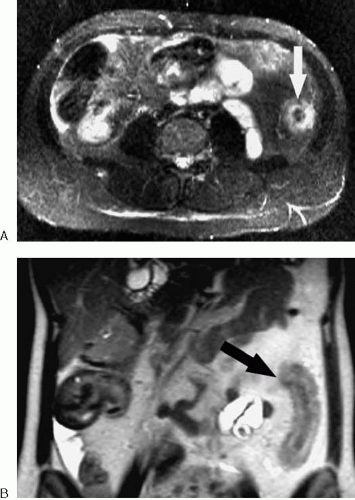
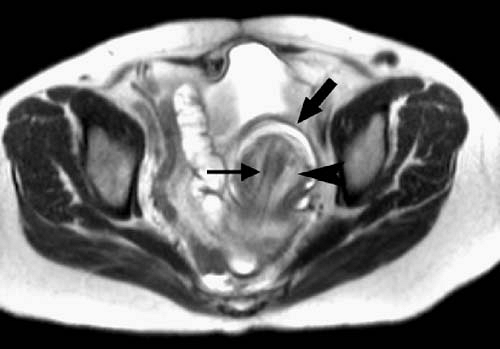
MRI is sensitive for detecting bowel obstruction and is accurate in determining its cause (Figs. 2.63, 2.64) (5,6,7). Fluid-sensitive fast MR techniques such as half-Fourier single-shot echo train spin echo (HASTE, ssFSE, ssTSE) are particularly useful for imaging of small bowel obstruction. The multiplanar capability of MRI should be exploited in determining the precise location of obstruction. In some cases, the ability of MRI to determine the location and cause of bowel obstruction may exceed that of helical computed tomography (CT).
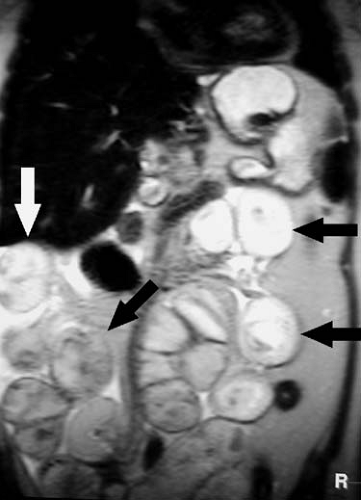 FIG. 2.63. Small bowel obstruction. Coronal HASTE image of abdomen demonstrates multiple dilated loops of small bowel (arrows) in patient with small bowel obstruction secondary to adhesions. |
Tumor Staging
Accurate staging of gastrointestinal tumors is critical to ensure that patients receive appropriate treatment. The role of MRI in staging gastrointestinal malignancies has not yet been firmly established. In recent years, however, data have accumulated supporting the use of MRI in the staging of gastrointestinal malignancies.
Gastric cancer is well demonstrated with MRI (Figs. 2.65, 2.66, 2.67). Gastric cancer staging is based on the TNM classification. Staging of the primary tumor (T) is based on depth of invasion into the gastric wall and MRI accurately predicts the T stage of gastric cancer in approximately 73% to 88% of patients (9,10,11,12). T1 disease invades the lamina propria or submucosa, T2 disease invades the muscularis propria or subserosa, T3 disease penetrates the serosa without invading adjacent organs, and T4 disease invades adjacent structures. Wall invasion is probably best appreciated on fat-suppressed, gadolinium enhanced, breath-hold T1-weighted gradient echo images. Dynamic and delayed (approximately 2 minutes) imaging typically reveals focal thickening of the stomach wall or a mass that enhances differently (greater or less) than the normal gastric wall. In general, increased enhancement of the tumor relative to the normal gastric wall is more common. Extraserosal invasion is suggested by disruption of the low signal intensity band normally present around the outer gastric wall (8). Extraserosal invasion is easier to detect when the gastric wall is surrounded by fat. Fat-suppressed, contrast-enhanced gradient echo images may demonstrate enhancing tumor extending into the perigastric fat. The sensitivity of MR for detecting extraserosal tumor invasion may be as high as 93% using dynamic contrast-enhanced techniques (13).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree