Fig. 6.1
Manual compression of the liver to temporarily control bleeding: by bimanual pressure on either side of the laceration (a), pressure between the thumb and fingers (b), compressing the liver lobe above and below (c), or squeezing together the liver tissue on either side of the injury
Simple Techniques of Repair
If the source of hemorrhage turns out to be a minor or modest injury (grade I or II) (Table 6.1), which is often the case, simple techniques of repair may be sufficient [3, 4]. Such techniques include drainage alone (for non-bleeding injuries), manual compression, high-energy surgical devices (electrocautery, argon beam plasma coagulation, etc.), topical hemostatic agents, and suture hepatorrhaphy. These basic techniques will suffice in 90 % of penetrating wounds and 60 % of blunt injuries [5, 6]. While these will be discussed individually below, they are often used in conjunction with one another.
Table 6.1
The American Association for the Surgery of Trauma (AAST) liver injury scale (1994 revision)
Grade | Injury description | |
|---|---|---|
I | Hematoma | Subcapsular, <10 % surface area |
Laceration | Capsular tear, <1 cm parenchymal depth | |
II | Hematoma | Subcapsular, 10–50 % surface area; intraparenchymal, <10 cm diameter |
Laceration | 1–3 cm parenchymal depth, <10 cm length | |
III | Hematoma | Subcapsular, >50 % surface area or expanding. Ruptured subcapsular or parenchymal hematoma; Intraparenchymal hematoma >10 cm or expanding |
Laceration | >3 cm parenchymal depth | |
IV | Laceration | Parenchymal disruption involving 25–75 % of hepatic lobe or >3 Couinaud’s segments within a single lobe |
V | Laceration | Parenchymal disruption involving >75 % of hepatic lobe or 1–3 Couinaud’s segments within a single lobe |
Vascular | Juxtahepatic venous injuries, i.e., retrohepatic vena cava/central major hepatic veins | |
VI | Vascular | Hepatic avulsion |
Drainage Alone (For Non-bleeding Injuries)
The drainage of minor non-bleeding hepatic injuries is rarely, if ever, required. If a minor capsular tear or laceration is identified, any avulsed biliary ducts should be directly visualized and suture ligated. As such, the vast majority of these injuries will not drain in the postoperative period and thus do not warrant drainage.
Manual Compression
Many minor injuries are associated with rib fractures. Often, a 5–10 min period of manual compression is all that is required to control any hemorrhage. If hemorrhage is controlled in this fashion, then no further therapeutic maneuvers are required, nor is drainage necessary.
High-Energy Surgical Devices (Electrocautery, Argon Beam Plasma Coagulation)
In addition to manual compression, high-energy surgical devices (electrocautery, argon beam plasma coagulation, etc.) are frequently utilized in minor or modest hepatic injuries, with varying degrees of success. Electrocautery should only be used to stop the bleeding of small vessels (larger vessels being ligated) or raw tissue surfaces. If individual vessels are appreciated, one is prudent to grasp them with tissue forceps and cauterize in this fashion. The argon beam has been utilized effectively to control local bleeding from raw edge surfaces [7]. The argon beam laser is less effective in controlling bleeding emanating from severed blood vessels of any significant magnitude (effective on small vessels only with a depth of penetration of approximately 2.5 mm).
Topical Hemostatic Agents
Topical hemostatic agents have gained tremendous popularity for use in emergency bleeding control, and that from hepatic trauma is no different. A number of agents are currently available and may be divided into two categories: those that act passively through contact activation and promotion of platelet aggregation and those that act on the clotting cascade in a biologically active manner (Table 6.2) [8, 9]. Such agents are applied directly to the site of bleeding and provide an excellent adjunct when standard means of hemostasis are ineffective or impractical [10]. Sealants function similarly.
Table 6.2
Topical hemostatic agents
Passive | Active | Tissue sealants |
|---|---|---|
Collagens | Thrombins | Fibrin sealants |
Cellulose | Polyethylene glycol | |
Gelatins | Albumin/glue | |
Combinations | ||
The agents should be applied directly to the site of bleeding followed by manual compression for 5–10 min. After releasing compression, remaining bleeders may be cauterized (using tissue forceps). There are several benefits to the topical hemostatic agents. First, they avoid the global effects of systemic hemostatic medications, i.e., unwanted blood clots [11]. Second, they may be titrated to the needed effect, i.e., used liberally with significant bleeding or sparingly with minimal bleeding. Ultimately, there is the prolonged benefit with respect to the postoperative blood loss and the need for excessive transfusions [12].
Suture Hepatorrhaphy
Approximately 50 % of all hepatic injuries managed operatively involve peripheral penetrating wounds or parenchymal lacerations 1–3 cm in depth (grade II). Suture hepatorrhaphy has traditionally been the mainstay therapy of such injuries. In doing so, it is important to initially enter the laceration and identify and ligate all injured blood vessels and bile ducts selectively. This may prove to be difficult if not impossible in coagulopathic patients or those with deeper lacerations. In such cases, it is best to proceed directly to mass closure, with the placement of large sutures through the liver parenchyma to arrest bleeding by coapting the two edges.
The suture of choice is an 0 chromic. If the laceration is 1–3 cm in depth, a standard (CT-21) needle may be used to loosely approximate the edges of the laceration in an interrupted fashion. For deeper lacerations, use a large blunt nose liver needle and perform a horizontal mattress closure. In order to prevent tearing through the liver, throw down two knots without tension. Have the first assistant hold the knots with a tonsil clamp, gently releasing it as the surgeon squares the knots. In this manner, tearing through the liver capsule rarely occurs.
Past personal experience with suture hepatorrhaphy has shown that tightly tied horizontal mattress sutures may result in necrosis of the underlying parenchyma during the postoperative period. For this reason, it is best to proceed with selective vascular and bile duct ligation followed by loose approximation of the liver edges rather than necrosing horizontal mattress sutures. Exceptions to this rule include patients (1) with multiple injuries and (2) who are coagulopathic, such that speed is critical and precise techniques are somewhat precluded.
Advanced Technique of Repair
The incidence of complex hepatic injuries (grades III, IV, and V) has remained stable over the past 25 years at 12–15 % [13]. These account for approximately 10 % of all penetrating injuries and less than 40 % of blunt injuries. Such injuries require a more advanced technique of repair involving the following six critical steps: (1) manual compression and resuscitation, (2) portal triad occlusion, (3) finger fracture of the parenchyma to identify and ligate lacerated blood vessels and bile ducts, (4) resectional debridement of nonviable hepatic parenchyma, (5) insertion of a viable omental pedicle into the injury site, and (6) closed suction drainage for grade III–V injuries.
Manual Compression/Resuscitation
Upon entering the peritoneal cavity, all efforts should focus on intraoperative resuscitation. As previously mentioned, this entails packing all four quadrants of the abdomen with laparotomy pads and manually compressing the liver with both hands (Fig. 6.1). This allows the anesthesiologists time to catch up with the resuscitation, ultimately working to prevent/correct the potentially lethal “bloody vicious cycle.” This step is absolutely critical, in that very few injuries cannot be temporized by such a maneuver, and yet few if any patients will survive without adequate resuscitation. It is only after this step that the surgeon should begin to assess the extent of the injury.
Portal Triad Occlusion (The Pringle Maneuver)
In 1908, J. Hogarth Pringle described occlusion of the portal triad for controlling hepatic hemorrhage [14]. Known as “the Pringle maneuver,” it entails placing an atraumatic vascular clamp (either a Satinski or curved DeBakey clamp) across the hepatoduodenal ligament (Fig. 6.2) [15, 16]. Prior to doing so, initial finger occlusion of the portal triad gives the operating surgeon an idea of whether or not this maneuver will be successful. It typically controls hemorrhage originating from intrahepatic branches of the hepatic artery and/or portal vein. Failure to do so implies that the site of injury is at the retrohepatic vena cava or hepatic veins.
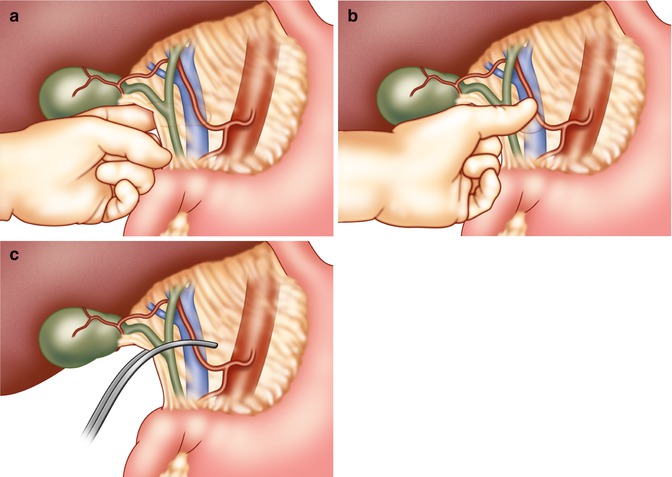

Fig. 6.2
Portal triad occlusion (the Pringle maneuver). (a) The surgeon slides his left hand with palm facing up into the foramen of Winslow and with the thumb feels the hepatic arterial pulse. (b) The hepatic artery in the free edge of the lesser omentum is compressed between the thumb and the other fingers, reducing hepatic blood flow. (c) A curved vascular clamp may now be applied to the lesser omentum
In order to avoid ischemic injury to the liver parenchyma, the safe cross-clamp time of the portal triad has been thought to be no more than 15–20 min in normothermic conditions [17–19]. In order to minimize this risk, the operating surgeon should clamp and release (for 5 min) the portal triad flow at 15–20 min intervals. Such “warm ischemia” decreases the time the liver is without blood supply, thereby minimizing ischemia.
Two methods examined to extend this time are (1) a bolus infusion of steroids (20–30 mg/kg of Solu-Medrol) and (2) topical hypothermia (cooling the liver to 30–32 °C). Steroids have been abandoned as experimental data have documented that (1) the percentage of high-energy phosphates within the liver at 60 min is lower than in control animals and (2) the incidence of sepsis is increased in injured patients receiving steroids [19, 20]. Topical hypothermia significantly prevents ischemia/reperfusion injuries to the liver, thus extending the safe cross-clamp time [21]. Using a “slush” solution or iced Ringer’s lactate directly applied to the surface of the liver, a temperature of 27–32 °C can quickly be attained. The surface temperature can be measured via a short intrahepatic temperature probe, appreciating that there will be temperature variations within the liver parenchyma. If this method is to be employed, it is essential to initiate a series of steps to prevent systemic hypothermia, such as covering the remaining abdominal viscera with warm laparotomy pads. If the patient becomes hypothermic, topical hypothermia should be abandoned. Likewise, if the patient is hypothermic prior to instituting topical hypothermia, it should not be undertaken.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








