Chapter 9 Laparoscopic Totally Hand-Sutured Roux-en-Y Gastric Bypass for the Treatment of Morbid Obesity
Indications for bariatric surgery
• The American National Institute of Health, along with European and international guidelines, recommend bariatric surgery in adults with either a body mass index (BMI) of 40 kg/m2 or higher or a BMI between 35 and 40 kg/m2 with obesity-related comorbidity. The comorbidities attributed to morbid obesity may include type 2 diabetes mellitus, hyperlipidemia, hypertension, coronary heart disease, cardiomyopathy, cerebrovascular disease, obstructive sleep apnea and hypoventilation syndrome, asthma, pseudotumor cerebri, osteoarthritis of spine and weight-bearing joints, female urinary incontinence and infertility, gastroesophageal reflux, cholelithiasis, malignancy (increased risk for colon, ovarian, and endometrial cancer in particular), and psychological disorders.
• Severe cardiorespiratory comorbidities, unless preoperatively remediable, may preclude safe bariatric surgery.
• Severe mental disease not responding to treatment, cognitive retardation, and malignant hyperphagia are generally considered absolute contraindications to bariatric surgery.
• It is advisable to consider patients with inflammatory bowel disease, pelvic or retroperitoneal radiotherapy, or large and complex abdominal wall incisional hernias for a sleeve gastrectomy or adjustable gastric band rather than a gastric bypass.
• A planned pregnancy is not a contraindication to gastric bypass because surgery does not usually affect the course of pregnancy and the health of the baby; however, a 12- to 18-month period of contraception until weight loss stabilizes is recommended.
• Bariatric surgery is available for selected adolescents who approached maturity, but only after extensive multidisciplinary workup; recently, a threshold BMI of 40 kg/m2 (with severe comorbidities) or 50 kg/m2 (with less severe comorbidities) has been proposed.
• Although there is no fixed age limit that is widely agreed on, consideration should be given to the established comorbidities and the risk for surgery in subjects older than 65 years.
Preoperative assessment
• The input of a psychologist or psychiatrist is invaluable to address psychiatric disorders such as psychotic personality, affective disorders, alcoholism, drug abuse, mental retardation, and eating disorders, especially bulimia nervosa and binge-eating disorder.
• Endocrine evaluation should focus on the possible diagnosis of hypothyroidism or Cushing syndrome.
• A history of ischemic heart disease calls for objective assessment such as myocardial perfusion scintigraphy and direct coronary angiography. Evaluation of cardiac function with trans-thoracic, or preferably trans-esophageal, echocardiography when clinically indicated should exclude patients with moderate to severe cardiac dysfunction from undergoing surgery.
• Obstructive sleep apnea and hypoventilation syndrome are common in morbidly obese people, and polysomnography is routine in most bariatric centers. These patients are at increased risk for postoperative complications, especially thromboembolic complications and anastomotic disruption. Patients with severe sleep disorders should be managed preoperatively with respiratory support to improve respiratory function and reduce right heart strain; admission to a high-dependency unit or to a ward with expertise in the management of continuous positive-airway pressure support is advised for the first night after surgery.
• Although routine upper gastrointestinal endoscopy is not necessary, the preoperative detection of iron deficiency—even in the absence of anemia—warrants upper and lower endoscopic or radiologic evaluation to exclude malignancy because the bypass will preclude endoscopic access to the excluded stomach, and morbidly obese patients are at increased risk for colonic malignancy. Although most hiatal hernias could be repaired at the time of the gastric bypass, consideration might need to be given to laparoscopic repair of a very large hiatal hernia as a first stage, with the bypass deferred for 3 to 6 months to reduce tension at the gastrojejunal anastomosis and the potential risk for anastomotic leak.
• Barium upper gastrointestinal fluoroscopy, done preoperatively, may diagnose ailments such as gastroesophageal reflux disease, hiatal hernia, gastric tumors, and duodenal ulcers.
• The role of routine preoperative ultrasonography of the gallbladder and concomitant laparoscopic cholecystectomy for asymptomatic cholecystolithiasis is controversial. At a minimum, symptoms of gallstones should be sought preoperatively and concomitant cholecystectomy carried out if these are confirmed.
• Consideration might need to be given to the insertion of a temporary caval filter, which is then removed 5 to 6 weeks after surgery, in the patient with a history of thromboembolism. Long-term anticoagulation might be a sufficient alternative in patients with recurrent deep venous thrombosis or hypercoagulable hematologic disorders.
Preoperative preparation
• A preoperative “liver-reducing” diet that is low in carbohydrates and fat is recommended for a minimum of 2 to 3 weeks before surgery and appears to reduce the size of the left lobe of the liver and therefore facilitate laparoscopic access to the stomach.
• Prophylaxis against thromboembolic complications is considered essential by most. Although there is no consensus, commonly applied measures include low-molecular-weight heparin administered preoperatively and continued for 1 to 4 weeks after surgery, graduated-compression stockings for 2 to 6 weeks, and the application of a pneumatic sequential venous compression system to the lower extremities during and after surgery.
• Single-dose intravenous broad-spectrum antibiotic prophylaxis at induction of anesthesia is sufficient for most. However, some surgeons who use the circular stapler for the creation of gastrojejunostomy recommend oral antibiotics (e.g., neomycin and erythromycin) as well as mechanical bowel preparation to “sterilize” the bowel lumen and minimize the risk for wound infection.
Operative technique
Patient Positioning and Placement of Trocars
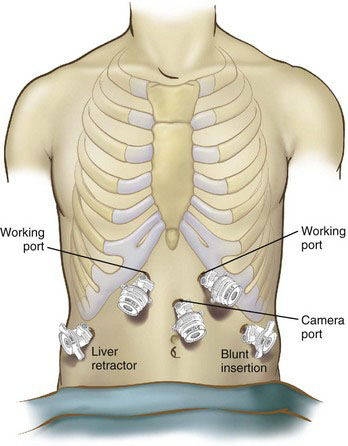
The patient is placed in Lloyd-Davies (French) position. Central venous and arterial lines are placed at the discretion of the anesthesiologist but are rarely necessary. We directly access the peritoneal cavity with a bladeless 5-mm-port trocar just below the left costal margin at the anterior axillary line, insufflate the abdomen to 20 mm Hg until five standard ports are safely placed (Fig. 9-1), and then reduce the pneumoperitoneum to 15 mm Hg CO2. In patients with previous abdominal surgery, once the pneumoperitoneum has been established, the initial 5-mm port can be exchanged with a 10- to 12-mm port, and further ports are placed under vision with a 30-degree laparoscope to enable adhesiolysis; standard ports are subsequently placed as shown in Figure 9-1.
Construction of the Jejunojejunal Anastomosis
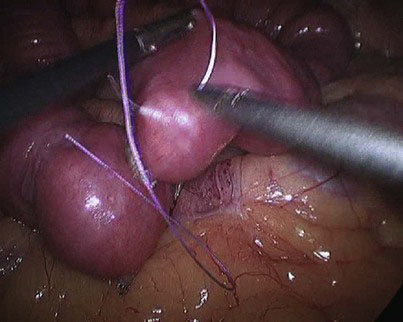
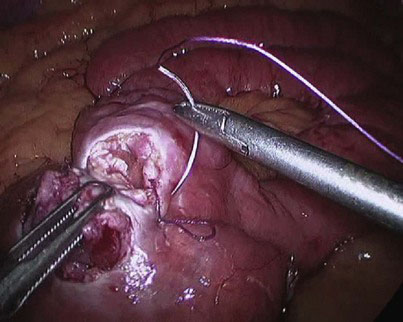
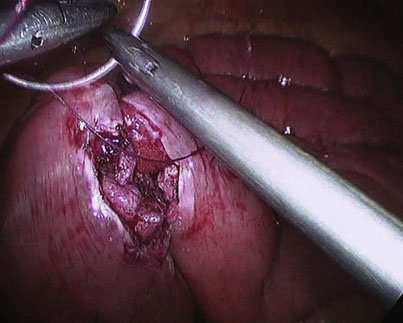
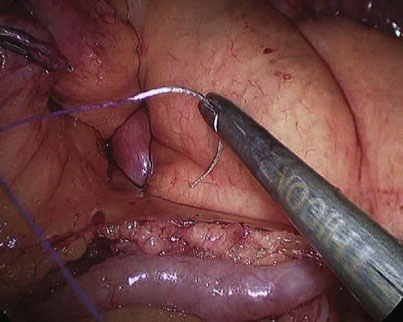
At the chosen distance, the jejunum is divided with an endostapler (we prefer 60-mm, 2.5-mm thick, white cartridge load), and the mesentery is split with the Harmonic scalpel down to its root to allow for a tension-free gastrojejunal anastomosis. The distal jejunal loop is marked with a Vicryl suture to avoid confusing it with the proximal end when the jejunojejunostomy is to be constructed and to facilitate its delivery to the gastric pouch for construction of the gastrojejunostomy (Fig. 9-2). The alimentary limb is measured, and a side-to-side jejunojejunal anastomosis is constructed. We prefer to hand-suture this anastomosis because we had encountered postoperative bleeding from the stapled jejunojejunostomy that necessitated laparoscopic reexploration to evacuate an obstructing hematoma, blood transfusion, and prolongation of hospital stay, as well as intussusception at the stapled jejunojejunostomy that appears to be related to the presence of a staple line acting as a lead point; the authors’ experience with both techniques indicates that the sutured anastomosis is safer. A seromuscular layer is fashioned using a 15-cm 3-0 Vicryl suture, the jejunal loops are then opened for approximately 2-cm distance using the Harmonic scalpel (Fig. 9-3), and a second posterior layer is then fashioned using a continuous 26-cm 3-0 Vicryl suture that takes full-thickness bites posteriorly (forming a second posterior layer) and seromuscular bites of a single layer anteriorly (Fig. 9-4). It is difficult to check for leak at the jejunojejunostomy, and we rely on meticulous technique. In the literature, the leak rate of the jejunojejunostomy is extremely low, and we have encountered none.
An alternative option is to double-staple the jejunojejunal anastomosis; this involves firing the stapler (45-mm long, 2.5-mm thick white cartilage) in one direction to fashion a side-to-side jejunojejunostomy, and then firing a second cartilage in the opposite direction before suture-closing or stapling the common enterotomy (the Frantzides-Madan triple-stapling technique is described in Chapter 6 of the Atlas of Minimally Invasive Surgery, 2009; see Suggested Readings at the end of this chapter). Double-stapling the jejunojejunostomy avoids stenosis at its proximal end where the alimentary limb joins, but care should be taken to ensure that the second stapler firing crosses the first staple line if a gap within the posterior layer of the jejunojejunal anastomosis is to be avoided with subsequent leak; the posterior staple line should always be inspected for such gap if a stapled technique is employed. It is also imperative to check the stapled anastomosis for bleeding before the common enterotomy is closed.
The mesenteric defect is then suture-closed using a 15-cm continuous 3-0 Vicryl suture (Fig. 9-5). Although some surgeons recommend closure of the defect with a nonabsorbable suture, we have not encountered any herniation at this mesenteric defect—having used absorbable sutures to close it—in more than 1500 cases, and have always found the defect closed at repeat laparoscopy in the occasional patient for investigation of abdominal pain with the intent to look for or exclude internal small bowel herniation.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree