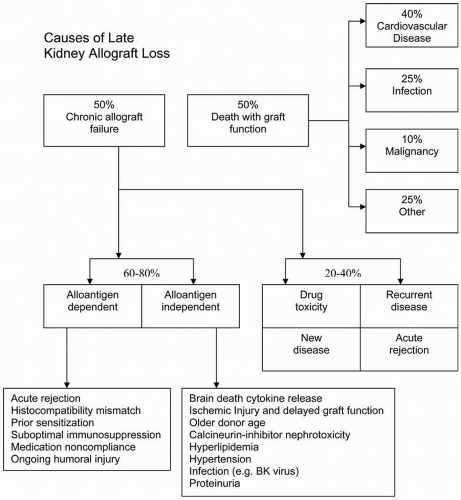
The previous chapter described the care of the patient during the first 3 post-transplantation months. By the end of that period, as patients commence the period of long-term management, the risk for surgical complications, acute rejection, and major infections is diminishing, although these issues continue to be of concern for the rest of the first year, and to a lesser extent for the life of the transplant. By the end of the third month, most patients have had their immunosuppressive doses reduced to the levels that will continue for many years, and the treatment of their hypertension, diabetes mellitus, hyperlipidemia, and other medical issues should be under stable control. Fewer patients now lose their allografts within the first year, and most centers report a 1-year graft survival rate of about 90% to 95%. There has been a gradual improvement in the time it takes for 50% of the grafts to fail, the graft half-life, and because of the thousands of transplantations, performed there are many patients whose grafts have been in place for more than 10 years, and indeed for more than 20 years. The half-life of two-haplotype living donor transplants has been estimated to be more than 20 years, and that of deceased donor grafts more than 11 years. Many of the factors that affect the longevity of the graft are determined by the features of the graft itself and by the early post-transplantation course. A major cause of graft loss is patient death (
Figure 10.1), predominantly from cardiovascular disease (CVD). To promote longevity of the graft, the intensive treatment of the medical complications from which transplant patients suffer, particularly those that increase the risk for CVD, are therefore as important as the long-term modification of immunosuppression.
The other major cause of graft loss is chronic allograft failure (CAF), the pathologic features of which are discussed in
Chapter 14. In that chapter, an argument is made to avoid the popular term
chronic allograft nephropathy because, as the graft fails, there is no specific nephropathy as such but rather features of many of the chronic processes that may impair its function (e.g., chronic rejection, chronic calcineurin inhibitor toxicity, hypertension or nephrosclerosis, chronic obstruction, viral infections, and recurrent diseases). The characteristic histologic features are interstitial fibrosis and tubular atrophy (IFTA).
Chronic rejection is another term that is often used loosely. The term does not adequately represent etiologic factors in allograft failure that can be considered both immune (alloantigen-dependent) and nonimmune (alloantigen-independent) factors (
Figure 10.1). The term
chronic allograft nephropathy has been removed from the most recent iteration of the Banff classification of renal transplant pathology.
This chapter is divided into two sections.
Part I describes the management of medical complications and considers strategies to improve patient and graft outcomes.
Part II describes the factors thought to cause CAF and strategies to reduce the rate of loss of kidney function. Inasmuch as CAF typically includes a component of vascular disease, those strategies that reduce CVD almost certainly are beneficial to the graft, as are control of blood pressure, hyperglycemia, and proteinuria.
Part II also describes other causes of late graft
loss from death after transplantation. Long-term immunosuppressive therapy and the immunosuppressive management of chronic allograft failure are discussed in
Chapter 5, Part V; post-transplantation infectious disease is discussed in
Chapter 11; post-transplantation liver disease is discussed in
Chapter 12; and medication nonadherence is discussed in
Chapter 20. Readers are also referred to the American Society of Transplantation’s
Guidelines on the Outpatient Surveillance of Renal Allograft Recipients (See Kasiske and colleague’s Selected Readings
Part II).
PART I: MANAGEMENT OF POST-TRANSPLANTATION MEDICAL COMPLICATIONS
Renal transplant recipients should be considered a subset of patients with chronic kidney disease (CKD). A small minority of these patients have normal kidney function, but the glomerular filtration rate (GFR) of the vast majority places them in one of the stages of CKD. Whereas there are factors particular to transplant recipients that may increase the risk for certain diseases and of their complications, in general, guidelines for the management of patients with CKD and those recommended for the general population are applicable to the
management of these patients. A continuous intensive and coordinated approach to the chronic conditions from which they suffer is an important part of their care. The transplantation center, the community physicians, and the patient are all part of the team that has to work to obtain optimal health for these patients and their transplants.
The great disparity between the demand for organs and the supply means that fewer patients than in the past will have successive transplants, and after the first transplant fails, most will return to dialysis for a considerable period of time, if not for the rest of their lives. Both physicians and their patients need to understand this. It should make the prevention of risk factors and adherence to the medication schedule a priority for all these stakeholders. The treatment of chronic conditions can be frustrating and arduous, but the rewards obtained from realizing the benefits of persistence are great. We know from studies in the general population (e.g., in the treatment of hypertension, an established risk factor for stroke and CVD) that even when the evidence is overwhelming that treatment is beneficial, a minority of patients at risk are treated, and a minority of these achieve the targets set in management guidelines. Transplant patients, however, are already well connected to a system that should provide their care, and this should enable more successful prevention strategies. We know the major causes of morbidity and mortality in the late transplant period and in some cases have evidence to suggest effective measures to prevent post-transplantation complications. When evidence is not available from transplant studies, the data from the CKD and general population should be used.
The success of treatment of chronic conditions is enhanced by frequent contact with the patient’s physicians. The intensity of care provided to transplant recipients should be tailored to their needs, but in general, it is recommended that after a gradual reduction in the frequency of visits from 2 times per month during the fourth month to 1 time per month at 6 months, this monthly scheduled should be maintained until the end of the first year. For the next year, the visits should be every 1 to 2 months and thereafter every 3 to 4 months as long as the transplant is functioning. Follow-up can occur at the clinic of the transplantation center, with the community nephrologist, or with a community internist or family practitioner with experience in the care of transplant recipients. There should be frequent and open communication between the community physicians and the transplantation center. The transplantation center should remain a source of care and expertise.
The following strategies address the most important management issues in the late transplantation period.
STRATEGY 1: REDUCE IMMUNOSUPPRESSION WHENEVER POSSIBLE
Death is a common cause of renal allograft failure in the late post-transplantation period. The ultimate goal is to have all our patients die with a functioning graft, but not prematurely, as is now too often the case. CVD, cancer, and infection are the leading causes of death in the late post-transplantation period, and immunosuppression plays a major role in the pathogenesis of each of these complications. Each immunosuppressive agent has both immune and nonimmune toxicity. Immune toxicity is usually nonspecific; that is, immune toxicity is the result of the total amount of all immunosuppression over a given period of time. Immune toxicity can only be avoided if patients became tolerant to their transplanted kidney. Unfortunately, most patients will reject their kidney if immunosuppression is completely withdrawn, and the best we can do is select the minimal amount of immunosuppression that prevents rejection. This minimal amount should ideally be tailored to the needs of specific patients, but we are able to do that only in a crude way.
The principal obstacle to reducing the overall amount of immunosuppression is acute rejection. A number of risk factors for acute rejection have been identified (
Table 10.1). These risk factors can be taken into account when determining the amount of immunosuppression that may be appropriate for individual patients in the late post-transplantation period. In general, outcomes are better for living donor kidneys. This is especially true for two-haplotypematched living related transplants. Even here one should be careful to provide adequate immunosuppression, and a protocol that uses less exposure to calcineurin inhibitor (CNI) and is more dependent on antiproliferative agents may reduce the long-term risk for CNI toxicity (See
Chapter 5, Part IV). For deceased donor kidney recipients, the number of major histocompatibility mismatches is associated with the rate of late allograft failure (see
Chapter 3,
Figure 3.3). In particular, patients with zero mismatches are at significantly lower risk for late graft failure when compared with patients with as few as one mismatch, and the number of HLA-DR mismatches may predict the incidence of acute rejection after elective withdrawal of a CNI. Patients who have had more than one previous transplant have a higher risk for graft failure. In addition, the chances of such a patient receiving yet another kidney are reduced, making the risk association with reducing immunosuppression higher. Patients younger than 18 years of age have a higher incidence of acute rejection and need more immunosuppression than do patients 30 to 50 years of age (see
Chapter 16). On the other hand, elderly patients are more likely to die of complications of immunosuppression than to lose their kidneys to acute rejection, and many transplantation centers attempt to use less immunosuppression in elderly transplant recipients (see
Chapter 5). This reduction in immunosuppression should not be too radical. African American patients are at increased risk for late allograft failure. The reasons for this are probably multiple but include possible differences in immunoreactivity to the graft and poor bioavailability of CNIs. Acute rejection is a strong predictor of outcome, particularly from CAF. However, not all acute rejections lead to graft failure. Characteristics of acute rejection that correlate to increased risk for graft failure and, by inference, increased needs for long-term immunosuppression include late rejections, severe rejections, and multiple acute rejections. All of the above factors can be used to judge the amount of immunosuppression that may be best for individual patients in the late post-transplantation period.
A number of randomized controlled trials have studied the feasibility of electively withdrawing individual immunosuppressive agents in the late post-transplantation period. Withdrawal of both prednisone and cyclosporine has
been studied extensively, yet total withdrawal of these agents remains controversial (see
Chapter 5, Part IV). Elective cyclosporine withdrawal is associated with about a 10% risk for acute rejection in the months following withdrawal. Most of these rejection episodes can be successfully treated and reversed. Despite this increased risk for rejection, controlled trials with long-term follo-wup have been unable to demonstrate an increased risk for graft failure after cyclosporine withdrawal. In contrast, acute rejection after prednisone withdrawal appears to increase the risk for late allograft failure in randomized controlled trials. Additional studies are warranted to define better circumstances under which immunosuppression withdrawal is advisable. For most patients, low-dose maintenance therapy is safer and less anxiety provoking than total withdrawal of individual agents. Total withdrawal of immunosuppression is not an acceptable or permissible option except in the circumstances of highly experimental tolerance-inducing protocols discussed in
Chapters 2 and
5, or in the face of life-threatening infection or malignancy.
In addition to deciding on the minimum amount of immunosuppression needed to prevent acute rejection, physicians and patients must also choose among the most effective, but least toxic, of several different agents. In general, it is prudent to tailor the choice of agents to the risk profile or adverse effects that are most troubling to the individual (
Table 10.2). For patients who have severe hyperlipidemia, especially those who are at high risk for CVD, it may be wise to minimize the use of cyclosporine, prednisone, and sirolimus. Each of these drugs causes hyperlipidemia. Switching a patient from cyclosporine to tacrolimus, for example, may reduce low-density lipoprotein cholesterol by the same amount as therapy with an HMG-CoA reductase inhibitor. Similarly, reducing cyclosporine or prednisone dose may help to control blood pressure. Patients with severe tremor will be especially eager to reduce or withdraw tacrolimus or cyclosporine in the late post-transplantation period if this is possible. A significant number of patients receiving cyclosporine develop gum overgrowth. This is made worse by poor dental hygiene and by the concomitant use of calcium antagonists. This reverses if patients are switched to tacrolimus. Similarly, patients with difficult-to-control diabetes may be good candidates for minimizing doses of prednisone. New-onset diabetes in a patient receiving tacrolimus may respond to switching to cyclosporine. Bone marrow suppression may be an indication for reducing doses of azathioprine, mycophenolic acid, or sirolimus. Patients with marginal renal function may sometimes delay starting dialysis by
decreasing or stopping calcineurin inhibitors. Patients with severe liver disease may benefit from lowering or discontinuing azathioprine. Patients with cosmetic complications may choose to switch calcineurin inhibitors. Allopurinol can dramatically increase blood levels of azathioprine; hence, azathioprine may need to be reduced or discontinued for patients with gout and switched to mycophenolic acid. Tacrolimus may be the better CNI for patients with gout. Finally, many patients cannot afford to pay the high cost of immunosuppression. The use of expensive medications for patients who cannot afford them increases the risk for nonadherence and graft failure. Prednisone and azathioprine are a fraction of the cost of newer immunosuppressive agents and yet may provide adequate immunosuppression for many patients (see
Chapter 5).
STRATEGY 2: ADOPT STRATEGIES TO PREVENT NONADHERENCE
There are few randomized, controlled trials to suggest how to prevent nonadherence with immunosuppressive medications. On the other hand, a number of observational studies have demonstrated that nonadherence is an important, preventable cause of allograft failure. These same studies have provided clues to preventive measures that are most likely to be effective.
▪ Minimize the number of daily doses of medication, and whenever possible, use medications that can be dosed once daily.
▪ Educate patients. In particular, dispel the common misconception that the immunosuppressive effects of medications extend beyond the dosing interval. Patients need to be reminded at every follow-up visit that failure to take medications regularly will eventually result in graft failure.
▪ Educate and update physicians and medical staff regarding immunosuppressive protocols and individual regimens and the potential for drug interactions (see
Chapter 5).
▪ Help patients to establish a system to remind them to take their medications. Enlist the help of friends, family, and public health aides. Use egg-carton-style pull containers or other mnemonic devices.
▪ Maintain close contact with patients throughout the late post-transplantation period. Insist that patients have routine follow-up with the transplantation center and make every effort to locate patients who are lost to follow-up. Clinic visits and laboratory checks are a valuable reminder to patients of the importance of taking medications. When negotiating contracts with providers, insist that patients be allowed to follow up with the transplantation center at regular intervals.
▪ Know whether your patients have trouble paying for their medications. If this is the case, assign someone to help them. Most transplantation programs have found that it is often necessary to have a dedicated social worker or pharmacist available to help patients (see
Chapter 20). Be prepared to offer less-expensive alternatives (see
Chapter 5).
▪ Identify patients who are at high risk for nonadherence. Adolescent patients are at increased risk, often because they are fearful of the cosmetic effects of prednisone and cyclosporine. Patients who are poorly educated are also at increased risk for nonadherence. Similarly, low family income is associated with nonadherence. Socioeconomic factors place members of racial minorities at increased risk for nonadherence. Studies show that patients who were nonadherent with medication, diet, and dialysis therapy before transplantation are more likely to be nonadherent after renal transplantation.
▪ Patients who are at high risk for nonadherence should be targeted with risk factor intervention in much the same way that we target patients
who are at high risk for CVD with intensive risk factor management. In both instances, the benefit is likely to be the greatest risk when the risk is the highest.
STRATEGY 3: MONITOR RENAL FUNCTION CLOSELY
Frequent monitoring of renal function in the late post-transplantation period helps to enforce adherence with immunosuppressive medications and provides the only reliable means to detect acute rejection at a time when it may still respond to treatment. A program requiring patients to make certain that serum creatinine is measured regularly and reported to the transplantation center also provides an indirect means for the center to monitor compliance. Patients should also keep a record of their own creatinine values and thereby learn to self-monitor for significant change. Patients who fail to have their serum creatinine level checked regularly should be contacted and reminded of the importance of close, ongoing follow-up to prevent graft failure. Patients and caregivers should be constantly reminded that acute rejection rarely presents with signs and symptoms in the late post-transplantation period. Although immune monitoring holds promise as a more sophisticated way of recognizing acute rejection before it manifests clinically, the serum creatinine level is currently the only practical tool that can be used to screen for acute rejection in the late post-transplantation period. It is not too much to ask patients to have their serum creatinine level measured regularly in the late post-transplantation period. Measurement of cystatin C may provide a more accurate estimate of GFR in transplant patients than creatinine-based estimations. Cystatin C measurements have yet to be used clinically in a widespread fashion.
At least once a year, and preferably more often, urine should be checked for protein excretion. Persistent proteinuria (i.e., more than 1 g in 24 hours for at least 6 months) is associated with an increased risk for graft failure. Proteinuria can be most reliably detected by either a timed urine collection (which is cumbersome) or a protein-to-creatinine ratio measured in a random “spot” urine sample (which is convenient). Dipstick screening is less reliable because the protein concentration is also dependent on the state of diuresis. There are two reasons why it is important to detect proteinuria.
1. Reducing high levels of proteinuria with an angiotensin-converting enzyme inhibitor (ACEI) or an adrenergic receptor blocker (ARB) may help to reduce levels of serum cholesterol and alleviate coagulation and other metabolic abnormalities associated with nephrotic-range proteinuria.
2. There is growing circumstantial evidence that proteinuria is injurious to the kidney and contributes to the progression of CAF.
STRATEGY 4: MAKE AN ACCURATE PATHOLOGIC DIAGNOSIS OF THE CAUSE OF GRAFT DYSFUNCTION
It is important to establish an accurate pathologic diagnosis in patients with deteriorating graft function. There is evidence to suggest that even low-grade tubulitis, or so-called borderline acute rejection, may increase the risk for CAF (see
Chapter 14). The evidence supporting the use of routine protocol biopsies, however, is not strong, and most programs do not perform them unless the patient is engaged in a research protocol. An increased serum creatinine level remains the prompt for biopsy and treatment. However, the message is clear. It is important to have a high level of suspicion for acute rejection and a low threshold for obtaining a renal allograft biopsy. An acute, sustained rise in serum creatinine should prompt immediate evaluation. The strategy of routinely
monitoring serum creatinine levels will only be successful if biopsies are obtained quickly and acute rejection is treated. Such a strategy will also avoid unnecessary intensification of immunosuppression when rejection is not present. Unexpected diagnoses, such as recurrent disease, calcineurin inhibitor toxicity, polyomavirus infection, and post-transplantation lymphoma, may require radically different therapeutic approaches. If a cause of CAF is established, repeated biopsies may be unnecessary because repeated treatment may be unwise (see
Chapter 5).
STRATEGY 5: TREAT HYPERLIPIDEMIA AGGRESSIVELY
Hyperlipidemia is common after renal transplantation. Elevations in total cholesterol are almost invariably accompanied by elevations in low-density lipoprotein (LDL) cholesterol. Triglycerides are also frequently elevated. Several studies have found correlations between hyperlipidemia and CVD after renal transplantation. Studies in the general population provide incontrovertible evidence that treatment of elevated LDL reduces the risk for ischemic heart disease events and decreases mortality. Transplant recipients with LDL cholesterol levels higher than 130 mg/dL should be considered for pharmacologic treatment, especially if they have preexisting CVD, diabetes, or other risk factors.
Tables 10.3 and
10.4 list recommendations for the primary and secondary prevention of CHD. Recognition of patients with the metabolic syndrome is important early after transplantation so that patients can be targeted for lifestyle modifications and drug therapy.
Reduction of the urine protein excretion with an ACEI or ARB may help to reduce lipid levels for patients with nephrotic-range proteinuria. Reduction or discontinuation of cyclosporine, sirolimus, or prednisone may also help lower lipid levels. Diet is effective in reducing cholesterol and LDL, but the effect is usually modest. A number of studies have shown that HMG-CoA reductase inhibitors are safe and effective in lowering LDL cholesterol after renal transplantation. In the ALERT (Assessment of Lescol in Renal Transplantation; see “
Selected Readings”) trial, fluvastatin lowered LDL by 32%, and
although there was no significant reduction in the rate of coronary intervention or mortality, the incidence of cardiac deaths and nonfatal myocardial infarction appeared to be reduced. About half of all kidney transplant patients receive these drugs. Plasma levels of HMG-CoA reductase inhibitors are increased in cyclosporine-treated renal transplant recipients, and it is generally prudent to use about half the usually prescribed dose. Patients who still have high LDL cholesterol levels may be candidates for combination therapy. Low-dose bile acid sequestrants can be combined with an HMG-CoA reductase inhibitor. Bile acid sequestrants should probably not be taken at the same time as cyclosporine and should not be used in patients with very high triglyceride levels. Fibric acid analogues, such as gemfibrozil, can also be used in combination with HMG-CoA reductase inhibitors. Some fibric acid analogues (but not gemfibrozil), however, are reported to increase the serum creatinine level. Combination therapy should be used with caution because it increases the risk for myositis and rhabdomyolysis.
STRATEGY 6: TREAT HYPERTENSION AGGRESSIVELY
Hypertension occurs in 60% to 80% of renal transplant recipients. It is associated with an increased risk for graft failure. Studies in the general population show that treatment with antihypertensive agents reduces the risk for CVD. There is good reason to believe that treating blood pressure elevations would be beneficial in renal transplant recipients.
All classes of antihypertensive agents can be used to lower blood pressure in renal transplant recipients. Although there are limited data on the effects of reduced dietary sodium chloride intake on blood pressure in renal transplant recipients, this is a reasonable first step. A low dose of thiazide diuretic is also reasonable for patients with creatinine clearance estimated to be greater than 25 to 30 mL per minute. Low doses of thiazides (e.g., 12.5 to 25 mg per day) are effective and do not generally perturb lipid or glucose metabolism. Both a lowsalt diet and thiazide diuretics may help with edema, which is a common problem after transplantation. A thiazide diuretic may also help in the management of the hyperkalemia that is common in CNI-treated transplant recipients. Transplant recipients may be sensitive to volume contraction; therefore, diuretics may cause a reversible increase in serum creatinine levels. Thiazides often potentiate the antihypertensive effects of other agents, especially ACEIs. Thiazides are inexpensive. β Blockers are also relatively inexpensive and are especially attractive for patients with ischemic heart disease, which is common after renal transplantation. Relative contraindications to β blockers (e.g., peripheral vascular disease, reactive airways disease, and hypoglycemic reactions) are rarely a reason to forego the use of this important class of medication.
Physicians are sometimes reluctant to use ACEIs and angiotensin II antagonists in transplant patients for fear of inducing hemodynamic impairment of allograft function. Several studies, however, show that these drugs are generally safe, effective, and well tolerated. They may reduce proteinuria and stabilize the deterioration in renal function in chronic allograft failure, possibly reducing the production of transforming growth factor-β (TGF-β). They may also have additional benefit in reducing the incidence of cardiovascular events in high-risk patients, and may also reduce the degree of insulin resistance. Occasionally, ACEIs may increase serum creatinine, but this is usually a transient and reversible effect. Hyperkalemia can often be managed by adding a thiazide diuretic or a loop diuretic to the treatment regimen. ACEIs may cause anemia in transplant recipients; this side effect can be exploited for the treatment of post-transplantation erythrocytosis. Cough occurs in about 15% of patients taking ACEIs but is much less frequent with ARBs. Otherwise, ARBs appear to have all of the advantages and disadvantages of ACEIs.
Calcium antagonists are also effective in renal transplant recipients. They can contribute to edema, which is already prevalent among transplant patients. Calcium antagonists appear to improve the preglomerular, arterial vasoconstriction that mediates cyclosporine-induced declines in renal blood flow. They may help to alleviate the propensity of the CNIs to exacerbate delayed graft function immediately after deceased donor transplantation. Nondihydropyridine calcium antagonists (e.g., diltiazem and verapamil) increase calcineurin inhibitor blood levels and can be used to help reduce the immunosuppressive drug cost. Dihydropyridine calcium antagonists have less effect on blood levels (see
Chapter 5, Part I). Calcium antagonists may cause gum overgrowth, particularly when used with cyclosporine. Vasodilators and α blockers are also effective in treating hypertension, although they can cause reflex tachycardia and may need to be used in combination with β blockers. Excess hair growth with minoxidil, the most potent vasodilator, limits its long-term usefulness in women. Other agents that are useful include sympatholytics, central and peripheral α antagonists, and combined α and β blockers. Readers are referred to the seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of hypertension (see “
Selected Readings”). Most patients require combination therapy. Some require several agents.
When hypertension cannot be controlled, particularly if attempts to reduce blood pressure result in decreased graft function, the possibility of renal
allograft artery stenosis should be considered (see
Chapter 8). In addition, the presence of diuretic-resistant peripheral edema, a loud allograft bruit, renal dysfunction after administration of ACEIs or ARBs, and polycythemia should engender consideration of this diagnosis. Color-flow Doppler examination of the renal artery may aid the diagnosis, but interpretation of this test is difficult, and false-positive results are common. Radionuclide scanning is usually not helpful. Magnetic resonance angiography or renal arteriography should be used for diagnosis when suspicion of renal allograft artery stenosis is high, paying attention to the risk for iodine-containing dyes worsening renal function, and that of gadolinium causing nephrogenic fibrosing dermatitis in patients with a reduced GFR (see
Chapter 13). The studies to exclude renal artery stenosis should also include studies of the proximal iliac artery because stenosis of this is not uncommon, and the effects may mimic those of renal artery stenosis. Percutaneous transluminal angioplasty may improve renal function and reduce the need for antihypertensive medications in 60% to 85% of cases. Restenosis may occur in up to 30%. Surgery should probably be reserved for critical stenosis that threatens the integrity of the graft.
The native kidneys often contribute to hypertension after renal transplantation. Studies to determine the role of the native kidneys in causing hypertension, however, are probably not useful. In particular, renal vein renins do not reliably predict blood pressure reduction after native kidney nephrectomy. Therefore, in difficult-to-control hypertension, consideration should be given to empirical removal of the native kidneys. Laparoscopic surgery may reduce the morbidity of post-transplantation native kidney nephrectomy.