and Armanda Tatsas2
(1)
Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
(2)
Pathology Group of Louisiana, Baton Rouge, LA, USA
Introduction
With the increasing use of imaging modalities to evaluate patients for primary diagnoses as well as to monitor patients with a history of malignancy, liver lesions are commonly identified. Autopsy studies indicate that in up to 50 % of patients, liver lesions may be detected and the vast majority of them are benign. Fine needle aspiration (FNA) of the liver is a useful tool to evaluate both solid and cystic lesions of the liver (Table 3.1). Benign and malignant lesions may be diagnosed by FNA, although the benign diagnoses may be difficult without a complementary needle core biopsy (NCB). Additionally, material obtained from FNA is well suited to microbiology cultures, flow cytometry studies, cytogenetic, and other molecular testing. FNA is not routinely indicated in the evaluation of hepatitis, cirrhosis, and metabolic diseases or for transplant monitoring.
Table 3.1.
Lesions that may be diagnosed on fine needle aspiration of liver.
• Fluid collections |
– Cysts |
– Abscesses |
• Benign nodules/proliferations |
– Focal Nodular Hyperplasia |
– Liver cell adenoma |
• Malignant neoplasms |
– Primary |
– Metastatic |
Approach
Liver FNA is most often performed transabdominally under ultrasound or computed tomography (CT) guidance, typically by a radiologist. The advantages of ultrasound over CT guidance include real-time needle guidance, speed of procedure, excellent discrimination between solid and cystic lesions, lower cost of equipment, portability, and lack of radiation exposure. CT guidance offers better visualization of some lesions, particularly small ones.
MRI-guided procedures are possible, but require special equipment and are thus very expensive, and they rarely have any advantage in liver FNA. Transjugular biopsies performed using interventional radiology are rarely indicated (except for patients with impaired clotting, morbid obesity, or gross ascites) but may be attempted. “Blind” percutaneous liver biopsies (without image guidance) may be used in evaluation of diffuse liver abnormalities, but they are not typically used for targeting space-occupying lesions.
The newest technique is endoscopic ultrasound-guided FNA (EUS-FNA), which has a sensitivity of 82–94 % and specificity 90–100 % for space-occupying lesions. In these procedures, the liver is accessed via the stomach or small intestine. The left lobe of the liver is easily accessed, and the liver hilum, proximal right lobe, gallbladder, and perihilar lymph nodes can potentially be accessed. The technique is good for detecting and assessing a number of lesions for staging, and the diagnostic yield is higher for metastatic lesions than for primary HCC.
The approach to image-guided FNA is discussed in further detail in Chap. 1.
Sensitivity, Specificity, and Accuracy
The sensitivity of liver FNA ranges from 67 to 100 % and the specificity from 87 to 100 %. The overall accuracy is 80–95 %. The sensitivity and specificity for liver malignancy are 90 % and 100 %, and positive predictive value for malignancy approaches 100 %. Combined FNA and NCB has a higher sensitivity (85–100 %) compared to either FNA (62–100 %) or NCB alone (69–91 %).
Complications
The risk of complications is low, but the most commonly encountered ones include pain, hemorrhage, and infection. To control the associated pain, local anesthetic is typically provided with lidocaine, but the liver parenchyma cannot be anesthetized. Hemorrhage occurs in less than 1 % of patients. Intra-abdominal bleeding is the most severe potential complication, however transfusion is rarely required. The procedure is typically performed using sterile technique, and the rate of infection is less than 1 %. Prophylactic antimicrobials are not routinely given. The risk of tumor seeding due to the procedure is generally regarded as <0.01 %, though small series have reported a higher incidence of seeding in HCC (0.003–5.0 %). The risk of mortality is approximately 1 in 10,000–12,000.
Contraindications
Though generally a low-risk procedure, there are certain patients in whom liver FNA is contraindicated. Patients with severe bleeding disorders that cannot be corrected with therapy and those unable to remain still during the procedure or cooperate with positioning may be ineligible. Morbid obesity or massive ascites may impair the ability to perform transabdominal percutaneous liver biopsy, so the transjugular approach may be indicated. Some authorities suggest that aspiration of hepatocellular carcinoma carries a higher risk of bleeding and/or seeding, thus the procedure risks should be evaluated carefully in a borderline patient who is suspected to have HCC.
Complications of and contraindications to FNA are discussed in further detail in Chap. 1.
Normal Components of Liver
Hepatocytes
The predominant cell type in the liver is the hepatocyte, thus hepatocytes are frequently encountered in aspirates of nonneoplastic liver as well as admixed with neoplastic cells, both primary and metastatic. Aspirates of normal liver typically show a combination of loosely cohesive tissue fragments, small clusters of evenly spaced cells, or single cells. Fragments and small clusters have well-defined edges, and individual cells have well-defined cell borders. Single cells are round to polygonal, and cytoplasm is abundant and granular. The nuclear to cytoplasmic (n/c) ratio is low given the abundance of cytoplasm. Hepatocytes display a single, round nucleus which is centrally placed with smooth nuclear contours and may show a single small to variably prominent nucleolus. Occasional binucleation is encountered and is not indicative of dysplasia or neoplasia. Perinuclear accumulation of lipofuscin pigment is commonly encountered and is normal, increasing with patient age. Intranuclear inclusions are occasionally seen in normal hepatocytes, and their presence should not be taken as evidence of a hepatocellular neoplasm (Figs. 3.1, 3.2, and 3.3).
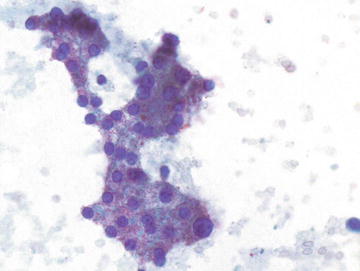
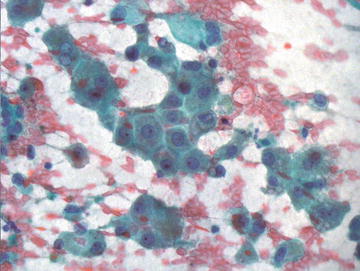
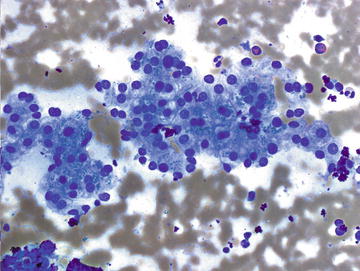

Fig. 3.1.
Normal Hepatocytes: Hepatocytes have a polygonal shape with abundant coarsely granular cytoplasm and round nuclei with variably prominent nucleoli. The nuclear to cytoplasmic ratio is low due to the large volume of cytoplasm. (Papanicolaou stain, medium power)

Fig. 3.2.
Normal Hepatocytes: these hepatocytes are cohesive, forming a flat sheet of cells. They are polygonal and show abundant granular cytoplasm. Nuclei are round and centrally placed and have intermediate sized nucleoli. (Papanicolaou stain, high power)

Fig. 3.3.
Normal Hepatocytes: A group of benign hepatocytes is shown demonstrating abundant granular cytoplasm and uniformly sized, round nuclei that are located in the center of the cell. There is some finely granular pigment present in the cytoplasm of some cells representing lipofuscin pigment. (Diff-Quik stain, medium power)
Key features:
Tissue fragments, loosely cohesive small clusters or single cells
Round to polygonal cells
Well-defined cell borders
Abundant granular cytoplasm
Low nuclear to cytoplasmic ratio
Centrally placed, round nucleus
Smooth nuclear contours
Single small to prominent nucleolus
Perinuclear accumulation of lipofuscin pigment
Scattered binucleation and intranuclear inclusions may be present
Biliary Ductal Epithelium
The second most common cell type encountered in aspirates of normal liver is biliary type epithelium, though it is usually present in much smaller quantity than hepatocytes. Ductal epithelium is typically present in flat, cohesive sheets of cells with evenly spaced nuclei showing no nuclear overlap or crowding. This “honeycomb” arrangement is similar to what is encountered in aspirates of pancreatic ductal epithelium. Small tubular or acinar structures are less commonly encountered (Fig. 3.4). The cells are overall smaller than hepatocytes, and the nuclear to cytoplasmic ratio is higher due to the presence of considerably less cytoplasm, though the nuclear sizes are similar in the two cell types. The cells are cuboidal to columnar in shape, nuclei are round to slightly oval and have absent to inconspicuous nucleoli.
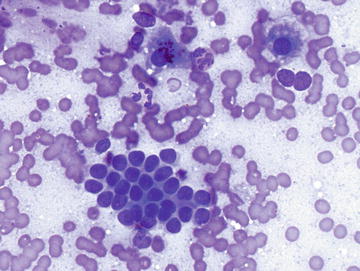

Fig. 3.4.
Normal Bile Duct: A cohesive fragment of epithelial cells is shown forming a flat sheet with evenly spaced nuclei showing minimal overlap. The nuclei are round to oval and show smooth nuclear contours. Biliary duct epithelium may present as flat sheets or acinar formations. (Diff-Quik stain, high power)
Key features:
Flat, cohesive sheets with “honeycomb” arrangement or small tubular or acinar structures
Evenly spaced nuclei with no nuclear overlap or crowding
Cuboidal to columnar cell shape
Round to slightly oval nuclei
Inconspicuous nucleoli
Nuclear to cytoplasmic ratio higher than hepatocytes
Endothelial Cells
While endothelial cells are recognized as a key diagnostic feature in hepatocellular carcinoma, they are reported to be present in up to half of all aspirates of normal liver. They are not usually abundant in aspirates of benign liver and may not be noted. The cells are small and have spindle-shaped nuclei with smooth nuclear contours and a scant amount of delicate cytoplasm.
Kupffer Cells
These specialized macrophages assist in the degradation of hemoglobin to unconjugated bilirubin. They are cytomorphologically indistinguishable from normal macrophages and are thus not definitively identifiable on aspirate smears.
Contaminants
Mesothelial Cells
The main pitfall associated with the presence of mesothelial cells in liver aspirates is overinterpretation of these cells as an epithelial neoplasm. Recognition of this normal contaminant is generally straightforward on aspirate smears. Mesothelial cells appear most often as flat sheets or small fragments of uniformly sized cells which are evenly spaced and show no overlap. The nuclear to cytoplasmic ratio is typically low, and clear spaces between the cells (“windows”) are a key defining feature. The nuclei are round to oval and may show occasional intranuclear grooves and small nucleoli (Fig. 3.5).
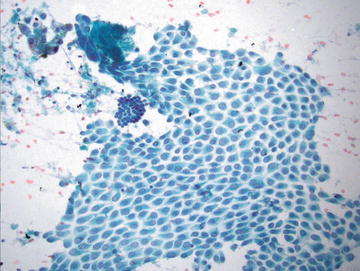

Fig. 3.5.
Normal Mesothelial cells: A large flat sheet of mesothelial cells is present and shows clear spaces (“windows”) between evenly spaced cells. The nuclei are round with evenly dispersed nuclei and occasional grooves. A small group of hepatocytes is present in the upper left corner, and a group of bile duct epithelium is present at 10 o’clock, just to the upper left of center. (Papanicolaou stain, medium power)
Key features:
Flat sheets or small fragments of uniformly sized and spaced cells
Nuclear to cytoplasmic ratio low
Oval nuclei with occasional grooves and small nucleoli
Characteristic clear spaces or “windows” between cells
Gastric Wall
Fragments of gastric epithelium may be aspirated in transabdominal aspirates targeting the left hepatic lobe. The epithelium is columnar and may display mucinous features. The overall appearance should be bland and nuclei should be small and well organized.
Pulmonary/Respiratory Epithelium
Just as hepatic tissue may be acquired unintentionally in sampling of the right lower lung, it is possible to encounter pulmonary or respiratory epithelium in aspirates targeting the hepatic dome. The epithelium may show cilia, or could show features of benign pneumocytes and alveolar macrophages. Knowledge of the precise lesion location may help confirm this contamination.
Skeletal Muscle and Skin
Skin may contaminate an aspirate of the liver if it is acquired transabdominally. Fragments of skin are typically cohesive and show organized epithelium with little nuclear overlap. Skeletal muscle may be encountered if the diaphragm is aspirated.
Pigments (Table 3.2)
Table 3.2.
Pigments encountered in liver aspirates.
Pigments | Lipofuscin | Bile | Hemosiderin | Melanin |
|---|---|---|---|---|
Location | Perinuclear | Cytoplasmic, extracellular | Cytoplasmic, macrophages | Cytoplasmic, macrophages |
Color on Papanicolaou stain | Golden-brown | Green to green-brown | Golden-brown | Brown |
Color on Diff-Quik | Dark green-brown | Dark green to black | Dark blue | Dark blue to black |
Size/texture | Fine | Amorphous/globular | Coarse | Finely granular |
Refractile | No | No | Yes | No |
Context | Normal, aging | Hepatitis, Cholestasis, HCC | Hemochromatosis, transfusions | Melanoma |
Lipofuscin
This normal “wear and tear” pigment is a result of debris produced from the breakdown of intracellular lysosomes. It is a normal component of the hepatocyte and accumulates with aging. It is found in a perinuclear location and is finely granular. Lipofuscin is golden-brown in color on Papanicolaou stain, dark green-brown on Diff-Quik and is not refractile. The absence of lipofuscin in all of the hepatocytes in an aspirated sample may raise the suspicion for a neoplastic process.
Bile
Bile is produced in the hepatocytes and stored in the gallbladder. Increased accumulation of bile occurs in a number of nonneoplastic and neoplastic settings in the liver. The presence of bile in a clearly malignant process strongly supports hepatocellular origin. Bile may be variable in color, texture, and density, but is generally coarse, irregular, and amorphous. It is not refractile and stains green to green-brown on Papanicolaou stain and dark green to black on Diff-Quik (Fig. 3.6).
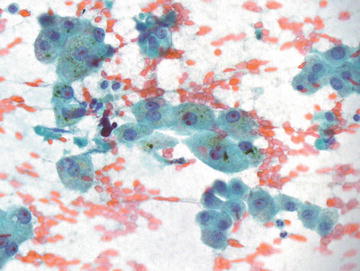

Fig. 3.6.
Hepatocytes, benign with bile: These hepatocytes show abundant granular cytoplasm with round nuclei, some showing prominent nucleoli. Globular green to brown pigment is present in many cells, most notable in the center of the image. Bile stasis may result from a number of neoplastic and nonneoplastic conditions. (Papanicolaou stain, high power)
Hemosiderin
Iron pigment, or hemosiderin, is encountered in hepatocytes, bile duct epithelial cells, and Kupffer cells. It is a coarse pigment, appearing golden-brown on Papanicolaou stain, but dark blue to black on Diff-Quik stain. It is refractile, in contrast to bile and lipofuscin pigments. Malignant hepatocytes lose ability to retain iron, so a Prussian blue iron stain may be helpful to confirm a diagnosis of HCC (negative staining), though it is not routinely used for diagnosis of HCC.
Melanin
Though not a normal component of liver aspirates, melanin may be present in cases of metastatic melanoma. The neoplastic cells of melanoma can show some morphologic overlap with hepatocytes, including prominent nucleoli and intranuclear inclusions. Melanin pigment is finely granular and brown on Papanicolaou stain and dark blue to black on Diff-Quik.
Nonneoplastic Processes and Benign Nodules
Infections
Hydatid Cysts
Though most commonly seen in South America, Australia, New Zealand, and Mediterranean countries, hydatid cysts are occasionally encountered in the United States. Overall, they are the most common cause of hepatic cysts worldwide. They are caused by dog tapeworm, Echinococcus granulosus, most commonly, or Echinococcus multilocularis. These cysts may remain asymptomatic until they become very large and can be fatal if left untreated. The cysts are typically not aspirated if suspected clinically as leakage of the contents may result in anaphylactic shock, though the actual likelihood of this is debated.
Radiographically, hydatid cysts typically present as a solitary lesion. Cyst wall calcification may be evident on imaging, and septae may be detected within the cyst. Grossly, they are usually unilocular. A peripheral rim of calcification is seen in 25 % of cases. On histologic sections, a laminated cyst wall is seen, often with acellular debris within the cyst. The wall of the cyst is surrounded by mixed acute and chronic inflammation with abundant eosinophils. Aspiration yields clear, colorless cyst fluid. Granular inflammatory debris is abundant with the presence of hooklets or scolices confirming the diagnosis. Hooklets are often degenerated, but are 20–40 μm in size, have a “shark’s tooth” shape, and are refractile (Fig. 3.7a). They are positive with acid-fast stains. Occasionally a large scolex, or worm head, composed of multiple hooklets is seen, but these may degenerate and be difficult to detect (Fig. 3.7b).
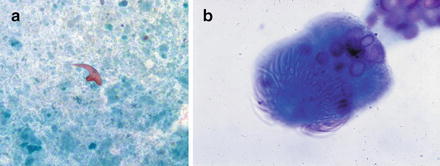

Fig. 3.7.
Hydatid cyst: (a) A hooklet from an echinococcal cyst is shown in the center of the field with a “whale’s tooth” shape. The background shows abundant granular, necrotic debris. (Papanicolaou stain, medium power). (b) A scolex from echinococcal cyst. (Diff-Quik stain, high power)
Key features:
Granular inflammatory debris
Hooklets
Often degenerated
Shark’s tooth shape
Acid fast positive
Occasional scolices
Differential diagnosis:
The differential diagnosis includes other parasitic cysts, including those caused by Entamoeba, Clonorchis, or Schistosoma species. In addition, other typically unilocular cysts which may occur in the liver include simple cyst (lined by biliary, ciliated, or squamous epithelium), ciliated foregut cyst, and pseudocyst.
Granulomatous Inflammation
In general, granulomatous inflammation is seen in a number of conditions including sarcoidosis, infection, drug reaction, primary biliary cirrhosis, and in the setting of some neoplasms (particularly hematopoietic). A specific etiology is not usually evident on cytopathologic material unless organisms are identified on aspirate smears. Cytopathologically, clusters of epithelioid histiocytes are seen admixed with variable numbers of multinucleated giant cells (Figs. 3.8 and 3.9). If granulomatous inflammation is seen on on-site evaluation, aspirates of the lesion should be sent for culture. If an accompanying CB or NCB is available, special stains for organisms can be performed, though culture is preferable.
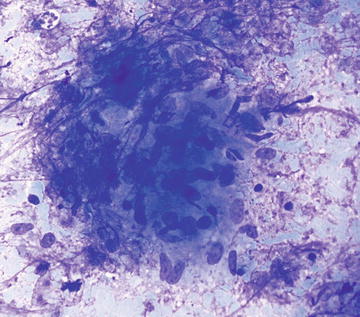

Fig. 3.8.
Granuloma: This is a loose aggregation of histiocytes showing abundant cytoplasm and oval to comma-shaped nuclei. There are scattered admixed small lymphocytes. The origin of the granulomatous inflammation cannot be determined from the aspirate smear in this case. (Diff-Quik stain, medium power)

Fig. 3.9.
Granuloma: A cluster of histiocytes is shown with abundant cytoplasm, ill-defined cell borders, and oval to comma-shaped nuclei. Small lymphocytes are embedded in the cluster consistent with a granuloma. (Diff-Quik stain, high power)
Pyogenic Abscess
Pyogenic abscesses are most often the result of bacterial infection, usually a polymicrobial infection. Escherichia coli is the most common infectious agent isolated from pyogenic abscesses. Radiographically, these lesions may show a “double-target sign” on CT. Aspirate smears show acute inflammation and cellular debris. Collection of material for bacterial, fungal, and acid fast cultures is essential.
Extramedullary Hematopoiesis
Although normally present in infants, extramedullary hematopoiesis (EMH) is an abnormal finding in adults and older children. It is seen in adults as a result of bone marrow failure. Aspirates are composed of all of the elements normally present in a bone marrow aspirate including erythroid, myeloid, and lymphoid cells at various stages of maturation (Figs. 3.10 and 3.11). The presence of megakaryocytes is the most striking feature and may be mistaken for neoplasm such as a high grade sarcoma or carcinoma if EMH is not considered (Fig. 3.12). The presence of a mixed hematopoietic population in the background should raise the possibility of EMH.
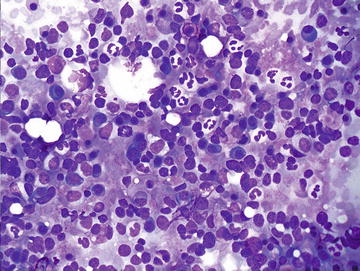
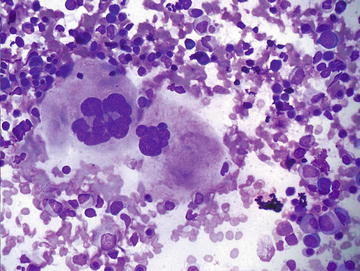
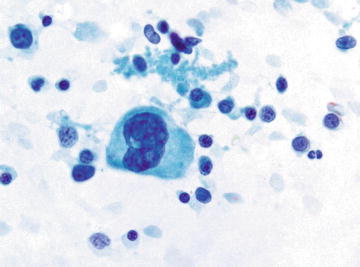

Fig. 3.10.
Extramedullary hematopoiesis: This field shows a mixture of immature hematopoietic cells including erythroid, myeloid, and lymphoid precursors. Mature neutrophils and a few plasma cells are also admixed. (Diff-Quik stain, high power)

Fig. 3.11.
Extramedullary hematopoiesis: Two megakaryocytes are shown in the center/left of the field surrounded by a mixed population of immature hematopoietic cells. Scattered megakaryocytes or bare megakaryocyte nuclei may be misinterpreted as a malignant neoplasm if EMH is not considered. (Diff-Quik stain, high power)

Fig. 3.12.
Extramedullary hematopoiesis: A megakaryocyte is shown at the center of the screen surrounded by immature hematopoietic cells. Many of the smaller cells in the background are more difficult to distinguish from mature lymphocytes on Papanicolaou as opposed to Diff-Quik preparations. (Papanicolaou stain, high power)
Hemangioma
Hepatic hemangiomas are very common lesions, found in up to 20 % of the general population. They have a female predominance and typically range from 1 to 10 cm in size. Small lesions are usually asymptomatic, but large ones may be symptomatic causing abdominal pain, anorexia, or nausea. There is a low risk of rupture, but these lesions may enlarge during pregnancy or during oral contraceptive use. They are most often solitary and have a characteristic radiographic appearance, thus they are not usually aspirated. If aspirated, cytopathologic features include abundant blood, scant paucicellular fibrous tissue, and, rarely, small groups of endothelial cells having spindle-shaped nuclei with tapered ends forming a swirling or streaming pattern (Figs. 3.13 and 3.14). CB or NCB can be quite useful if even a small amount of the lesion is obtained. The diagnosis should be considered in samples deemed “inadequate.”
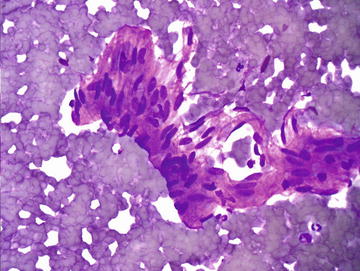
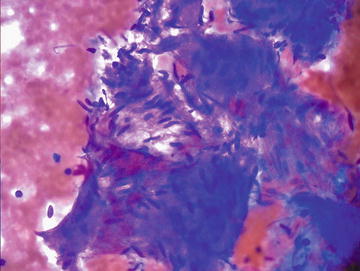

Fig. 3.13.
Hemangioma: A small fragment of cohesive spindle cells is shown and shows a suggestion of lumen formation. Aspirates of hemangiomas are typically sparsely cellular and show a bloody background. (Diff-Quik stain, high power)

Fig. 3.14.
Hemangioma: Fibrous tissue with embedded bland spindle cells is present in a background of blood. A low grade spindle cell neoplasm would be included in the differential diagnosis. Even a small NCB can be very helpful in making the diagnosis in this situation. (Diff-Quik stain, high power)
Key features:
Very scant cellularity
Bloody background
Scant fibrous stroma
Few spindled endothelial cells
Differential diagnosis:
The differential diagnosis includes unsampled neoplasm as the lesion may appear insufficient for diagnosis. If spindle cells are encountered, one should consider spindle cell neoplasms such as angiosarcoma, epithelioid hemangioendothelioma, metastatic gastrointestinal stromal tumor (GIST), or leiomyosarcoma. The degree of pleomorphism is typically much greater in the spindle cell neoplasms, and CB or NCB may be quite useful in distinguishing these lesions.
Cirrhosis
Over half of cases of cirrhosis in the United States are caused by alcoholic liver disease. Other common causes of cirrhosis include viral hepatitis, hemochromatosis, nonalcoholic steatohepatitis, and autoimmune diseases. FNA is not useful to grade cirrhosis, but cirrhotic liver may be sampled as part of a workup for a solid mass, thus knowledge of the cytopathologic features is essential. Cirrhosis is characterized histologically by diffuse fibrous bands separating nodules of hepatocytes that show varying degrees of atypia including increased nuclear size and prominent nucleoli (Fig. 3.15). Masson trichrome stain highlights the fibrosis (Fig. 3.16). Similarly, morphologic features range from normal appearing to variably atypical hepatocytes. Focal marked anisocytosis may be present, and binucleation is not uncommon. The nuclear to cytoplasmic ratio may be normal to slightly increased. Prominent nucleoli and intranuclear inclusions are commonly seen. Typically there is some fibrous tissue aspirated and admixed with hepatocytes, and there is usually abundant bile duct epithelium present, in contrast to hepatic adenomas and hepatocellular carcinoma (Figs. 3.17, 3.18, and 3.19).
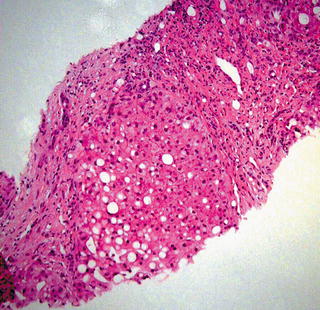
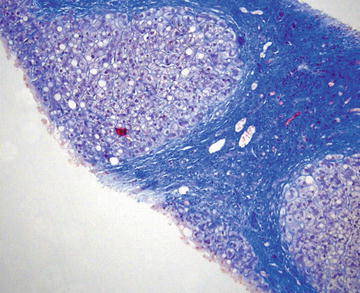
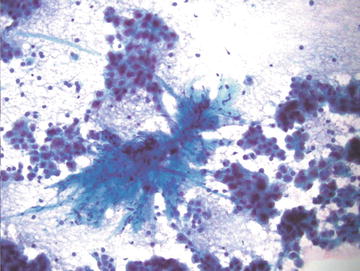
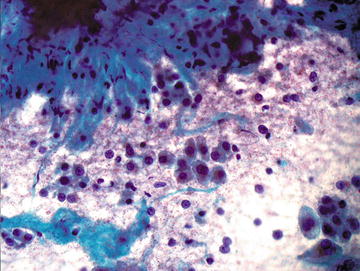
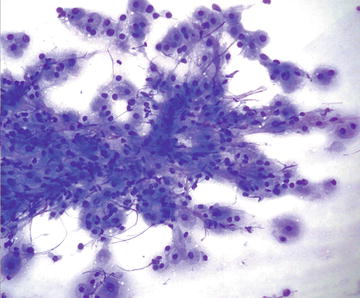

Fig. 3.15.
Cirrhosis: A nodule of hepatocytes surrounded by dense fibrous bands is shown. The hepatocytes show steatosis and some nuclear enlargement. These features of mild atypia are also seen in aspirate smears from cirrhotic livers. (Core biopsy. H&E stain, medium power)

Fig. 3.16.
Cirrhosis: This Masson trichrome stain highlights the dense fibrous tissue surrounding nodules of hepatocytes. Steatosis and nuclear atypia including enlargement can be seen. (Core biopsy, Masson trichrome stain, medium power)

Fig. 3.17.
Cirrhosis: A fragment of dense fibrous tissue is shown in the center of the field surrounded by small fragments of hepatocytes. The hepatocytes show scattered nuclear enlargement and occasional binucleation. There are very few single cells present. (Papanicolaou stain, medium power)

Fig. 3.18.
Cirrhosis: Single hepatocytes with mild nuclear enlargement and scattered binucleation are shown adjacent to a fragment of dense fibrous tissue. (Papanicolaou stain, high power)

Fig. 3.19.
Cirrhosis: This fragment shows hepatocytes with mild nuclear atypia intimately admixed with dense fibrous tissue. There is no vascular pattern or marked atypia to suggest a hepatocellular neoplasm. (Diff-Quik stain, high power)
Key features:
Normal to variably atypical hepatocytes
Focal marked anisocytosis
Binucleation
Prominent nucleoli
Intranuclear inclusions
Normal to slightly increased n/c ratio
Scant to moderate fibrous tissue
Abundant bile duct epithelium
Regenerative or Dysplastic Nodules
Macroregenerative Nodules
Macroregenerative nodules are nodules 1 cm or larger in a background of cirrhosis. They are also known as large regenerative nodules or adenomatous hyperplasia. Histologically they resemble cirrhotic nodules with an intact reticulin framework and cell plates one to two cells thick. Cytopathologically they show a uniform population of hepatocytes with some nuclear atypia. Whole cellular and nuclear enlargements are common, but the nuclear to cytoplasmic ratio is maintained (approximately 1:3). The nuclei show smooth nuclear membranes. It is also possible to encounter Mallory bodies, bile stasis, iron deposition, or focal fatty change depending on the underlying etiology of cirrhosis. The differential diagnosis includes focal nodular hyperplasia and well-differentiated hepatocellular carcinoma, though distinction is not possible on cytology.
Dysplastic Nodules
Dysplastic nodules also occur in a background of cirrhotic liver and may display either “large cell” or “small cell” change. Large cell change morphologically consists of cellular enlargement, nuclear pleomorphism, and multinucleation, and it is controversial as to whether it is a direct precursor lesion to HCC. Small cell change, on the other hand, is considered premalignant and is present in up to 50 % of cirrhotic liver explants. Morphologically, hepatocytes show overall decreased cytoplasmic volume, cytoplasmic basophilia, mild nuclear pleomorphism, and hyperchromasia. There is an increased nuclear to cytoplasmic ratio given the reduction in cytoplasmic volume. These nodules show focal loss of reticulin framework, which must be evaluated on tissue sections. The differential diagnosis includes a well-differentiated HCC, and tissue evidence of stromal invasion is needed to distinguish these two.
Focal Nodular Hyperplasia (FNH)
This typically asymptomatic condition is often discovered incidentally in patients undergoing imaging for an unrelated complaint. It is more common in women, particularly in the age range of 20–40 years. Formation of FNH is not associated with oral contraceptive use, however use may result in enlargement and increase in vascularity. There is no association with hemoperitoneum. They are usually less than 3 cm in size and arise in noncirrhotic livers. This condition is either treated with local or segmental liver resection or followed radiographically (small lesions). Radiographically, a characteristic central stellate scar is usually evident, and tissue diagnosis is often not necessary. Histologically these lesion resemble “focal cirrhosis,” that is nodules of bland hepatocytes surrounded by bands of fibrosis with bile duct proliferation at the periphery of the nodule. No portal tracts or central veins are present within the lesion. Cytopathologically, normal to hyperplastic hepatocytes may show binucleation and may be present as two cell thick groups. Otherwise the hepatocytes show normal morphology and nuclear to cytoplasmic ratio. Fatty change may be seen, and numerous bile ducts are present, some forming long tubules. Fibrous tissue is typically sparse on aspirate smears. In contrast to HCC, there is no endothelial proliferation and the reticulin framework is intact (Figs. 3.20 and 3.21).
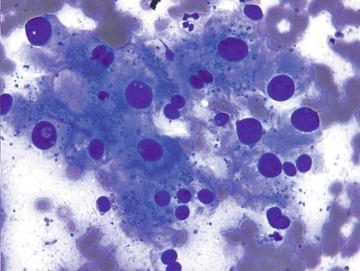
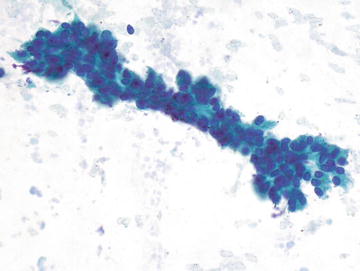

Fig. 3.20.
Focal nodular hyperplasia: These hepatocytes show anisonucleosis with some marked nuclear enlargement. The nuclei are still round and centrally placed in the cells. (Diff-Quik stain, high power)

Fig. 3.21.
Focal nodular hyperplasia: This long tubular structure is a bile duct. The nuclei are small and uniformly sized with smooth nuclear contours. Abundant bile duct epithelium, some forming large tubular structures are common in FNH. (Papanicolaou stain, high power)
Differential diagnosis:
Correlation with radiographic findings to rule out cirrhosis or normal liver is essential to the diagnosis. Based on morphology alone, the differential includes well-differentiated HCC, which typically occurs in a cirrhotic liver and shows increased thickness of cell plates (>2 cells), which can be confirmed with reticulin. A hepatic adenoma is also in the differential, but the two cannot be reliably distinguished based on cytology alone.
Hepatic (Liver Cell) Adenoma
Hepatic adenomas (HAs) are much more common in women than men and are seen most often in patients under 30 years of age. They are strongly associated with oral contraceptive use (>5 years). They are often symptomatic, and abdominal pain is common, particularly with intratumoral hemorrhage. Spontaneous rupture and hemoperitoneum occur in 10 % of cases, especially during menstruation, pregnancy, or postpartum. Radiologically, they are seen to arise in noncirrhotic livers and are most often solitary. They are homogeneous lesions with no central scar, and hemorrhage within the lesion suggests adenoma. On histologic sections, HAs are well-defined neoplasms consisting of hepatocytes with normal (nonthickened) hepatic plates (one to two cells thick). They lack portal triads and central veins, and steatosis is common. FNAs are hypercellular and show a monotonous population of normal hepatocytes. Hepatocytes occur singly and in cords two cells thick and show minimal nuclear atypia with normal nuclear to cytoplasmic ratio. Bile ducts should be absent, though the diagnosis of HA is not completely excluded by the presence of bile ducts, as they may be sampled from surrounding normal liver.
Differential diagnosis:
Just as in FNH, it is essential that one correlate the cytomorphology with radiographic findings to rule out cirrhosis or normal liver. The differential includes a well-differentiated HCC and immunostains, including CD34, can be helpful in making the distinction. FNH is also in the differential, but the two cannot be distinguished reliably on FNA.
Bile Duct Adenoma
These are small (<2 cm) lesions, commonly encountered during intra-abdominal surgery due to their subcapsular location. They are well circumscribed and histologically composed of a bland bile duct proliferation and fibrosis. They are not often aspirated, but when they are, they show abundant bile duct epithelium in flat sheet and scant hepatocytes.
Differential diagnosis:
The differential diagnosis includes von Meyenburg complex, which typically show multiple lesions and have intraluminal bile present. Well-differentiated cholangiocarcinoma or metastatic adenocarcinoma may be considered, though bile duct adenoma should not have nuclear atypia. Radiographic and clinical correlation is also important.
Angiomyolipoma
Angiomyolipoma (AML) is a rare primary liver tumor. It occurs in male and female patients with similar incidence, and is most common in patients 30–40 years of age. Most are asymptomatic. They display similar histologic and cytomorphologic findings to AML in kidney, including a combination of fat, smooth muscle and blood vessels. The fat component is more often scant or absent in liver versus kidney. Spindle cell lesions are considered in the differential. The main pitfall is the epithelioid variant, which may be misinterpreted as carcinoma, particularly HCC, due to cells with abundant eosinophilic cytoplasm and formation of trabeculae. The distinction is based on absence of cirrhosis in background liver and lack of nucleoli, bile, and fatty change within tumor cells. Immunoreactivity for HMB-45 and SMA, along with negativity for cytokeratin, confirms the diagnosis of AML.
Hepatobiliary Cystadenoma
This rare lesion is seen predominantly in middle-aged women and is associated with cystadenomas of the pancreas and ovary. Patients may present with abdominal pain. They have a thick septated wall and histologically are lined by a single layer of mucinous epithelial cells (flat, cuboidal, or columnar). The stroma in women shows ovarian morphology and is positive for ER and PR. Importantly, up to 25 % undergo malignant transformation to cystadenocarcinoma, so surgical resection is indicated. On cytology smears, they are composed of benign-appearing ductal epithelium with mucinous cells and goblet cells having bland, uniformly sized nuclei without nucleoli. There is often extracellular mucin. Ovarian stroma is not typically aspirated, and there should be absent or very few hepatocytes (from needle pickup only). Hepatobiliary cystadenocarcinoma is the main differential diagnostic consideration and shows atypical glandular epithelium. Surgical resection may be required to distinguish the two.
Primary Hepatic Malignant Neoplasms
Improvements in dynamic imaging techniques, such as contrast-enhanced ultrasound and dynamic magnetic resonance imaging (MR), have greatly increased the accuracy of diagnosis of hepatocellular carcinoma in patients with cirrhosis, obviating the need for tissue confirmation in many cases with the classic presentation of HCC. Thus, the nodules that cytopathologists are asked to evaluate are smaller (less than 2 cm) and show fewer of the characteristic clinical and radiographic features of HCC than hepatic nodules evaluated in the past.
Cholangiocarcinoma is the second most common primary liver malignancy, though far less common than HCC. It should be distinguished from metastatic carcinomas, which are discussed in detail later in this chapter.
Hepatocellular Carcinoma (HCC)
Though more rare in the United States, HCC is the sixth most common malignancy and third leading cause of cancer deaths worldwide. It is most common in Asia and sub-Saharan Africa due to endemic hepatitis B and aflatoxin B1. Incidence is rising in the West, however, as a consequence of increased Hepatitis C infection and nonalcoholic fatty liver disease. Risk factors for HCC include cirrhosis as 90–95 % of HCCs arise in a cirrhotic liver and the incidence of HCC is 1–6 % in cirrhotic patients. Particular associations include alcohol abuse, Hepatitis B and C viral infection, metabolic liver disease, environmental carcinogens, smoking, and hereditary hemochromatosis. It is important to realize, though, that in the United States metastases are 20–40 times more common in noncirrhotic livers. Primary hepatic lesions, primarily HCC, do outnumber metastases in the cirrhotic liver by three to one. Over 80 % of masses larger than 2 cm in a cirrhotic liver are HCC and 75 % of masses <2 cm in a cirrhotic liver are HCC. Overall the tumor has a very poor prognosis. FNA has a high sensitivity for the diagnosis of HCC, but in those with a negative (false) diagnosis, repeat FNA only returns a definitive diagnosis in approximately one-third due to sampling of small lesions or vast necrotic areas in large tumors.
Clinical Features, Signs, and Symptoms
Clinically, patients with HCC most often have underlying cirrhosis. Symptoms include new abdominal pain, recent hepatomegaly, hemoperitoneum, persistent fever, weight loss, a sudden increase in alkaline phosphatase, increased AST to ALT ratio, and persistent leukocytosis. Serum alpha-fetoprotein (AFP) is typically elevated, though 20 % of all HCCs produce no AFP. An AFP greater than 400 ng/mL suggests HCC, but 30 % of patients with HCC smaller than 2 cm have normal AFP. The presence of these signs and symptoms, particularly in a cirrhotic patient, should prompt consideration of a diagnosis of HCC.
Radiographic Features
HCC most often presents as a single large mass but may have multiple smaller nodules. Rarely, diffuse liver enlargement is present. Dynamic imaging modalities (US and MR) have very high accuracy, sensitivity, and specificity (99.6, 100, and 98.9 %) for HCC. In fact, no tissue confirmation is required if there is intense enhancement in the arterial phase with contrast washout in the delayed venous phase on one dynamic imaging modality. Nodules between 1 and 2 cm in cirrhotic livers require concurrence of two imaging modalities, otherwise tissue confirmation is required. Some suggest an every 3-month surveillance approach in lesions <1 cm, but 68 % of these nodules detected in cirrhotic livers are HCC.
Histology
On tissue sections, HCC shows a loss of normal hepatic and sinusoidal architecture, including cell plates greater than two hepatocytes thick, absent portal triads and central veins, and hepatocyte atypia, which increases with increasing grade. The architecture may be trabecular (most common), acinar, or solid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







