Fig. 50.1
Conversion (%) according to body mass index (From Denost et al. [27]; used with permission
Tumor Staging
The TNM system, as defined by the American Joint Committee on Cancer, is the most commonly used system and is based on the depth of local tumor invasion (T stage), the extent of regional lymph node involvement (N stage), and the presence of distant metastasis (M stage) [28].
Local Tumor Staging
As part of a full physical examination, rectosigmoidoscopy should be performed in conjunction with a digital rectal examination to determine the length of anal canal, the distance of the lesion from the anal verge and the anal ring, tumor mobility, and to assess tumor position in relation to the sphincter complex.
Clinical staging of the primary tumor by endorectal ultrasound (EUS) or dedicated high resolution rectal MRI should be performed. Circumferential resection margin (CRM), defined as the shortest distance between the rectal tumor (including noncontiguous tumor) and the mesorectal fascia (TME) is considered positive when it is ≤1 mm. EUS and MRI are almost equivalent for accurate measurement of the depth of extramural tumor spread or nodal status involvement. Demonstration of accurate measurement in predicting CRM status with MRI compared with the histopathological reference standard in the MERCURY Study enabled accurate preoperative prognostication [29]. Thus, preoperative MRI are now often preferred to EUS. However, the accuracy of radiological restaging post-CRT is impaired due to the difficulty in differentiating fibrotic or reactive tissue from residual [30]. Endoscopic ultrasonography has been dismissed outright as a restaging [31–33]. Diffusion-weighted MR [34, 35] and fusion positron emission/computed tomography [36] have shown some promising results but larger scale studies are needed.
Distant Metastases Staging
The liver and lungs are the most frequent sites of metastatic disease from rectal cancer [37, 38]. Preoperative chest, abdomen, and pelvis CT scan should be routinely performed before the surgical resection of rectal cancer to detect and assess local organ penetration or synchronous metastases, which may require a change in the treatment strategy.
Preoperative Treatment
The preoperative management of high rectal cancer is very close to the management of colon cancer. No preoperative treatment is recommended except for T4 tumor. The patients with T3, T4 or N+ mid or low rectal cancer should receive long-course preoperative radiotherapy (45 Gy in 25 fractions during 5 weeks) in association with concomitant chemotherapy in first and fifth weeks [39]. The chemotherapy comprised a continuous infusion of fluorouracil (5FU: 350 mg/m2/day during 5 days) in association with leucovorin (20 mg/m2/day in bolus just before the infusion of fluorouracil) [40]. Some T2 lesions close to the anal canal could also receive neoadjuvant treatment in order to facilitate ultralow sphincter preservation [41]. High rectal tumors (>10 cm from the anal verge) do not receive neoadjuvant treatment. Since 2006, patients with mid rectal cancer and a circumferential margin >3 mm at the magnetic resonance imaging could be operated by TME surgery alone [39]. Surgery was performed 6 weeks after the end of radiotherapy. The ongoing French multicenter GRECCAR six trial is evaluating the optimal interval between irradiation and surgery (between 7 and 11 weeks) [42].
Surgical Strategies
Surgical Management of High and Mid Rectal Cancer
For high and mid rectal tumors, the rectum was transected 5 cm below the lower edge of the lesion [20]. Partial mesorectum excision is recommended for high rectal cancer and Total mesorectum excision is recommended for mid rectal cancer with stapled colorectal anastomosis.
Surgical Management of Low Rectal Cancer
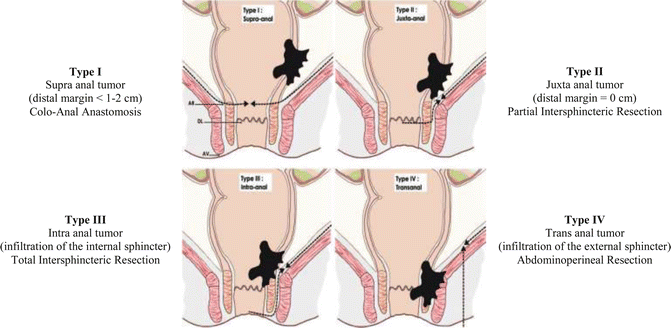
Management of low rectal cancer is very different of high and medium rectal cancer. This management is composed by the three following steps: firstly, classification of low rectal cancer in four types [43], secondly, standardization of surgery in four operations, and finally, anticipation of surgery before and decision after neoadjuvant treatment.
The Surgical Classification of low rectal cancer separates patients with rectal cancer below 6 cm from the anal verge in four groups according to the location of the tumor from the anal sphincter (surgical anal canal) (Fig. 50.2):
Type I: Supra anal tumors: lesions located >1 cm from the anal ring
Type II: Juxta anal tumors: lesions located ≤1 cm from the anal ring
Type III: Intra anal tumors: lesions with infiltration of the internal anal sphincter
Type IV: Trans anal tumors: lesions with infiltration of the external anal sphincter or levator ani muscles
Standardization of surgery defines four surgical procedures, each dedicated to the four types of low rectal cancer:
Type I: Coloanal anastomosis (CAA), the internal sphincter is preserved
Type II: Partial intersphincteric resection (pISR)
Type III: Total intersphincteric resection (tISR)
Type IV: Abdominoperineal excision (APR)
Classification of low rectal cancer is part of the initial staging of the tumor and is performed by consensus including digital examination by the surgeon, endorectal ultrasound and MRI. It must be performed before neoadjuvant treatment. Rectal palpation is performed with and without voluntary anal contraction to check the exact distance of the tumor from the top of the anal canal. Examination under anesthesia is sometimes necessary, especially when the anal canal is involved or in case of fixed tumors. Rigid rectoscopy informs on tumor location and on distance between the tumor and the dentate line. Endorectal ultrasound and magnetic resonance imaging are necessary for tumor staging and to confirm clinical examination, in term of relation and distance between the tumor and the anal sphincter. Preservation of the intersphincteric plane at MRI is the key point to differentiate types I, II and III low rectal cancers suitable for conservative surgery, from type IV treated by APR.
The advantages of this classification are to facilitate decision making between sphincter preservation and APR in low rectal cancer, in order to propose more sphincter preservation by using techniques of partial and total intersphincteric resection. This classification will allow to assess more homogeneously treatment of low rectal cancer and outcomes between institutions. Finally, it could also be used to convert an APR to a sphincter saving procedure in case of downstaging after irradiation.
Organ Preservation for Rectal Cancer
Rectal excision is the standard treatment of rectal carcinoma. Local excision removing the tumor transanally and leaving in place both the rectum and the mesorectum is a common option at present only in some early rectal cancers. Those are T1 tumors infiltrating the superficial part of the submucosa (Sm1). For the other tumors, i.e. T1Sm2–3, T2, T3 and T4 rectal cancers, rectal excision is conventionally the only chance of cure for the patient. A new concept is to propose local excision in good responders after neoadjuvant chemotherapy in locally advanced rectal cancer. This strategy is called organ preservation. The preliminary results of the ACOSOG US trial suggest feasibility in T2 low rectal cancer with 44 % of pathologic complete response and low surgical morbidity [44]. Long-term results are not yet available. The ongoing French GRECCAR 2 trial (end of inclusion in January 2013) aims to clarify if a T2 tumor could be include in this strategy [45].
50.3 Perioperative Management
Perioperative management involves dieticians, nurses, surgeons and anesthesiologists. All patients had a preoperative bowel preparation. When an ostomy is a consideration, potential site of the ostomy should be marked preoperatively to ensure optimal fitting of the device. Postoperative analgesia was ensured by intravenous morphine chlorhydrate (patient-controlled administration) at a maximum of 4 mg per hour with a single dose of 1 mg and free interval of 10 min for 1–2 days. Postoperative protocol involves nasogastric tube removal at the end of the surgical procedure, fluids intake on postoperative day 1, oral solid food at postoperative day 2, and pelvic drain and catheter removal on postoperative day 3. Postoperative evaluation of C-reactive protein (CRP) is systematically realized at day 3. A CT-scan is performed when abscess or anastomotic leakage is clinically (fever, abdominal pain, anal, vaginal or drain purulent output) or biologically (CRP >100 mg/L) suspected.
50.4 Surgical Technique of Laparoscopic Rectal Surgery
Installation
The patient is positioned in lithotomy position. The surgeon stands to the right of the patient and the monitor is located in his line of vision at the left of the patient. The camera assistant and the scrub nurse stand to the right of the patient, respectively at the left and at the right of the surgeon. A second assistant stands between the legs of the patient. The operative technique was achieved by a five-port procedure (Fig. 50.3). The laparoscopic procedure was standardized:

Fig. 50.3
Laparoscopic port sites
Step 1: Vascular Ligation
The first step of the procedure consists in grasping the inferior mesenteric vein, which correspond to the first anatomical landmark, and dissecting adhesions between the vein and the proximal jejunum. The primary dissection of the vein from the posterior attachments is particularly useful to identify the plane of dissection of the told fascia, and to dissect the upper border of the inferior mesenteric artery. This step avoids pitfalls during the artery dissection. In order to facilitate the inferior mesenteric artery (IMA) ligation, the second anatomical landmark is the presacral area. Once the vein is completely free, the dissection continues by the exposition of the presacral area using a grasper placed on the sigmoid mesentery giving an optimal view of the promontory. An incision of the peritoneum is performed exactly 2 cm in front of the promontory allowing the beginning of the dissection of the upper part of the mesorectum, which is also the distal part of the IMA. To insure the good plane of dissection, the ‘angel hairs’ of the mesorectal space are opened and the proximal mesorectum is dissected posteriorly on a few centimeters. The objective is to connect by an horizontal incision the area of the proximal part of the mesorectum and the area located under the vein already dissected. This step allows identifying the artery exactly 2 cm from the aorta in order to preserve the sympathetic nerves and facilitate the end of the dissection of the IMA by using an accurate instrument (Fig. 50.4a). The IMA must be completely free before performing ligation or transection. A various kind of ligation can be used such as clips, suture or modern devices. Thermal fusion can be used in vessels no more than 7 mm without calcification, but requires to stop any tension of the artery before transecting to avoid incomplete sealing. After IMA ligation, the next step is left colonic mobilization.
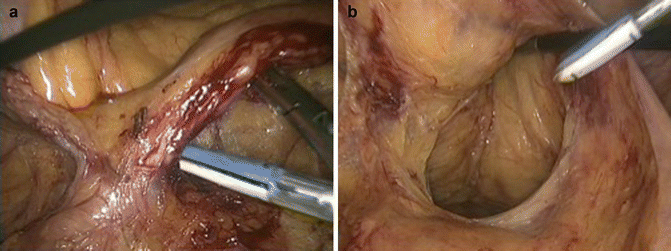

Fig. 50.4
Laparoscopic steps of rectal excision. (a) High ligation of the inferior mesenteric artery; (b) extra-fascial dissection of the mesorectum
Step 2: Mobilization of the Left Colon and the Splenic Flexure
Both surgeon and assistant push up the mesentery to dissect the told fascia via a medial approach. The dissection using scissors begins in the left iliac fossa, preserving the gonadal blood vessels and the left ureter. The advantage of the medial approach is an optimal view and exposure of the planes of dissection. The told fascia dissection is continued as high as possible, especially in front of the Gerota’s fascia and close to the splenic flexure. During this step, the IMV is preserved and served as potential landmark to identify the pancreas. By dissecting from the posterior approach, the incision of the transverse mesentery permits to identify the pancreas and to enter in the lesser sac. In some difficult cases, the complete dissection of the terminal part of the IMV improves the view of the pancreas giving the plane to open the lesser sac. The complete anterior dissection of the pancreas makes the transverse mesentery totally free and finishes the medial approach. The IMV is then transected close to the pancreas, or 5 cm below if a left transverse colic artery is visualized in this area, to avoid colonic ischemia. The last part of colonic mobilization is the lateral mobilization. By pushing the sigmoid on the right, a peritoneal incision is performed along the left border of the sigmoid, including the rectosigmoid area. The division of the lateral peritoneum continues along the descending colon and finishes at the splenic flexure. In order to avoid splenic decapsulation, adhesions of the greater omentum and the splenocolic ligament must be dissected. The medial approach greatly facilitates this last step of mobilization.
Step 3: Dissection of the Mesorectum
The first step of the dissection of the mesorectum is the high posterior dissection. This extra facial anatomical dissection of the mesorectum can be performed by using scissors and monopolar coagulation, harmonic scalpel (UltracisionTM, Ethicon Endosurgery, Cincinnati, Ohio, USA) or thermal fusion (LigasureTM, Covidien, Mansfield, Massachusetts, USA). The rectum exposure is achieved by pulling up vertically the rectum using a supra-pubic grasper. The anatomical landmarks are the retrorectal space medially, and the hypogastric nerves laterally. The dissection begins medially 2 cm in front of the sacral promontory and continues caudally and posteriorly at 45 °C along the presacral area, using scissors. The good plane of dissection is located between the mesorectum (yellow tissue) and the presacral fascia (gray tissue). The medial dissection must be stopped 10 cm below the promontory at the level of the retroscral ligament corresponding to the fusion between the presacral and the mesorectal fascias. Adequate tension to the tissues facilitates dissection. Non-traumatic instruments are used without grasping the mesorectum to preserve its integrity. This medial posterior dissection avoids injuries of both superior hypogastric plexus and hypogastric nerves.
The second step is the right lateral dissection after identification of the hypogastric nerves. A cephalic traction of the superior hypogastric plexus at the sacral promontory level induces tension of the hypogastric nerves, which can be visualized as a “fiber” through the soft pelvic tissues or below the peritoneum. An incision of the peritoneum along the right side of the rectum is performed down to the anterior reflection. Lateral pelvic dissection from the hypogastric nerve to the presacral area is then performed connecting the previous medial high posterior dissection. After full right lateral mesorectum mobilization, the same dissection is performed on the left side.
The third step consists in the high anterior dissection. Seminal vesicles in men and cervix in female are the landmarks, which need to be identified. The upper rectum is grasped with cephalic and left lateral traction giving a tension to the opposite anterior and right sides of the rectum. A peritoneal incision is performed anteriorly 2 cm above the Douglass pouch and connects the lateral right incision performed during the previous step. Distal dissection from 1 to 2 cm of right anterior peritoneum allows discovering the right seminal vesicle, which must be completely isolated. In order to preserve both small efferent nerves along the vesicles and the mesorectum which is very thin and fragile at this level, the dissection must be lead close to the seminal vesicles. In female, pelvic exposure is facilitated by uterus fixation to the anterior abdominal wall by using a supra-pubic stitch. The anterior incision of the peritoneum permits to identify the cervix and then to connect the high lateral dissection.
The fourth step is low lateral dissection using the inferior hypogastric plexus as anatomical landmark. The objective is to transect the lateral ligament of the rectum. By following dissection along a virtual line between the seminal vesicle and the hypogastric nerve, the pelvic plexus appears as a 2–3 cm triangle located along the lateral sidewall of the pelvis. To free the mesorectum from the pelvic plexus, some attachments due to vessels and nerves coming from the plexus to the mesorectum have to be cutted. To prevent from plexus injury, perfect hemostasis and adequate traction of the tissue are essential.
Then, the rectum is pulled up for optimal visualization of the low pelvis. After incision of the rectosacral ligament, dissection is continued distally and posteriorly along the levator ani muscle. Presacral nerves (S2–S4) are identified at the mid part of the sacrum and preserved by dissecting close to the mesorectum. For a sphincter saving procedure, the dissection is performed until the top of the anal canal. For an APR, dissection is stopped earlier at the coccyx to avoid disconnection between tumor adhesion and levator ani muscles.
The end of the TME procedure consists in dissecting anteriorly and caudally taking care not to injure Denonvilliers’ fascia, the seminal vesicles, the prostate and the vagina. This last step is particularly challenging in men. The oncologic low anterior dissection must be performed in front of the Denonvilliers’ fascia, which can be considered as the anterior part of the mesorectum. Care is taken to do an anatomical dissection which preserves both the Denonvilliers’ fascia and the anterior mesorectum, which needs to be removed together to achieve a radical oncologic laparoscopic TME excision.
Step 4: Rectal Transection and Reconstruction
For high and mid rectal cancer, the rectum is transected 5 cm below the lower edge of the lesion [20], achieving respectively a partial or a total mesorectal excision. After full mobilization of the rectum, pushing on the perineum facilitates rectal division. Stapler is introduced by the 10 mm supra-pubic port (Fig. 50.5a) [46]. The specimen is then removed from a supra-pubic 6 cm incision. A wound protector is necessary to decrease risks of port-site and local recurrences. Reconstruction with a colonic pouch or a straight colorectal anastomosis is performed laparoscopically (Fig. 50.5b). A colonic pouch is performed if the rectal stump is shorter than 5 cm. The quality of the anastomosis is checked by the completeness of the doughnuts and by a transanal air test.
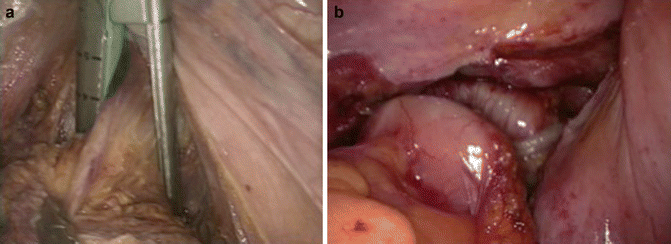

Fig. 50.5
Colorectal anastomosis. (a) Supra-pubic stapler; (b) circular colorectal anastomosis
In low rectal cancer, a low colorectal stapled anastomosis could be very difficult to perform laparoscopically, especially for men, narrow pelvis, and bulky tumors. The alternative is to perform a manual coloanal anastomosis instead of stapled anastomosis which can compromise oncologic margins and anastomosis healing. For very low tumors, intersphincteric resection is used to achieve sphincter preservation with safe distal margin [47]. The low pelvic dissection during the laparoscopic coloanal procedure can be achieved either by the laparoscopic approach or by the perineal approach. In both approaches, rectal transection is performed transanally. The anal canal is exposed by a self-holding retractor (Lone Star Retractor®, Lone Star Medical Products Inc., Houston, TX). The specimen is removed through the anal canal except for thick mesentery or anal stricture, and a hand-sewn side-to-end coloanal anastomosis is performed (Fig. 50.6). For the first laparoscopic approach, surgical procedure was previously described. For the first perineal approach, instead of doing high rectal dissection laparoscopically followed by dissection of the low rectum through the perineum, the procedure begins by a transanal perineal step [48]. A gauze was introduced into the rectum to limit the risk of seeding. The rectum was transected at least 1 cm below the lower edge of the tumor. Then, rectum was closed transanally by suture to avoid intraoperative tumor spillage and facilitate exposure. The perineal dissection of the low rectum was performed transanally along the levator ani muscles. The plane of dissection began into the intersphincteric plane and continued posteriorly to the sheath of the pelvic floor for at least 5 cm. The sheath of the levator ani muscles, which was usually thickened due to irradiation, was then transected to join the plane of the abdominal dissection. Thus, the perineal dissection permitted to dissect as far as possible from the distal rectal wall, where the mesorectum is usually lacking, and therefore to maintain optimal distance between the tumor and surgical resection. The perineal dissection was conducted posteriorly and anteriorly up to 10 cm from the anal verge. It was easier in female than in male due to the shorten length of the anal canal in the former. After the perineal dissection of the distal rectum, a conventional laparoscopic procedure is performed. Surgery is usually easier and faster due to previous low rectal dissection. The primary transanal perineal step represents an interesting surgical alternative option to avoid problems associated with stapling and to decrease the rate of conversion to an open procedure.
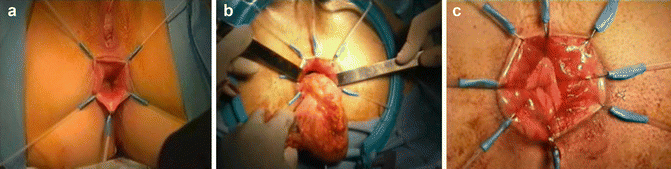

Fig. 50.6
Perineal step. (a) Perineal exposure; (b) transanal specimen extraction; (c) hand-sewn coloanal anastomosis
A presacral suction drain and a temporary loop ileostomy are used in all TME procedures. The loop ileostomy is closed 8–12 weeks after surgery.
50.5 Quality of Surgery
Criteria of surgical resection quality for rectal cancer included circumferential resection margin [49], number of lymph nodes harvested and Quirke’s graded assessment of completeness of mesorectal excision protocol [4].
Grade 3: Good, intact mesorectum with only minor irregularities of a smooth mesorectal surface and no defect deeper than 5 mm;
Grade 2: Moderate, moderate bulk to the mesorectum but irregularity of the mesorectal surface, moderate coning of the specimen towards the distal margin;
Grade 1: Poor, little bulk to the mesorectum with defects down into the muscularis propria and/or very irregular circumferential resection margin.
50.6 Outcomes of Laparoscopic Surgery in Rectal Cancer
For two decades, the outcomes of laparoscopic surgery for rectal cancer have been well documented. Data from high standard of evidence studies are available to validate its feasibility, safety and oncologic validity.
Operative Outcomes
Laparoscopic resection for rectal cancer is more time-consuming than open rectal resection, but associated with lower blood loss based on the main randomized controlled trials (Table 50.1) [17, 18, 50–54]. Conversion to open surgery rates range from 1.2 to 34 % [17, 18, 50–53]. Male sex, higher body mass index, stapled anastomosis and intraoperative rectal fixity are the main factors of conversion [27, 51, 55]. With regard to the results of the CLASICC trial, conversion has a negative impact on postoperative morbidity. In our institution, further analysis revealed that men with a stapled anastomosis had a threefold higher rate of conversion than all other patients (34 % vs 11 %; P < .001) [55]. We recommend not to begin experience in laparoscopic TME in difficult cases i.e. men with high BMI, bulky or fixed tumors, to avoid high risk of conversion to open procedure. Inexperienced surgeons in TME should prefer the laparoscopic approach in favorable cases, i.e. for high rectal cancer, mid rectal cancer in women and low rectal cancer treated by APR. Laparoscopic surgery for mid rectal tumors in men and low rectal cancer treated with sphincter preservation should be reserved for experienced colorectal surgeons.
Table 50.1
Main operative and postoperative outcomes from randomized controlled trials comparing laparoscopic versus open surgery for rectal cancer
Study | Year | Patients | Surgical time | Blood loss | Conversion | Overall morbidity | Anastomotic leakage | Wound infection | Hospital stay | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
N | (min) | P value | (ml) | P value | (%) | (%) | P value | (%) | P value | (%) | P value | (Days) | P value | ||
Zhou et al. [54] | 2004 | L = 82 | 120 | 0.051 | 20 | <0.050 | NA | 6.1 | 0.016 | 1.1 | NA | NA | NA | 8.1 | 0.001 |
O = 82 | 106 | 92 | 12.4 | 3.4 | 13.3 | ||||||||||
Guillou et al. [51] | 2005 | L = 253 | 180 | NA | NA | 34 | 18 | NA | 10 | NA | 13 | NA | 11 | NA | |
(CLASICC trial) | O = 128 | 135 | 14
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||||||||||