High risk
Low risk
Poor differentiation
Well and moderate differentiation
Size ≥3 cm
Size <3 cm
Circumferential involvement ≥30 % of lumen
Circumferential involvement <30 % of lumen
Presence of lymphovascular invasion
Presence of perineural invasion
Margins <2 mm
pT2
However, sometimes these features are only confirmed after review of the pathological specimen following a local excision. In this case, additional treatment should be considered. One option includes completion of total mesorectal excision or salvage resection in order to provide radical lymphadenectomy. Alternatively, adjuvant radiation or chemoradiation may be used for the management of these patients [17].
Techniques of Local Excision
Preoperative Preparation
Patients are admitted at the same day or day before of the procedure after full bowel preparation. Antibiotic prophylaxis is performed at the time of anesthetic induction. The procedure is performed under general or regional anesthesia. However, in patients undergoing transanal endoscopic techniques (TEM or TAMIS) for upper lesions with higher risk of peritoneal entry, general anesthesia is preferable. Positioning of the patient is determined by primary tumor location: the lesion should preferably be located downwards. Therefore, jack-knife prone for anterior or lithotomy for posteriorly located lesions. In transanal endoscopic techniques, the lateral position should be considered in tumors located at the right or left rectal walls.
Traditional or Standard Transanal Local Excision
An anal retractor is used to dilate the anus and obtain an adequate exposure. A lone-star retractor, may provide excellent access to the lower rectum for this purpose. Selectively, traction sutures may be placed laterally to the lesion to enhance exposure. A line of dissection with a margin of 1 cm is made with electrocautery circumferentially. The depth of resection should always reach the mesorectal fat to provide a maximal radial margin (Figs. 9.1 and 9.2). The specimen should always be fixed to a cardboard for better assessment by the pathologist (Fig. 9.3). The defect in the rectal wall is then closed transversely in a running suture, preferably with an absorbable material (PDS® or caprofyl®).
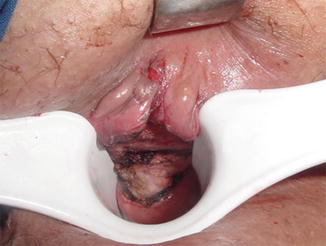
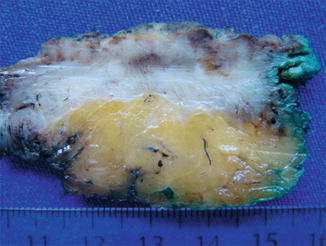
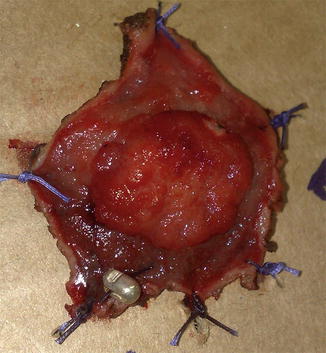

Fig. 9.1
Standard local excision: the mesorectal fat can be seen after the excision of the surgical specimen

Fig. 9.2
After fixation, a significative amount of the perirectal fat can be seen providing an appropriate radial margin

Fig. 9.3
The surgical specimen should be fixed to a surface in order to provide orientation for the pathologist
Minimally Invasive Options
Two relatively new techniques have been introduced in order to approach rectal tumors using the transanal approach with the use of rectal CO2 distention (pneumorectum), endoscopic view and minimally invasive instrumentation [18, 19]. These techniques may provide improved surgical field view and access to lesions in the middle and upper rectum. Implementation of these endoscopic microsurgical approaches has resulted in significant improvements in quality of the resected specimen. In a review of 171 patients undergoing transanal endoscopic or standard local excision, margin clearance, specimen fragmentation, and local recurrence were all consistently higher among the patients operated by the traditional approach. Considering that the postoperative morbidity between the approaches is similar, the authors concluded that transanal endoscopic surgery is the procedure of choice for the local excision of rectal masses [20, 21]. Finally, this approach provides proper access to safe resection of upper rectal lesions and closure of peritoneal defects created by full-thickness excision in the anterior wall, particularly in males or post-histerectomy females.
Transanal Endoscopy Microsurgery (TEM)
The procedure is performed using a special proctoscope of 4 cm in diameter available in lengths of 12 and 20 cm. The rectum is insufflated with carbon dioxide at 10–15 mmHg. This can be achieved with the use of specific or usual laparoscopic CO2 insufflators. The optical 6-fold increase and the stability provided by the equipment, attached to the operating table, allows for an excellent view of the rectum and lesion. The proctoscope is frequently repositioned to allow best visualization of the lesion during the procedure. Once setup is complete, special endoscopic instruments are introduced through the proctoscope (usually four ports for entry) and resection is performed. In addition to the scope and two instruments manipulated by the surgeon, suction may be used through the fourth portal entry for aspiration of the smoke created by cautery (Fig. 9.4). Marking of 1 cm circumferential margins around the primary lesion prior to resection is advised to avoid disorientation. Full-thickness resection is performed using electrocautery avoiding direct manipulation of the tumor. Alternative energy sources may be used for this resection including harmonic or sealing devices. Once the specimen is removed, final check for hemostasia is performed and bleeds are carefully dealt with. In most cases, attempt to close the rectal defect is done with the use of an absorbable running suture.
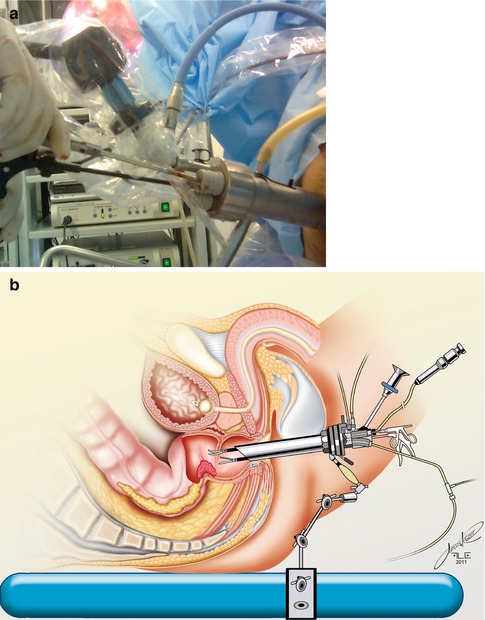

Fig. 9.4
(a) TEO/TEM equipment, with camera and insufflation in place. (b) The position of TEO/TEM equipment fixed to the surgical table and with instruments inserted (From Kosinski et al. [76])
The use of specific TEM equipments requires a significant investment and cost-effectiveness becomes a relevant issue. In a recent retrospective case-control study, patients undergoing TEM were compared to standard rectal resection [22]. Even though the initial investment was significantly higher for TEM, decreased costs related to disposable instruments, hospital stay and stoma takedown clearly compensated differences. Ultimately, TEM resulted in a less expensive approach for the management of rectal lesions when compared to standard surgical resection despite the need for equipment purchase. In that study, the authors suggested that savings with TEM would provide compensation of the initial investment after 11–12 cases.
TAMIS (Transanal Minimally Invasive Surgery)
More recently, a variation of the previous technique has been proposed to allow transanal endoscopic microsurgery with the use of standard laparoscopic equipment [19]. This would potentially avoid the need of considerably expensive and exclusively dedicated TEM equipment. Instead, the use of simple and readily available laparoscopic equipment would allow similar efficacy with considerably lower associated costs. Also, learning curve of the procedure could be minimized by the use of routinely used as opposed to specific TEM instruments.
Several transanal ports have been suggested for this approach including disposable or reusable single-ports. After connection with the regular laparoscopic insufflator, a 5 mm laparoscopic scope is inserted to provide endorectal view. In contrast to TEM, TAMIS requires an assistant to control camera and therefore, stability of the image is lost. Once the additional instruments are inserted, the surgeon may perform the procedure very similar to the TEM technique. However, most single ports have only 3 portal entries and therefore, smoke aspiration is not continuous. Finally, access to the lower rectum may be difficult due to significant need for instrument angulation. On the other hand, access to the upper rectum may be limited by rectal folds in some patients. Middle rectal lesions are best suited for this technique.
Transcoccygeal Excision
The posterior approach via trans sacral popularized by Kraske, was especially useful for lesions on the posterior wall within the middle or distal thirds of the rectum [23]. However, it also allows resection of lateral and anterior lesions. The advantage of this approach is that it provides exposure of the mesorectum. Therefore, perirectal nodes could potentially be removed for histopathological examination.
The patient should always be placed in jack-knife prone position. The coccyx is tackled by a longitudinal incision from the perineum to the second or third sacral vertebra. Gluteal muscle insertions are released and the anococcygeal ligament is transected. Removal of the coccix is performed after complete exposure. At this point, the middle sacral artery should be ligated. The rectum is approached through the perirectal fat, and through the levator ani, separated at midline. This provides a complete mobilization of the rectum within the intraperitoneal pelvis. For posterior lesions, it is useful to use the digital rectal examination to guide the resection. This gives the orientation of the lower edge of the tumor in order to achieve 1 cm circumferential margin. For anterior lesions, posterior incision of the rectum is required, allowing resection under direct vision of the primary tumor. All the defects in the rectal wall are closed transversely in order to prevent stenosis, using running absorbable suture. Finally the levator ani are approximated at the midline and the anococcygeal ligament is reattached to the sacrum. The subcutaneous tissue and the skin are closed. Morbidity rates for this procedure are higher than for transanal excision approaches. Development of rectocutaneous fistulae ranges from 15 to 25 %, and sometimes a temporary diverting stoma is required. Other complications include urinary dysfunction, wound infection and transient fecal incontinence. In this setting, transsacral approach is being progressively less used. However, the procedure remains a viable option particularly to patients that are not amenable to a transanal approach [24].
Morbidity and Mortality
Mortality after local excision is very low, with most studies showing no mortality and others up to 2–3 % [25].
Overall morbidity has been reported to range from 9 to 45 %. Major complications are uncommon and occur in around 1.5 % of cases. Bleeding is the most common major complication, eventually requiring reintervention. Infectious complications may rarely require a diverting stoma in around 1 % of cases [26].
The single most relevant risk factor for postoperative complication is the use of neoadjuvant CRT. When preoperative CRT is delivered, TEM resection leads to a rectal wound that allows primary suturing without any technical difficulty, unless the distal margin is very close to the anal canal/verge. In this situation, even though the upper border of the wound may retain its considerable elasticity, the lower border of the wound of the anal canal is rather fixed and with little mobility. If the resection is wide enough to result in significant separation of the proximal and distal borders, significant tension will be present, a known feature to contribute for wound dehiscence. Also, the anal canal has ectodermic as opposed to endodermic nerve supply to rectum. Therefore, wound separation and mucosal discontinuity in this region may be quite painful. Finally, regardless of the level of suturing (rectal or anal canal), the borders to be sutured after a TEM resection in previously irradiated rectum will necessarily put together two previously irradiated borders. This is actually quite different from a coloanal anastomosis following neoadjuavant CRT, where the proximal colon is never included in the radiation field and therefore a NORMAL colon is sutured to an abnormal anal canal previously treated with a significant amount of RT [27]. In fact, even after a coloanal anastomosis is constructed, the risk of dehiscence is so significant that a loop ileostomy is almost always recommended [28]. One can imagine the risk of wound dehiscence after suturing together two previously irradiated borders of rectum or anus, sometimes with significant tension depending on the level of the suture.
In fact, few studies compared the risk of wound separation and its consequences with or without previous exposure to CRT. However, retrospective studies have suggested that the risk of wound dehiscence was significantly higher when CRT was delivered preoperatively. In one of these studies, diagnosis of wound dehiscence was made after more than 1 week following TEM and healing of the dehiscence took an average of more than 8 weeks to complete. An operation that otherwise would almost never require a stoma, in this situation diversion is occasionally required [29]. In another study, even though none of the dehiscences required stomas, pain management was quite significant requiring readmission for analgesia in a significant proportion of patients [30].
Ultimately, these findings raised the issue whether any attempt to close the wound defect created by TEM should even be performed. Leaving the wound open could potentially avoid the complication of wound dehiscence and minimize its consequences. However logical this may seem, there is no good evidence to support this idea and the author’s clinical experience with unclosed wounds showed no significant differences in pain control after TEM following neoadjuvant CRT for rectal cancers [30].
Outcomes
T1 Rectal Cancer – Local Excision Alone
Local excision alone was considered a valid treatment alternative for T1 rectal cancer for a long time. In the absence of prospectively randomized studies comparing full-thickness local excision to radical total mesorectal excision, most of data arises from retrospective analysis and case-series. Retrospective reviews of selected patients undergoing FTLE, oncological outcomes (local recurrence, survival and cancer-related death rates) were inferior to radical surgery for T1 disease, including higher risk for cancer-related deaths [31, 32]. Even though none of these patients were managed by transanal endoscopic techniques and there was no distinction between favorable and unfavorable tumors, the authors suggested that local excision should be restricted to patients with prohibitive medical contraindications to major surgery.
The only prospective study on local excision alone for T1 rectal cancer was performed in the United States under CALGB [33]. Between 1990 and 1995, 59 patients with T1 rectal cancer were managed by local excision alone. Ten-year local and distant recurrence rates were 8 and 5 % respectively. These encouraging results were followed by less successful outcomes in following studies. A number of studies with variable inclusion criteria, inconsistent pretreatment staging assessment and no standard pathological reporting reported on a wide range of local recurrence rates (0–30 %). The retrospective comparison of local excision to radical surgery for stage I rectal cancer consistently showed worse oncological outcomes after local excision, even though no randomization or case-matched was ever possible [31].
More recently, with significant improvements in local pretreatment staging accuracy and refinements in technical aspects of the procedure with transanal endoscopic techniques, local recurrence rates after local excision alone for selected pT1 rectal cancers remains between 10 and 20 % [34]. In addition to the considerably high local recurrence rates, salvage procedures after local recurrence offer poor oncological outcomes. A recent review of 88 patients with pT1 undergoing TEM, local recurrences were observed in 20 % of the cases [35]. Of these recurrences, only a minority had unfavorable pathological features (Sm3 invasion, lymphovascular invasion, poor differentiation). More than 80 % had advanced stage disease at the time of recurrence and even though R0 resection was possible in most cases, 3-year disease-free survival was disappointing (58 %). Alternatively, immediate salvage resection following local excision seemed to have not compromised oncological outcomes of patients with early stage rectal cancer. In a retrospective study of patients undergoing local excision followed by radical salvage resection within 30 days revealed that outcomes were similar to a matched control group of patients undergoing straight to radical surgery and comparable pathological staging [36].
Even though there is a suggestion that early or immediate salvage provides acceptable oncological outcomes for these patients, the procedure (salvage or completion TME) is not trivial. The quality of the resected mesorectum in this setting may be significantly compromised in a significant proportion of cases (moderate or poor in 36 %). Also, some features of the original procedure such as distal location of the tumor and long interval after local excision (≥7 weeks) were all associated with the risk of poor quality of the specimen.
Distant metastases, when found after FTLE for T1 tumors, usually appear synchronically with local recurrence. Although salvage surgery after local excision is feasible in most patients with T1 tumors, survival might be limited, mainly because of distant metastases [35].
Local Excision and Adjuvant Therapy
In patients that final pathology after local excision reveals high-risk features in the surgical specimen, an alternative to completion of total mesorectal excision is the use of adjuvant RT or CRT. Most studies have considered the presence of T2 tumors, close or positive resection margins, lymphovascular invasion and poor differentiation for such purposes. In the CALGB study, negative margins pT2 cancer patients were offered adjuvant 5FU-based CRT (54 Gy). In that study, 10-year local and systemic recurrence rates were 18 and 12 % respectively. Curiously, median time to recurrence for pT2 cancers was nearly 2 years, significantly shorter than for pT1 cancers (nearly 4 years) in the same study (treated by local excision alone).
The RTOG study showed slightly better results for local excision. CRT was given when local excision specimens showed unfavorable histopathological features in T1 tumors or T2 and a higher dose of CRT when margins were involved. Low risk T1 tumors were only observed without further surgery. The overall local recurrence rate was 16 % and local recurrence free-survival was 86 % for patients treated with adjuvant therapy in 5 years. These rates are similar with the ones seen for TME in the literature. There was no difference in disease free survival or overall survival between patients who received adjuvant chemoradiation and those who did not. The local recurrence rates were 1/14 (7.1 %) patients who were only followed and 2/51 (3.9 %) in those who received CRT [37].
Chakravarti et al. published a retrospective cohort of T1/T2 rectal tumors with adjuvant radiation following FTLE or FTLE alone. In the irradiation group, local control rates for high-risk T1 tumors were 100 %, while 85 % for T2 tumors. In the FTLE alone group local control rates were, respectively 89 and 33 % for T1 and T2 tumors. The addition of systemic chemotherapy with 5FU did not significantly improve local control or recurrence free survival in the irradiation group. With these results, they recommended only adjuvant CRT for high-risk tumors after local excision [38].
Neoadjuvant CRT Followed by Local Excision
Even though postoperative (adjuvant) therapy would have the benefit of offering patients treatment after confirmation of “unfavorable” pathological findings, the observation of decreased toxicity and improved local disease control in prospective randomized trials of rectal cancer in the setting of radical surgery led to the utilization of radiation and chemotherapy in the pre-operative period (neoadjuvant) [39–41]. In addition, the exposure of healthy and well-oxygenated tissue, as opposed to post-operative fibrotic tissue, to radiation would theoretically improve its anti-neoplastic effects. Finally, perhaps one of the most beneficial aspects of offering patients preoperative neoadjuvant therapy would be the effect on tumor shrinkage. The decrease in tumor size (downsizing) and shifts in tumor stage (downstaging) have been well documented after neoadjuvant therapies with radiation and chemoradiation (CRT) [42–45]. In fact, the addition of chemotherapy to radiation has been shown to significantly increase the effects on tumor size and stage when delivered preoperatively [42]. Also, this downsizing and downstaging seem to be time-dependent and therefore, at least 6, 8 or even 12 weeks may be required to obtain maximal results tumor regression [46–48].
It appeared that neoadjuvant therapy, particularly CRT, was the answers to all prayers for TEM in rectal cancer: improve local disease control, minimize toxicity, decrease tumor size, downstage cancers and allow a minimally invasive approach without all of downsides of radical total mesorectal excision (TME).
However, the expected benefits of this strategy came at a significant cost in terms of wound healing (as mentioned previously) and salvage possibilities. Also, local recurrences may still be a concern depending on baseline and post-treatment characteristics.
Local Recurrence
As mentioned earlier, local recurrence rates have historically paralleled the risk of lymph node metastases in patients treated by FTLE for rectal cancer. pT status is one of the most relevant determinants of the risk of perirectal nodal metastases both with or without chemoradiation [49–52]. In fact, studies have suggested that the risk of lymph node metastases is <5–10 % for ypT0, 10–15 % for ypT1 and nearly 20 % for ypT2 [53]. Therefore, one could expect these rates of local recurrence after treatment with CRT followed by local excision regardless of the original baseline staging.
However, radiological imaging has evolved significantly over the years and nodal staging has improved. Even though accuracy is still far from 100 %, magnetic resonance (MR) and endorectal ultrasound have been studied extensively in order to improve detection of lymph node metastases. It has been suggested that MR could safely assign patients after CRT that would be appropriate candidates for FTLE by correctly identifying ycT0-2N0 (accuracy ≥90 %) [54]. This suggests that ypT0, ypT1 and ypT2 would all be appropriate candidates for FTLE or TEM, once nodal metastases have been ruled out. In a review of patients with ycT0-2 N0 following long-course CRT and TEM, local recurrence rates were nearly 15 % [55]. In this study, most patients had ypT1/ypT2 whereas ypT0 were very few. In a recent report from a multicenter study in Italy (Phase II), 63 patients underwent CRT for cT2-3N0-1 disease at baseline [56]. Of these, 42 had ypT0 and were treated by FTLE alone with no recurrence. One patient with ypT1 and TRG2 also did not recur. However, of the 9 patients with ypT2 who refused radical TME, 2 developed local recurrences after FTLE alone (22 %). Bonnen et al., in 2004, published their results comparing 26 patients with T3 tumors submitted to neoadjuvant chemoradiation followed by local excision and 405 patients submitted to neoadjuvant CRT followed by TME. In the local excision group the pCR was 54 %. The 5-year local recurrence rate was 6 % in this group while 8 % in the TME group, and 6 % in the subgroup of complete responders in the TME group. Overall survival was 86 and 81 % in the local excision and TME groups, respectively. An update on their data [57] showed that results were maintained after a longer follow up of 63 months. They suggest that highly selected patients that respond well to CRT might be submitted to FTLE [58].
Other authors have suggested that baseline staging is also important and only cT2N0 followed by neoadjuvant CRT would be appropriate candidates for FTLE or TEM [59, 60]. In fact, the single randomized study that compared cT2N0 followed by neoadjuvant CRT and TEM or TME found in its first report advantages in early/immediate outcomes favoring TEM (less transfusion and stoma requirements, less hospital stay and less need for ICU). Local recurrence rates were similar between groups [61]. In a more recent update, local recurrence rates were still similar between groups. However, TEM resulted in more early recurrences when compared to TME. Also, TEM was considered to be an independent risk factor for the development of recurrent disease (metastatic or local recurrence) after multivariate analysis [62]. Ultimately, local recurrence rates were all <10 % in both groups. Still, it should be noted that nearly 1/3 of the patients in each group (total of 50 patients in each group) had complete pathological response (ypT0), a known predictor of low risk for LN metastases. Also, all of the local recurrences were among patients with ypT2 residual cancers. Finally, there is still an ongoing study specifically dealing with cT2N0 rectal cancer patients managed by long-course CRT followed by FTLE (including but not necessarily TEM) [63]. One could expect that local recurrence rates will ultimately depend on the effectiveness of CRT. If CRT was highly effective, with many ypT0, local recurrences will probably be low. However, if ypT2 were frequent, one could expect higher local recurrence rates.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






