Fig. 5.1
Thick-slab HASTE image enteral contrast outlining the small bowel up to the ascending colon
Table 5.1
MRI sequences for enterography examination
1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
MR sequences | HASTE with fat saturation | True-FISP with and without fat saturation | HASTE with fat saturation | VIBE/FLASH 3D with fat saturation | High-resolution imaging |
True-FISP with fat saturation | |||||
Plane | Coronal | Coronal and axial | Coronal and axial | Coronal and axial | Coronal and axial |
Breath hold | No | Yes | Yes | Yes (multi) | Yes |
Timing | In last part of study (unenhanced and enhanced [60 s after contrast administration]) | ||||
No. of slices | 1 | 19–25 | 19–25 | 60–80 | 12–15 |
Slice thickness (mm) | 50 | 4 | 4 | 2.5 | 2 |
Slice distance | 0 | 0 | 0 | 0 | |
Field of view (mm2) | 512 × 512 | Coronal: 512 × 400 | Coronal: 512 × 512 | Coronal: 280 × 320 | 160 × 160 |
Axial: 350 × 240 | Axial: 350 × 350 | Axial: 350 × 262 | 160 × 160 | ||
TR (ms) | 5,000 | 2.5–4.0 | 1,200 | 2.5–5.12 | 2.5–4.0 |
TE (ms) | 1,000 | 1.6–1.8 | 80 | 1–2.5 | 1.6–1.8 |
Flip angle (°) | 90–140 | 50–80 | 90–140 | 10–20 | 50–80 |
If bowel obstruction is observed on the initial thick-slab HASTE images, MR fluoroscopy of the affected segment is performed to assess for inflammatory adhesions or strictures before injection of antiperistaltic drugs (Video 5.2).
Patients can be scanned in the supine or prone position. The author prefers to perform MR enterography in the supine position, as this is more comfortable for patients. Prone positioning is advocated by some studies as it provides compression of the bowel loops with better loop separation, although this has not been proven to provide any significant diagnostic advantages [29].
MRE Findings in Crohn’s Disease
Ulcers and Intestinal Fold Abnormalities
Acute inflammation in CD is characterized by ulcers and mucosal fold abnormalities. Intestinal folds may also have a thickened or polypoid appearance and folds may be absent in the chronic phase of CD (Fig. 5.2). Edema within the mucosal folds is seen as hyperintense signal on HASTE and true-FISP sequences (Fig. 5.2). In patients with active CD, the bowel wall may have a higher signal intensity compared to non-affected bowel wall. Early ulcers (aphthous ulcers) may be seen as a ring of edema around a tiny ulcer crater (Fig. 5.3).
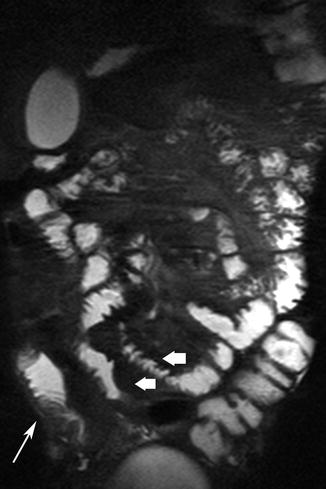
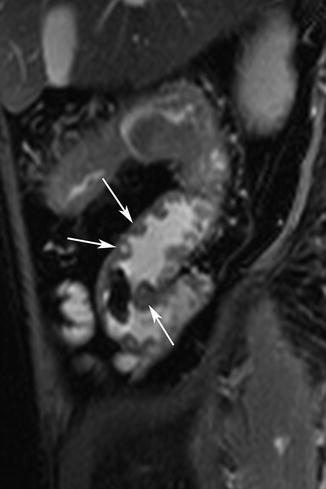

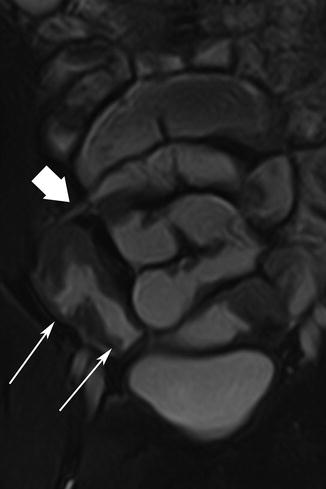
Fig. 5.2
Acute inflammatory CD. Thickened, distorted mucosal folds are readily visible against the bright signal from enteral contrast (thick arrows). Mural edema is seen as linear high signal (thin arrow)

Fig. 5.3
Ulcers. Sagittal true-FISP image shows multiple aphthous ulcers as high signal lesion surrounded by ring of edema (arrows). Note mural thickening and pseudopolypoid appearance of residual mucosa
Fissuring ulcers initially manifest as areas of breakdown in the mucosal lining (Fig. 5.4). These ulcers may extend in the submucosal space and cause undermining of the mucosal lining. Larger transmural ulcers are outlined by luminal contrast and appear as linear, high signal intensity protrusions into the bowel wall (Fig. 5.5) [5, 6, 33]. Residual mucosal islands between ulcerated mucosa may have a polypoid appearance (pseudopolyps) (Fig. 5.3). Linear ulcers are pathognomonic for CD [26]. They typically run parallel to the mesenteric border and may cause fibrosis and rigidity of the bowel leading to obstructive symptoms (Fig. 5.6). Confluent, intersecting longitudinal and transverse ulceration with residual mucosal islands leads to the formation of a “cobblestone” appearance. The presence of aphthous ulceration in combination with thickened intestinal folds has high specificity for CD [1].
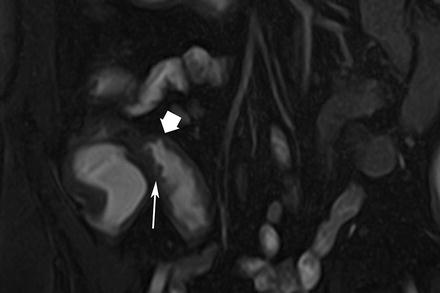
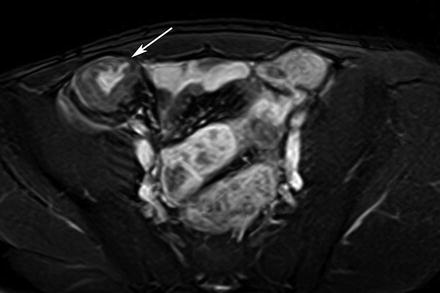
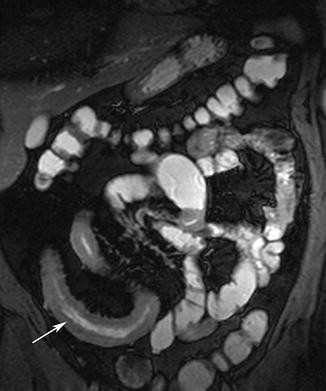

Fig. 5.4
Ulcers. Coronal true-FISP image shows thickened terminal ileum with fissuring ulcers causing break in the mucosal outline (arrow). Note deeper ulcer (thick arrow) outlined by enteral contrast. Hypointense submucosa of the descending colon in quiescent phase of CD (arrow)

Fig. 5.5
Ulcers. Axial true-FISP image multiple transmural ulcers as linear high signal tracks traversing the inflamed bowel wall (arrow)

Fig. 5.6
Linear ulcer. Coronal true-FISP image shows inflamed ileum with a linear ulcer visible as high signal track along the bowel lumen (arrow)
An ulceration pattern with edema within mucosal folds is more readily visible on MRE compared to CT, as generally MR imaging has higher tissue specificity than CT. T2-weighted sequences facilitate detection of ulcers due to high contrast obtained between luminal contrast and bowel wall. Abnormalities in the mucosal fold pattern can also be readily visualized on HASTE sequences against the background of the high signal from the luminal contrast.
Mural Thickening
Mural thickening is a significant feature of CD, although not entirely specific as several other disease entities can also cause bowel thickening. Mural thickness >3 mm should be considered to be abnormal and has been reported to have sensitivity and specificity ranges of 83–91 % and 86–100 %, respectively, in the detection of CD [1]. Good correlation between mural thickening and the CD activity index (CDAI) has been reported [34]. A recent comparative study of MRE and histopathology reported that using a mural thickness cutoff of >4.5 mm distinguished between severe and mild inflammation in CD [25].
Mural and Mesenteric Enhancement, Fibrofatty Proliferation
Engorged mesenteric vessels supplying inflamed bowel segments produce the “comb sign” on MRE examinations (Fig. 5.7) [35]. A secondary finding associated with bowel inflammation is “fat-wrapping” or “fat proliferation” around the inflamed bowel, which is a discriminating feature of CD [1, 36, 37]. Increased enhancement of the mesenteric fat around a bowel segment is a secondary sign of active bowel inflammation [38]. Active inflammation causes mucosal hyperemia that manifests as intense enhancement after intravenous contrast administration (Fig. 5.8). Increased mural enhancement has been reported to have good correlation with CDAI [25, 34, 39].
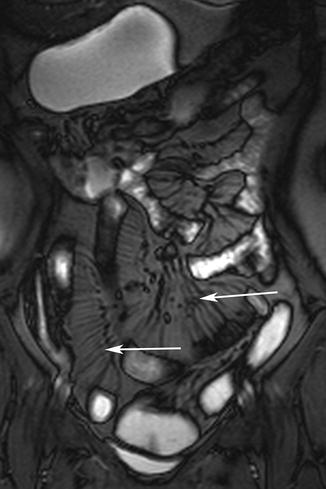
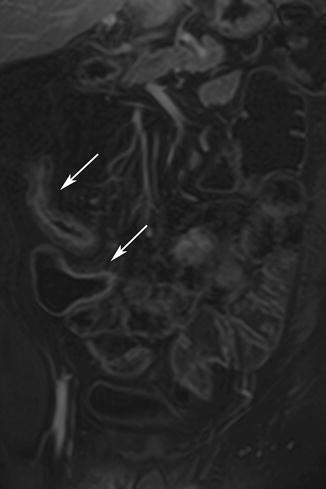

Fig. 5.7
Comb sign. Coronal true-FISP image shows engorged vessels as linear structures against background of mesenteric fat (arrows). Note extensive mesenteric fat proliferation surrounding inflamed bowel

Fig. 5.8
Enhancement. Axial 3D VIBE image shows marked enhancement of inflamed ileum and cecum (arrows)
Enhancement can be homogeneous (all bowel wall enhancing equally), layered (both mucosal and serosal bowel wall layers enhancing with a central band of relatively reduced enhancement), or irregular. A layered pattern of bowel enhancement has good correlation with active inflammation, although an overlap exists, and this pattern may also be seen in chronic or inactive disease [25, 40]. A similar appearance may also be produced by a low signal intensity “halo” produced by fat hypertrophy and fibrosis of the submucosa in chronic inflammatory bowel disease (Fig. 5.9). In these cases the submucosa has a dark, hypointense signal, especially on fat-suppressed sequences.
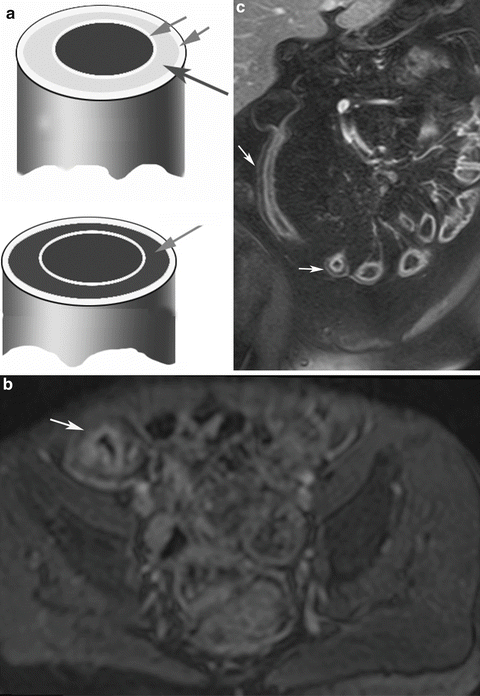

Fig. 5.9
Enhancement patterns. (a) Schematic diagram. (b) Layered enhancement shows enhancing serosa and mucosa and isointense submucosa (arrow). (c) Halo sign in chronic CD shows markedly hypointense submucosal layer between enhancing mucosa and serosa
Fibrotic strictures have been reported to demonstrate irregular or reduced mural enhancement [1]. This pattern of different enhancement has been attributed to differently expressed mediators in active and inactive CD [25]. MRE may be better suited to distinguish between a fibrotic stricture and one that is primarily due to acutely inflamed submucosa, as MR sequences can detect fibrotic change with greater facility than CT or ultrasound. Making the distinction between inflammatory strictures with spasms and the fat-halo sign of fibrotic CD is important, as obstruction and spasm in active disease are best treated with medical therapy, whereas chronic strictures may require surgical intervention.
Fistulating Disease
In advanced inflammation, deep ulcers penetrate the intestinal wall and cause inflammation in the adjacent mesenteric tissue leading to formation of small peri-intestinal abscesses and blind-ending sinus tracts. Once these tracts perforate through the wall of an adjacent hollow organ a fistula is formed (Fig. 5.10). Sinus tracts manifest as nodular irregularities and spiculations adjacent to the serosal surface of the bowel and they are the precursors of fistulating disease [41]. Small sinus tracts may be better seen on high-resolution MR images and high-quality multiplanar reconstruction (MPR) images are useful in their assessment [25, 26]. Larger sinus tracts and fistulae may be outlined by enteral contrast and are seen as linear tracts of high signal intensity on MRE; however, the majority of fistulous tracts do not contain air or fluid within their lumen [41]. Fistulas occur in up to one-third of patients with CD at some time during the course of their disease and the lifetime risk ranges from 20 to 40 % [41–43]. Internal fistulae are more common and enteroenteric fistulae are usually asymptomatic [44]. The most common location of fistulae is the perineal region (54 %). The reported sensitivity and specificity values of MRE for detection of internal fistulae range between 83.3–84.4 % and 100 %, respectively [30, 45, 46].
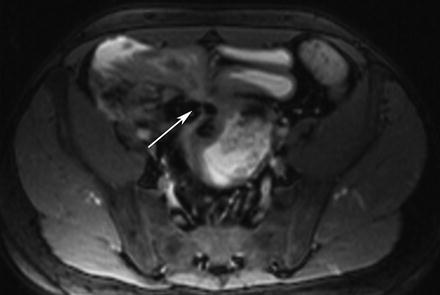

Fig. 5.10
Fistula. Axial true-FISP image shows multiple ileo-ileal fistulae arising from inflamed ileal segments (arrow)
MR imaging has also been reported to be highly accurate for detection and depiction of perineal fistula. This is due to the inherent higher tissue contrast resolution of MR imaging. It is difficult to accurately assess perianal fistula on CT as the attenuation value and appearance of fistula tracts, fibrosis, and sphincter muscles are similar to each other. Furthermore, compared with endorectal ultrasound, MR imaging provides a wider field of view and is better suited for assessment of complex branching tracts, lateral extension, and extension above the levator muscles.
Surgical intervention may be required if fistulae cause recurrent infection or if they lead to significant malabsorption [47]. Fistulae may have a stellate (“star”) appearance due to fibrotic and desmoplastic reaction in the mesentery around the inflamed fistulous tract (Fig. 5.11) [36]. Abdominal abscesses and inflammatory masses are less frequent than fistulae but are more likely to need intervention. Smaller abscesses may be treated with antibiotics or drained under imaging guidance, while larger ones may benefit from surgery.
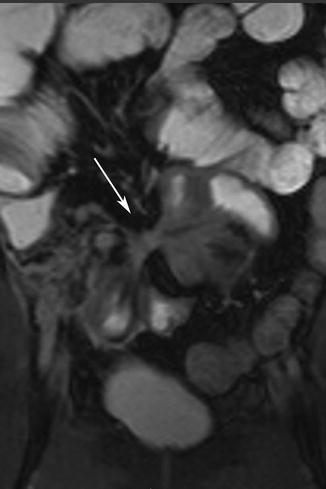

Fig. 5.11
Fistula. Coronal true-FISP image shows interloop fistula forming the star sign (arrow)
Fibrostenotic Disease
Fibrostenotic disease typically presents with bowel obstruction. Fibrostenotic strictures are seen as a fixed narrowed segment of bowel with proximal bowel dilatation (Fig. 5.12). Chronic fibrotic strictures are typically hypointense on both T1- and T2-weighted sequences. Fibrotic strictures may show minor, inhomogeneous contrast enhancement without any evidence of edema or surrounding mesenteric inflammation or hyperemia. MR fluoroscopy can be used in conjunction with MRE to provide functional assessment of bowel obstruction and strictures similar to those obtained on enteroclysis with less patient discomfort [31].
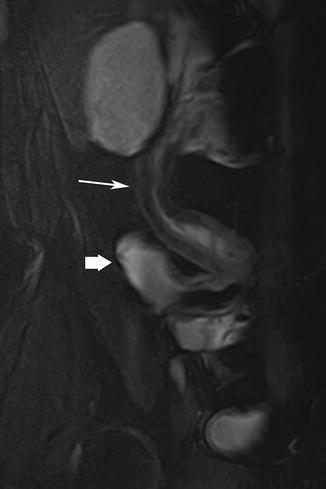

Fig. 5.12
Stricture. Coronal true-FISP image shows stricture in the distal ileum showing marked hypointensity in the submucosal suggestive of fibrosis (arrow). Thick arrow = distended proximal bowel segment
Negaard et al. reported that the sensitivity for detecting stenosis of the terminal ileum on MRE was 86 % versus 100 % for MR enteroclysis, although the higher diagnostic accuracy was not statistically significant [3]. This higher accuracy is likely to be due to the better luminal distension in MR enteroclysis. However, in routine practice, symptomatic stenoses are easily detected on MRE as non-distending bowel segments with proximal dilatation.
Cancer in CD
There is an increased incidence of adenocarcinoma in intestinal segments affected with CD [48]. The risk for developing colorectal cancer in those with Crohn’s colitis is between 4 and 20 times greater than in the general population [49, 50]. There is also an increased risk for developing cancer in excluded bowel segments [48]. Carcinoma in CD typically appears as a stricture, which may be difficult to differentiate from benign inflammatory strictures [50]. Some adverse features that may indicate underlying tumor are asymmetric mural thickening, shouldering, mesenteric infiltration, ascites, and lymphadenopathy (Fig. 5.13). Any bowel obstruction that does not resolve with conservative treatment and nasoenteric decompression should arouse the possibility of an underlying cancer. Imaging features that are out of keeping with clinical parameters should also raise concerns regarding an underlying tumor. Diffusion-weighted imaging may be a useful aide to differentiate between acute inflammatory strictures and underlying cancer [51].
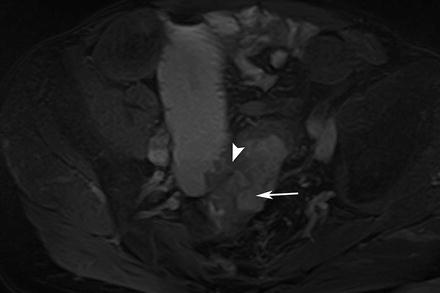

Fig. 5.13
Cancer in CD. Axial true-FISP image shows a thickened segment of distal ileum with fistulation into the sigmoid (arrowhead). The sigmoid thickening is eccentric with a shouldered appearance suggestive of a neoplastic growth (arrow). Adenocarcinoma was found at histology
MRE Findings in Other Small Intestinal Diseases and Disorders
Other clinical uses and roles for MRE are evolving and currently being investigated. There have been reports of the use of MRE in the evaluation of celiac disease, benign and malignant neoplasms arising in polyposis syndromes such as Peutz-Jeghers, and inflammatory conditions such as vasculitis, infectious processes, systemic sclerosis, and bowel obstruction [52, 53].
Tumors
High sensitivity and specificity have been reported in the detection of small-bowel neoplasms with MR enteroclysis on several studies [54, 55]. MRE has been reported to be a feasible alternative to capsule endoscopy for small-bowel surveillance in adults with Peutz-Jeghers and other familial polyposis syndromes [56, 57]. Benign tumors of the small intestine include leiomyomas, adenomas, lipomas, hemangiomas, inflammatory polyps, and hamartomas [58].
Benign tumors such as adenomas appear as well-defined sessile or pedunculated lesions that show homogeneous enhancement after intravenous contrast administration. Usually benign tumors, such as adenomas, protrude into the bowel lumen without causing obstruction and have a smooth border with no mesenteric infiltration (Fig. 5.14) [59].
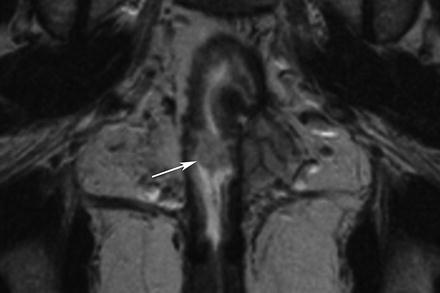

Fig. 5.14
Benign tumor. Coronal true-FISP image shows a small, well-defined polypoid lesion projecting into the bowel lumen of an ileo-anal pouch (arrow). This was a benign adenoma
Malignant lesions include gastrointestinal stromal tumors (GISTs), adenocarcinomas, carcinoid tumors, lymphoma, sarcomas, and metastases. GISTs in the small bowel most often originate from the muscular layer and commonly demonstrate an exophytic growth. GISTs have a strong association with neurofibromatosis type 1 [60, 61]. Tumors usually show brisk enhancement. GISTs may be intraluminal, submucosal, or subserosal in location and appear as smooth, well-defined masses (Fig. 5.15). After intravenous contrast administration, GISTs are typically enhancing masses with areas of low attenuation from hemorrhage, necrosis, or cyst formation [61].
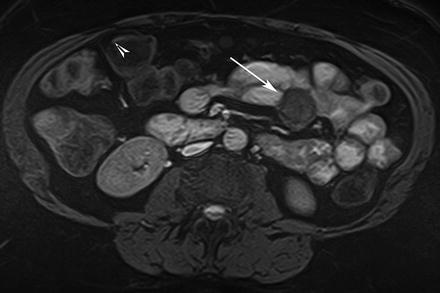

Fig. 5.15
GIST. Axial true-FISP image shows a rounded, well-defined tumor with extra-serosal growth (arrow)
Adenocarcinomas are the most common primary malignancies of the small bowel. They most often arise in the duodenum (50 %), followed by the jejunum (30 %) and ileum (20 %) [59]. Adenocarcinomas typically show eccentric involvement of a short segment of bowel, and often lead to partial or complete bowel obstruction (Fig. 5.16). MRE findings of adenocarcinomas include annular or eccentric mural thickening with adjacent infiltration and lymphadenopathy. Adenocarcinomas typically demonstrate moderate enhancement [62]. Lymph node enlargement in metastatic adenocarcinoma is not as marked as in lymphomatous disease. Distant metastases from adenocarcinomas to the liver and peritoneum may also be depicted on MRE examinations [36, 51–53].
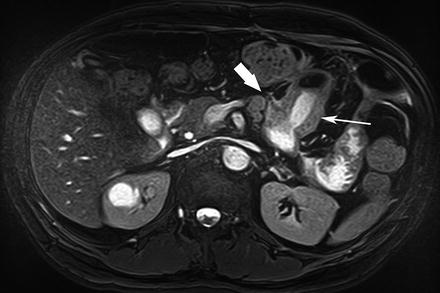

Fig. 5.16
Adenocarcinoma. Axial true-FISP image shows a concentric stricture in the jejunum (arrow) with lymphadenopathy and mesenteric invasion (thick arrow). Adenocarcinoma was found on histopathology
Most (50 %) carcinoid tumors occur in the appendix and about one-third (33 %) arise from the small bowel. One-tenth of patients develop carcinoid syndrome [54]. Carcinoids vary in appearance from small submucosal lesions to large ulcerating masses. These tumors often involve the adjacent mesentery, causing a desmoplastic reaction with in-drawing of adjacent bowel segments (Fig. 5.17). Carcinoid tumors are typically isointense to that in muscle on T1- and T2-weighted images, and sometimes exhibit radiating spicule-like strands of tissue.
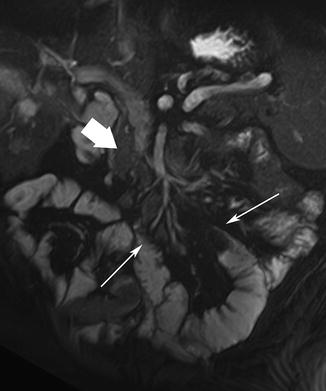

Fig. 5.17
Carcinoid tumor. Coronal true-FISP image shows an infiltrative mesenteric mass (thick arrow) causing in-drawing of surrounding bowel segments (arrows)
On MRE, small intestinal lymphoma usually appears as circumferential bowel wall thickening involving a long segment. Extensive adjacent lymphadenopathy, aneurysmal dilatation, and lack of obstruction despite a large tumor mass are suggestive of lymphoma as the primary diagnostic consideration (Fig. 5.18).
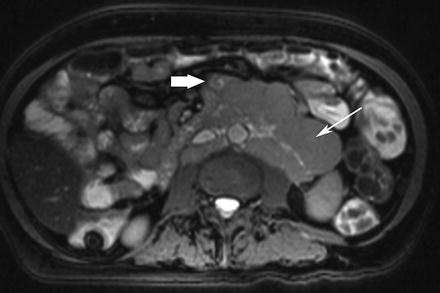

Fig. 5.18
Small-bowel lymphoma. Axial true-FISP image shows an infiltrative mass involving the small bowel (thick arrow). Note the extensive lymphadenopathy in the mesentery and retroperitoneal regions (arrow)
Obstruction
MRE can be used in the diagnostic work-up of suspected small bowel obstruction. The diagnosis of a mechanical small bowel obstruction is based on the visualization of an abrupt change in bowel caliber without evidence of another cause of obstruction at the transition point from the dilated segment to the collapsed segment of bowel.
Adhesions and adhesive bands are not usually associated with thickening of the small-bowel wall [63] (Fig. 5.19). Occasionally adhesive bands, seen as hypointense linear structures, may be seen coursing through mesenteric fat on T2-weighted images. Clumping or kinking of bowel loops also may be seen [64].
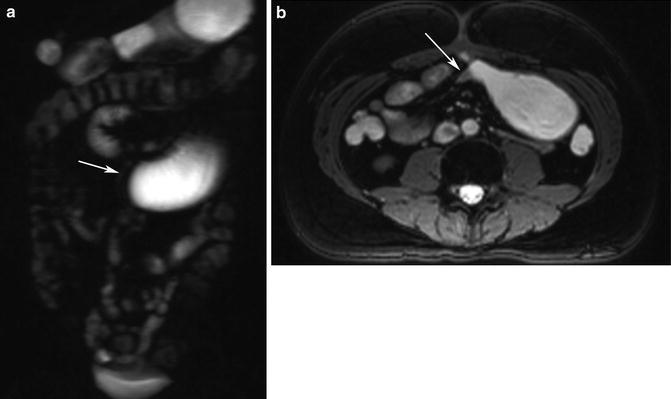

Fig. 5.19
Small-bowel obstruction. (a) HASTE image shows obstructed and dilated segment of proximal small intestine (arrow). (b) Axial true-FISP image shows the distended bowel loop and a kinked segment (arrow) causing mechanical obstruction. Note lack of inflammation or any other finding at the site of obstruction. Adhesive band was found at surgery
MR imaging has been shown to have accuracy for the detection and characterization of malignant versus benign strictures in the small bowel [65]. Investigators have used MR imaging to map adhesions preoperatively using a visceral slide technique with a sensitivity of 87.5 % and a specificity of 92.5 % [66].
Celiac Disease
Celiac disease predominantly involves the duodenum and proximal jejunum [67, 68]. MRE findings in celiac disease include inflammatory thickening of the bowel wall, lymphadenopathy, and mesenteric vascular engorgement. Complications of the disease may include intussusception and extensive ulcerative jejunoileitis with marked, circumferential thickening of the small bowel (Fig. 5.20).
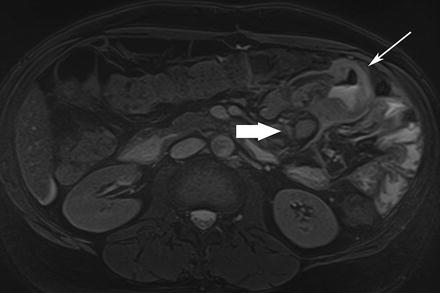

Fig. 5.20
Celiac disease. Axial true-FISP image shows inflamed segment of jejunum with large ulcers (arrow) and lymphadenopathy (thick arrow). Ulcerative jejunitis was found at surgery
Infective Diseases and Disorders
MRE is not routinely indicated for the diagnosis of small intestinal infections. Usually, patients present with nausea, vomiting, and/or diarrhea and CT or ultrasonography is carried out in the acute phase [69, 70]. Infectious ileitis may manifest on MR images as nonspecific, segmental, or circumferential wall thickening of the terminal ileum and cecum with moderate or marked enlargement of the mesenteric lymph nodes [36].
GI tract tuberculosis typically involves the ileocecal region with the cecum and ascending colon usually involved to a greater degree than the terminal ileum [69]. Tuberculosis causes asymmetric thickening of the ileocecal valve and medial wall of the cecum with contraction of the cecum [69]. Enlarged lymph nodes with central areas of necrosis are often seen. Peritoneal disease and ascites are often associated with ileocecal tuberculosis [71]. The most common type of peritoneal disease called “wet-type” manifests as large amounts of viscous ascitic fluid that shows a high signal due to its high protein and cellular content.
It may be difficult to differentiate small-bowel tuberculosis from CD. Differentiating between these entities is important as corticosteroids are used for treating CD that can provoke fulminant, catastrophic infection in patients with tuberculosis. Ulcers in tuberculosis tend to be axial (girdle) or oval. Linear, longitudinal ulcers of CD are not seen. Contraction of the cecum and a prominence of cecal involvement more than ileal involvement suggest tuberculosis, whereas the ileum is predominantly involved in CD. Ascites and necrotic lymph nodes are commonly seen in tuberculous infections, but are uncommon in CD. Fat proliferation of the mesentery around the affected bowel is indicative of CD rather than tuberculosis.
Recent Advances
High-Resolution MR Enterography (HR-MRE)
High-resolution MR enterography (HR-MRE) has recently been reported as a refinement to the standard MRE technique [26]. High-resolution true-FISP images with fat suppression are acquired using contiguous thin slices (2–3 mm), using 160–250 mm field of view and matrix sizes of 128–256 × 128–256 providing in-plane resolution of 1–2 mm and small field of view [26]. HRE-MRE images are obtained after aligning the MR imaging plane either parallel or perpendicular to the affected bowel segment. Images aligned parallel to the bowel segments allow better visualization of mucosal irregularities and abnormalities. Images aligned perpendicular to the bowel provide accurate visualization of transmural ulcers, fistulae, sinus tracts, and para-intestinal abnormalities (Figs. 5.21 and 5.22) [38]. The higher in-plane resolution achieved on HR-MRE has been reported to be the main factor that increases the diagnostic accuracy. HR-MRE images also allow high-quality MPR images that increase diagnostic confidence in the detection of smaller fistulae and ulceration. A comparative study has reported the sensitivities of MRE versus HR-MRE in the detection of superficial ulcers as (50 % versus 69 %) deep ulcers (69 % versus 94 %), fistulae (75 % versus 96 %), and abscesses (77 % versus 100 %), respectively [25].