Author and year
Patient with TEM
LR
Winde [7] (1996)
24 T1
4 %
Mentges [8] (1996)
56 T1
7 %
Lee [9] (2003)
52 T1
4 %
Palma [10] (2004)
18 T1
6 %
Duek [11] (2005)
25 T1
0 %
Floyed [12] (2006)
53 T1
7.5 %
Guerrieri [13] (2006)
58 T1
0 %
Lezoche [14] (2012)
50 T2 with nRCT
8 % (distant Rec. 4 %)
Ganai [15] (2006)
21 T1, 1 pt RCT
9.5 %
Stipa [16] (2006)
23 T1, 4 with RT
8.6 %; only TEM 11 %
21 T2, unknown number with RT
9.5 %
Maslekar [17] (2007)
27 T1 (all R0)
0 %
22 T2 (19 R1)
18 %
Bretagnol [18] (2007)
28 T1
10.7 %
Whitehouse [19] (2007)
23 T1
26 %
Zacharakis [20] (2007)
14 pts T1
7.1 %
11 pts T2
42.8 %
Moore [21] (2007)
39 pts T1-2-3 (TEM + RT in some T2-3)
8 % (overall) (2 pts with LR staged preoperative cT3)
Speake [22] (2008)
31 T1
5.7 % (overall)
4 T2
(all R0)
(only distant Rec. 5.7 %)
Serra-Aracil [23] (2008)
16 pts T1
6.2 %
9 pts T2
22.2 %
Koebrugge [24] (2008)
20 pts T1
4.1 % (overall)
1 pt T2
1 pt T3
Bach [25] (2009)
230 T1
18.6 %
107 T2
29.3 %
Jeong [26] (2009)
16 T1
5 % (overall)
3 T2
T1 0 %
1 T3
T2 16 %
(3 pts nRCT + TEM)
T3 0 %
De Graff Ej [27] (2009)
80 pts T1 (all R0)
24 %
Doornebosch [28] (2010)
88 pts T1 (all R0)
20.5 %
Christoforidis [29] (2009)
25 pts T1
12 %
12 pts T2
25 %
Tsai [30] (2010)
51 pts T1
9.8 %
17 pts T2: 5 pts TEM + RT
23.5 %
Allaix [31] (2012)
9 pts T2: nRT + TEM
0 %
32 pts T2: TEM
26 %
Since 1992 we have introduced this technique in our institution, and more than 950 operations have been performed by TEM for benign and malignant lesions of the rectum. The lesson we learned from this experience is that in order to obtain good clinical results two conditions must be met:
1.
A correct patient selection
2.
An adequate local exeresis
Patient Selection at the Admission
In case of malignancy, the lesion must be localized in the extraperitoneal portion of the rectum. Usually neoplasia can be easily palpated at finger exploration and if the lesion is adherent to the narrow structures or fixed, the patient is not eligible for the LE.
The diameter must not exceed 4 cm, preferably 3.
The use of the vital dye technique during flexible endoscopy is advisable to define the tumor limits especially in flat neoplastic lesions and in case of multifocal adenomas with degenerated areas. The routinary use in the clinical practice of NBE, and of image analysis systems seems promising in accurately identifying the neoplasia margins.
According to our protocol, during vital dye endoscopy, 5/6 biopsies are performed all around the lesion at a minimum distance of 1 cm from the identified tumor margins on apparently normal mucosa. Each biopsy site is identified with a number and then sent to the morphologist. Tattooing is performed at each biopsy site so that during endoluminal surgery the black spots will guide the excision line (Fig. 9.1).
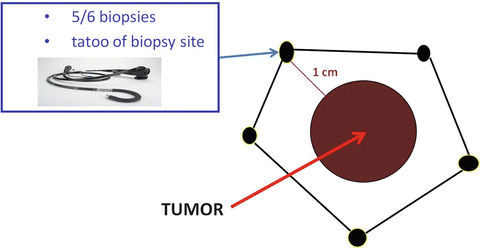
Fig. 9.1
Tattooing at each biopsy site so that the black spots will guide the excision line
Rigid rectoscopy must be performed in all patients candidates to TEM for two reasons: (1) to define the circumferential tumor involvement and consequently the most appropriate patient’s position on the operative table (Fig. 9.2): patient must be placed in prone position for anterior and supine for posterior lesions. For lateral tumors in lateral position lying on the side that is ipsilateral to the lesion. In other words, if the lesion is on the right side of the rectum, the patient is placed in 90° right lateral jack-knife position with legs’ support (Fig. 9.3). (2) Furthermore, rigid rectoscopy is routinely employed to perform macrobiopsies using the old shape forceps (Fig. 9.4) to provide to the morphologist an adequate amount of tissue for grading and histological evaluation.
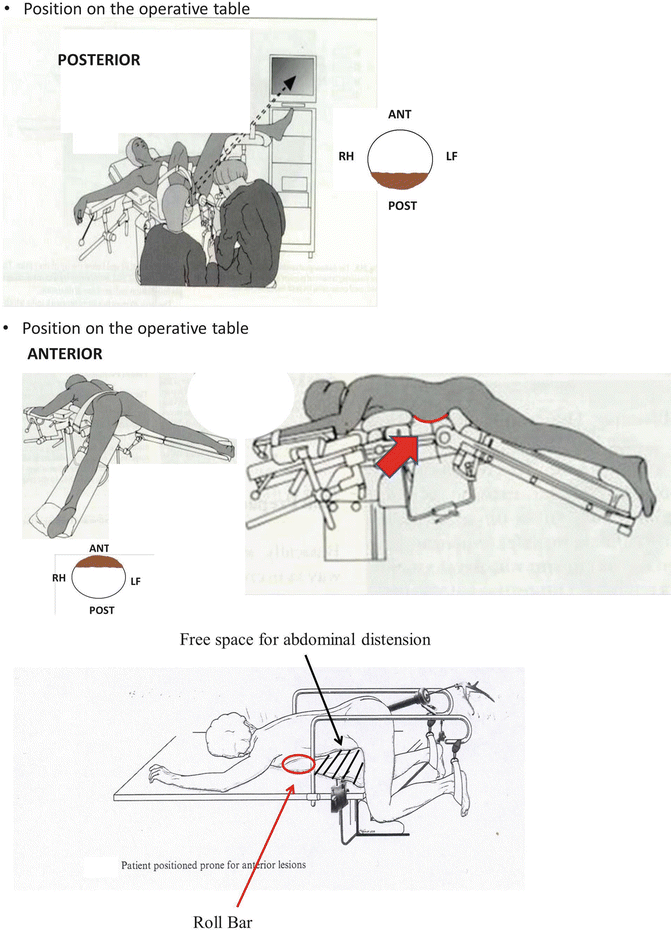
Fig. 9.2
Position on the operating table
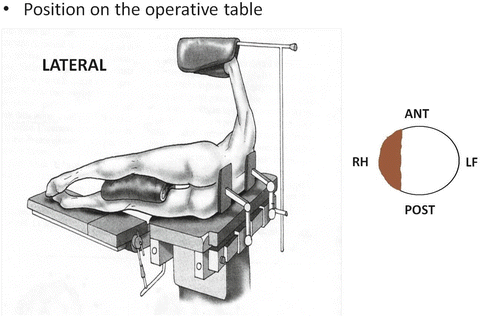
Fig. 9.3
Lateral position on the operating table
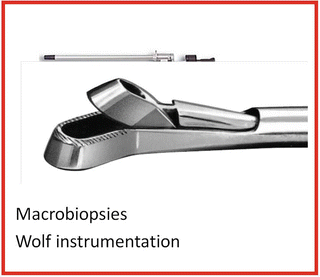
Fig. 9.4
Macrobiopsy using Wolf instrumentation
Histology: Undifferentiated and mucous tumors are not eligible for LE and must be treated with TME; therefore morphologist must work on macrobiopsies that involve muscolaris propriae in order to give an accurate evaluation of T stage and G grading (lymphatic, neuronal, and vessel infiltration). According to recent observations, after neoadjuvant therapy (NT) tumor grading is not predictive of clinical evolution. In case of T1, it is mandatory that the morphologist check the invasion of tumoral cells into the submucosa (Sm 1–3). In T1 Sm3 the risk of metastasis in loco-regional lymphatic nodes is too high to limit the treatment to an LE (Fig. 9.5). In selected cases with the tumor close to dentate line or in high risk patients, it is reasonable to combine LE with radiotherapy (RT) to avoid more aggressive surgery as TME.
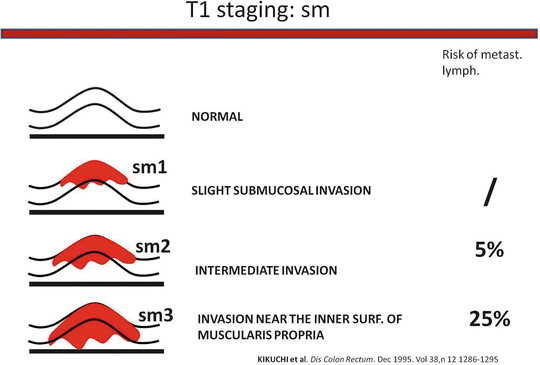
Fig. 9.5
T1 staging: sm
Endoscopic mucosectomy: In the last years mucosectomy by flexible endoscope has been performed more and more frequently. This technique utilizes electrocoagulation to dissect and the high temperature destroys the submucosa layer making; it is impossible for the morphologist to determine the Sm grading. The lack of this crucial parameter makes the full thickness LE unwise. All T1 patients who previously had mucosectomy must undergo an operation to remove at least the nodes adjacent the tumor such as TME (or ELRR see specific chapter). Consequently before performing a mucosectomy of a potentially malignant lesion the patient should be informed that the technique could cause a loss of an important prognostic parameter. Therefore the use of flexible endoscopy mucosectomy should be discouraged in low rectal lesions with high risk of malignancy (presence of a diameter >2 cm, ulceration, etc.)
Imaging tumor stage (iT): Endorectal ultrasound (EUS) is mandatory to differentiate T1 vs. T2 RC; unfortunately as EUS is unable to evaluate the submucosa infiltration despite the technological improvement, macrobiopsies are necessary. It is advisable that before performing any endoluminal operation the surgeon carry out an EUS to give him the spatial location and dimension of the lesion. It follows then that the use of a 3D ultrasound system is recommended. Pelvic MR is mandatory in every stage of RC. New MR technology gives a reliable differentiation between T2 and T3 lesions (such as EUS), and it is able to identify adjacent organs infiltration and suspicious nodes (false negative inferior to 10 %). In near future, the introduction in the clinical practice of new MR dyes or new MR techniques such as diffusion-weighted imaging (DWI) will significantly improve the diagnosis of malignant nodes. Low rectal tumors have a significant risk of lung and/or bone metastasis. Therefore total-body CT provides useful information. Although the role of PET-CT is still controversial in staging, it seems to be promising for early detection of recurrences. In our protocol, all patients who underwent local treatment perform a PET scan at 6 months after surgery.
Manometry: Preoperative study of the sphincter function is advisable in patients with low rectal tumors. In all elderly patients it is important to study preoperatively the sphincter to evaluate risk of postoperative incontinence that can onset in the first months after TEM procedure. Furthermore it is useful for any possible medical-legal implications.
From 1992 we have employed radiotherapy for local treatment of RC and in absence of literature data regarding the risk of suture dehiscence after TEM we employed the so-called “sandwich” radiotherapy (2.5 G preoperatively and 2.5 G 1 month after TEM procedure). After 1995 NT has been routinely employed in all T2 and more advanced RC. The indications to NT and local treatment for T1 and T2, N0 low rectal cancer are reported in Figs. 9.6 and 9.7 respectively.
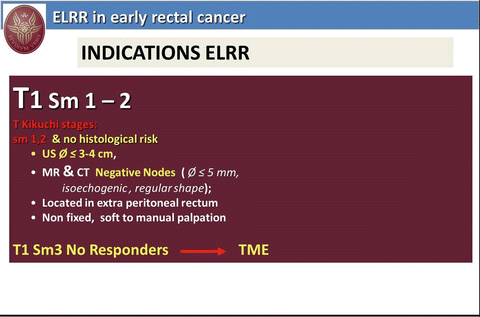
Fig. 9.6
ELRR in early rectal cancer: indications
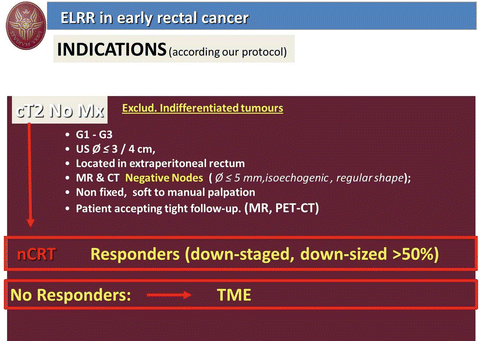
Fig. 9.7
ELRR in early rectal cancer: indications
Neoadjuvant Treatment
From the literature, it is evident that neoadjuvant treatment (NT) reduces the risk of local recurrence and may increase survival. In patients eligible for LT long-course high-dose radiotherapy (lchdRT) is preferable to the short one for several reasons: (a) lchdRT increases down staging and tumor downsizing percentage. In a series of 100 non-advanced RC (iT2-T3 N0) lchdRT determines down staging in over 40 % and in 90 % of patients a tumor downsizing over 30 %. Tumor mass reduction is particularly helpful during endoluminal manipulations required for local excision; (b) lchdRT presumably gives a “sterilization” effect on metastatic deposits of loco-regional lympho nodes. (c) After lchdRT (combined with chemio) at 45 days a complete clinical response is observed in 15 % of cases and these patients can be recruited for “watch and wait” strategy. The full dose of 50,4 Gy can be administered also in elderly patients. Contraindications are rare; the possible side effects are generally well tolerated and in such cases the length of treatment is prolonged. Ninety-five percent of patients complete the full course.
Preoperative Staging (After NT Staging)
No data are available in the literature to define when is the best timing to restage the tumor after NT. According to our protocol at the end RT the patient is submitted to a clinical examination to evaluate if the lesion has responded or not to radiotherapy. In case of no significant responses a further image evaluation is performed at 3/4 weeks and then the patient undergoes TME or APR!
In the event of a significant clinical response the staging usually is scheduled at 45 days after the end of NT. In the last years it has been observed that delaying the post-NT staging of further 2/3 weeks the percentage of patients with complete clinical response (CCR) increases.
After radiotherapy the evaluation of tumor response is mainly based on two parameters: tumor downsizing by imaging and Tumor Regression Grading (TRG) by histology.
Imaging after NT is a complex and difficult matter requiring the services of specifically trained radiologist. The actinic-induced edema and scar tissue make the interpretation of imaging and the correct evaluation of tumor volume difficult. EUS and MR are routinely employed to evaluate the tumor mass reduction after NT.
Tumor response grading: This score follows the same criteria proposed by Mandard criteria for the esophageal cancer. To have a reliable TRG, the morphologists need of an adequate volume of tissue. Therefore “macrobiopsies” must be performed.
In our series of patients, recurrences were observed only in patients not responding to NT. Now we consider eligible for local treatment only responders to NT (downstaged or tumor downsized >50 %). Following this criteria, no recurrences were observed at a 3 years follow-up in the last 40 T2 patients treated with endoluminal loco-regional resection (ELRR).
Several authors suggest to prolong the period of time, between the end of radiotherapy and local resection. Now, in case of significant response to RT, we wait at least 2 months, and this policy seems not to increase the intraoperative difficulties, the risk of suture leaks or other postoperative complications.
Informed Consent
Written and detailed information regarding state of the art of local treatment must be provided to the patient. The informed consent form will include all the therapeutic options (APR, TME, local excision, ELRR, watch and wait in case of complete clinical response), the risk of each approach in terms of intra- and postoperative complications, as well as the oncological long-term results.
If the patients choose an endoluminal treatment, they must accept to be submitted to a close follow-up.
Adequate Local Excision/Resection by TEM
Surgeon Skill
TEM is a complex and difficult procedure that requires a specific skill that can be achieved only after prolonged training on the simulator. Several different exercises are recommended.
Dissection generally is easily acquired as soon as a good coordination of the instruments is achieved.
To perform a fluent suturing it is necessary to spend at least 2/3 h by day at the simulator during 3 or 4 weeks. Particular attention must be dedicated to another exercise whose aim is to improve the surgeon’s ability to reach in a blind fold manner a predetermined point. This exercise is very important! In fact, brisk bleeding may occur from hemorrhoidal arteries during resection of the mesorectum. These vessels have a relevant flux that in a few seconds can fill the operative field and obscure the vision. The amount of blood delivered from the artery can be higher than the volume that the suction cannula can remove. Consequently the surgeon must be able to localize and coagulate the bleeding source under every circumstances. In the meantime, blood can cover the lens thereby completely obscuring the vision. I like to underline that only the TEM optics can be washed under the pedal control.
To develop this ability, its marking with a black spot the operative field of the simulator can be useful; then the operator must touch the spot several times under 3D vision. The exercise is then repeated in blind conditions as to verify the possible errors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








