and Chao-Hui Zheng1
(1)
Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou, China
Abstract
The lymph nodes (LNs) that lie along the celiac artery (CA) system in the suprapancreatic area include LNs around the root of the right gastric vessels (No. 5 and No. 12a) and those around the root of the left gastric vessels (No. 7, No. 8a, No. 9, and No. 11p). There is a high incidence of lymph node metastasis (LNM) in the suprapancreatic area for gastric cancer. Thus, complete clearance of LNs in this area is of great importance during radical gastrectomy for gastric cancer. It is also a critical procedure during laparoscopic D2 lymphadenectomy.
5.1 Review of Laparoscopic Suprapancreatic Area Lymph Node Dissection for Gastric Cancer
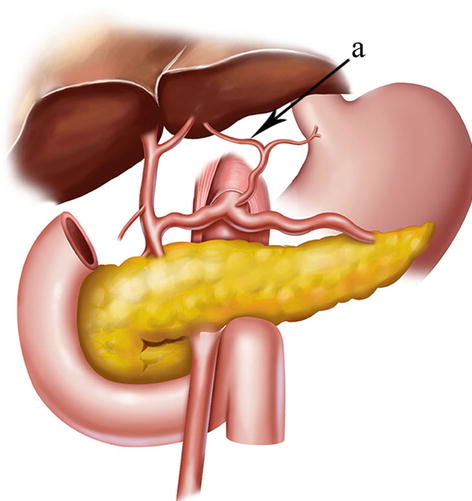
The lymph nodes (LNs) that lie along the celiac artery (CA) system in the suprapancreatic area include LNs around the root of the right gastric vessels (No. 5 and No. 12a) and those around the root of the left gastric vessels (No. 7, No. 8a, No. 9, and No. 11p) (Fig. 5.1). There is a high incidence of lymph node metastasis (LNM) in the suprapancreatic area for gastric cancer. Thus, complete clearance of LNs in this area is of great importance during radical gastrectomy for gastric cancer [1]. It is also a critical procedure during laparoscopic D2 lymphadenectomy [2].

Fig. 5.1
Suprapancreatic area
The frequency of No. 7 LNM varied between 19.0 % and 35.0 % [1, 3–7], while the 5-year survival rate among patients with positive No. 7 LNs was only 19.0 % [3]. The rate of No. 7 LNM differed depending on the location of the primary tumor. The metastatic rates of No. 7 LNs in proximal gastric cancer were reported to be between 19.0 % and 33.0 % [3–5], while the frequency in middle gastric cancer varied between 7.4 % and 22.0 % and in lower gastric cancer between 3.1 % and 23.0 % [3, 8]. In addition, the incidences of No. 7 LNM in early middle and lower gastric cancer were determined at 2.5 % and 0.8 %, respectively [9]. Dissection of the No. 7 LNs is part of D1 lymphadenectomy, as defined by the 3rd edition of Japanese Gastric Cancer Treatment Guidelines [10]. Therefore, No. 7 LNs should be routinely removed.
There are no strict boundaries among the No. 8, No. 9, and No. 7 LNs. The incidence of No. 8a LNM was reported to be between 9.7 % and 36.0 % [5, 6, 11], while the metastatic rate varied between 4.9 % and 24.0 % in No. 9 LNs [4–7, 11]. The 5-year survival rate was shown to be 16.0 % when No. 8a or No. 9 LNs are positive [3]. The incidences of No. 8a and No. 9 LNM were also associated with tumor location, T staging, and other factors. In proximal gastric cancer, the metastatic rates of No. 8 and No. 9 LNs were 7.0–16.7 % and 4.2–13.0 %, respectively [3, 8]. The incidence of No. 8 LNM was the highest in advanced proximal gastric cancer, at 68.0 % [12]. In middle gastric cancer, the metastatic rates of No. 8 and No. 9 LNs were 2.4–11.0 % and 2.2–8.0 %, respectively [3, 8]. Even in early middle gastric cancer, the incidences of No. 8 and No. 9 LNM were as high as 1.3 % [9]. Furthermore, in distal gastric cancer, the frequency of No. 8 LNM varied between 4.1 % and 13.4 % [8, 13], while the incidence of No. 9 LNM was 13.0 % [3]. And 1.6 % of patients with early distal gastric cancer exhibited metastasis to No. 8 LNs [9]. Dissection of the No. 8 LNs and No. 9 LNs is included in D1+ or D2 lymphadenectomy despite gastric resection according to the 3rd edition of Japanese Gastric Cancer Treatment Guidelines [10]. Therefore, excision of No. 8 LNs and No. 9 LNs should be given sufficient attention.
The incidence of No. 11 LNM was also high, varying between 12.0 % and 13.7 % [3, 4], and the 5-year survival rate among patients with positive No. 11 LNs was 6.0 % [3]. The incidence of No. 11 LNM can reach up to 15.5 % in advanced proximal gastric cancer [5]. No. 11 LNs are divided into No. 11p LNs (along the proximal splenic artery (SpA) near the CA) and No. 11d LNs (along the distal SpA near the splenic hilum). The former has a higher metastatic rate. In middle gastric cancer, the metastatic rate of No. 11p LNs was 20.0 % [1], and the frequencies of No. 11p LNM in early middle and lower gastric cancer were 2.0 % and 0.8 %, respectively [9]. Hence, No. 11p LNs should be also excised during D2 dissection based on the treatment guidelines.
The 5-year survival rate among patients with positive No. 5 LNs was 17.0 % [3], and the incidence of No. 5 LNM varied, 16.6–36.0 %, in patients with tumors located in middle and upper gastric cancer [4, 6, 11, 14]. Hence, there was no objection against complete clinical clearance of No. 5 LNs for middle and upper gastric cancer. There were rare reports of No. 5 LNM in early proximal gastric cancer [15, 16], while the incidence of No. 5 LNM varied between 6.8 % and 9.0 % in advanced proximal gastric cancer [5, 17]. Thus, No. 5 LNs should be routinely removed in all patients except those with early proximal gastric cancer. Dissection of No. 12a LNs is still considered part of D2 lymphadenectomy during distal or total gastrectomy based on the 3rd edition of Japanese Gastric Cancer Treatment Guidelines [10]. It has been reported that the incidence of No. 12a LNM is between 2.9 % and 22.0 % in patients with gastric cancer [3, 5–7, 11]. The 5-year survival rate among patients with positive No. 12a LNs was 9.0 % [3]. Therefore, No. 12a LNs should be resected attentively during distal or total gastrectomy.
In conclusion, LNs along the CA system in the suprapancreatic area should be critically excised during either D1+ or D2 lymph node dissection based on the 3rd edition of Japanese Gastric Cancer Treatment Guidelines [10]. These LNs have a strong relationship with gastric cancer metastasis and are the critical components during lymphadenectomy. Lymph node dissection in the suprapancreatic area is an important and technically demanding procedure during radical lymphadenectomy for gastric cancer.
5.2 Anatomy Associated with Lymph Node Dissection in the Suprapancreatic Area
Most of the gastric arteries originate from the celiac trunk, which generally has three branches: the splenic, common hepatic, and left gastric arteries. Each in turn has its own branches that supply the greater and lesser curvatures of the stomach. The efferent lymphatic vessels of the stomach follow the adjacent arteries and their branches in reverse to gather at their roots. There are many LNs that drain these efferent vessels lying along the arteries. Thus, the LNs along the celiac artery system in the suprapancreatic area are intimately associated with metastasis in gastric cancer.
5.2.1 Fascia and Intrafascial Space in the Suprapancreatic Area
5.2.1.1 Pancreatic Fascia and the Intrafascial Space
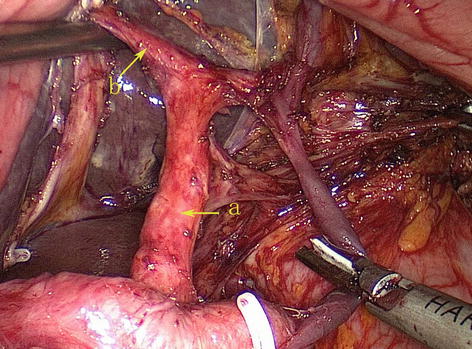
The pancreatic fascia encompasses the pancreas, forming the anterior and posterior pancreatic fascia (APF and PPF). The space between the APF and the PPF is called the pancreatic intrafascial space (PIS) (Figs. 5.2 and 5.3). The pancreatic parenchyma and related vessels along with their branches are situated in the PIS. The APF passes to the right of the pancreatic head and becomes continuous with the transverse and ascending mesocolon at the right colic flexure. It extends below the pancreas to come into contact with the PPF and joins the anterior lobe of the transverse mesocolon (ATM) to form the lower part of the posterior wall of the omental bursa. It passes left along the anterior pancreatic tail to become continuous with the gastrosplenic ligament (GSL) at the top as well as the lateral splenorenal ligament (SRL). The anterior pancreatic space (APS) is the space between the APF and the inherent pancreatic fascia. The pancreatic capsule can be completely peeled along the APS.
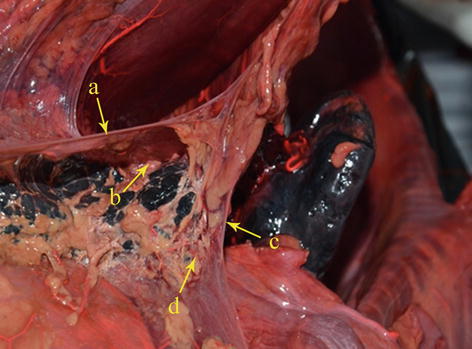
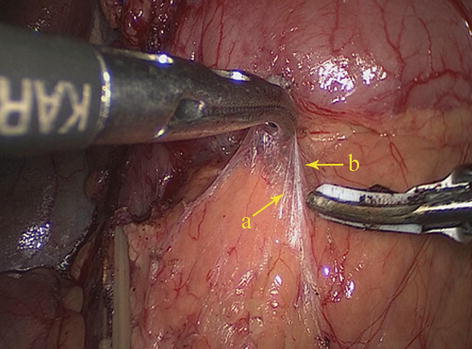

Fig. 5.2
APS (a), APF (b), PPF (c), and RPS (d) (in autopsy)

Fig. 5.3
APF (a) and APS (b)
The PPF is formed by the posterior layer of the posterior leaf of the dorsal mesogastrium (DM), which attaches to the posterior abdominal wall. The PPF covers the posterior aspect of the pancreas and extends below it to fuse with the APF. The space between the PPF and the inherent pancreatic fascia is the retropancreatic space (RPS), which is in the suprapancreatic area and contains the three branches of the celiac trunk and their concomitant lymphatic vessels and LNs.
5.2.1.2 Gastropancreatic and Hepatopancreatic Folds
At the anterior upper side of the pancreas, the peritoneum behind the omental bursa covers the posterior abdominal wall, part of the pancreatic head, the pancreatic body and neck, the anterior part of the left kidney, most of the adrenal gland, the initial part of the abdominal aorta, the celiac trunk, and part of the diaphragm. The section extending from the middle upper border of the pancreatic body to the posterior wall of the gastric lesser curvature is called the gastropancreatic fold (GPF). It passes along the upper border of the pancreas and becomes continuous with the left edge of the posterior HDL to form the hepatopancreatic fold (HPF) (Figs. 5.4, 5.5, and 5.6). The GPF and HPF form the isthmus of the omental bursa, dividing it into two parts (the lesser sac on the right and the greater sac on the left). The GPF provides a pathway for the left gastric vessels, vagus nerve, and lymphatic vessels to penetrate the lesser sac (the posterior and front walls) and enter the mesogastrium attached at the lesser curvature of the stomach. The HPF provides a pathway for the common hepatic artery (CHA), gastric coronary vein, vagus nerve, and lymph vessels to penetrate the lesser sac. It extends to the furcation of the CHA and SpA from the celiac trunk.
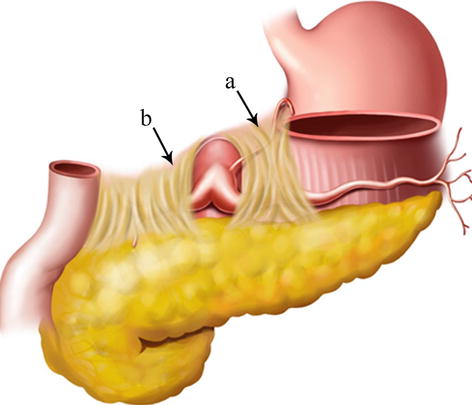
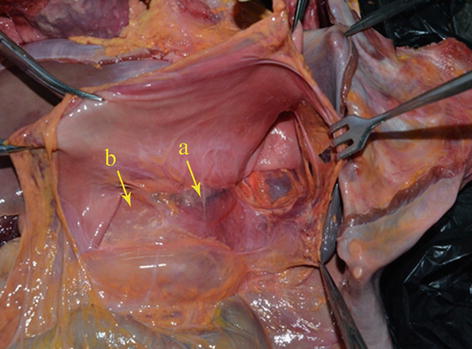
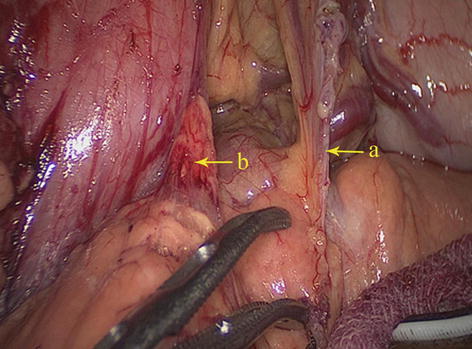

Fig. 5.4
GPF (a) and HPF (b)

Fig. 5.5
GPF (a) and HPF (b) (in autopsy)

Fig. 5.6
GPF (a) and HPF (b)
5.2.1.3 HDL
The HDL consists of two layers of peritoneum, which cover the anterior and posterior walls of the lower part of the gastric lesser curvature, the porta hepatis and the upper edge of the duodenum. It is the portion of the lesser omentum that extends between the liver and the superior part of the duodenum. The right free border of the HDL forms the anterior boundary of the epiploic foramen, and its left border is continuous with the hepatogastric ligament (HGL). The HDL contains the hepatic pedicle (the common bile duct, hepatic artery, and PV) and the No. 12 LNs. In addition, the HDL and the falciform ligament are mutually linked (Figs. 5.7 and 5.8).
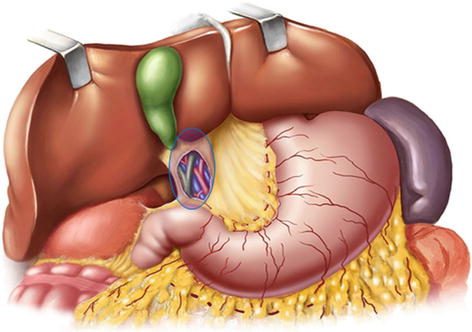
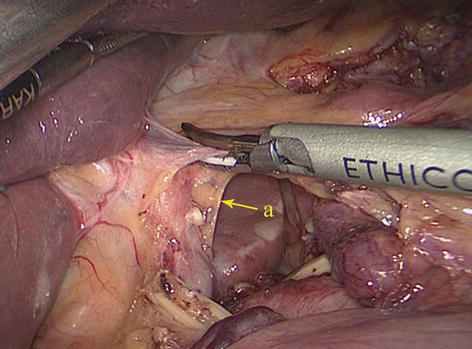

Fig. 5.7
HDL

Fig. 5.8
HDL (a)
5.2.1.4 Retrogastric Space (RGS)
The potential space at the superior border of the pancreas, between the parietal peritoneum and the posterior abdominal wall, is called the RGS. It is full of loose connective tissue and contains the No. 9 LNs and the celiac trunk (Fig. 5.9).
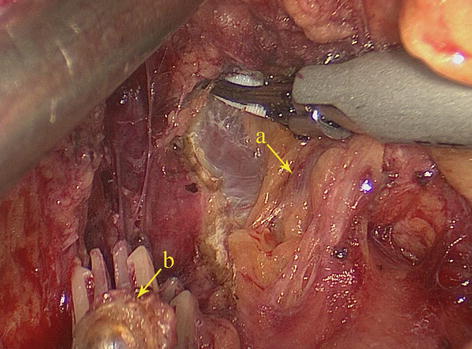

Fig. 5.9
RGS (a). The end of the left gastric artery (LGA) (b)
5.2.2 Vascular Anatomy Associated with Lymph Node Dissection in the Suprapancreatic Area
5.2.2.1 Arteries Associated with Lymph Node Dissection in the Suprapancreatic Area
CA
The CA, also known as the celiac trunk, is the first unpaired branch of the abdominal aorta. It is only 1–2 cm in length and branches from the aorta anterior to the level of T12, slightly below the aortic hiatus of the diaphragm. The gastric arteries originate from the CA, which has three main divisions: the LGA, CHA, and SpA (Figs. 5.10, 5.11, and 5.12).
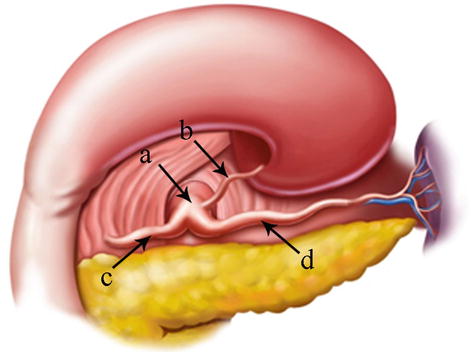
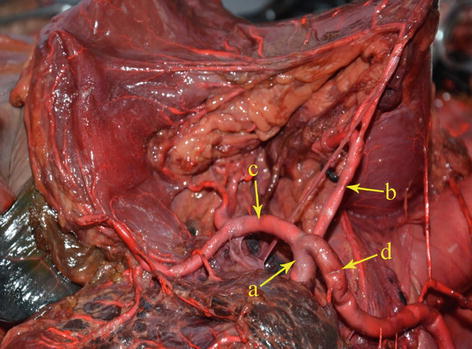
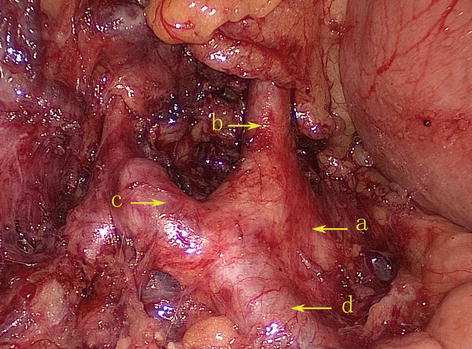

Fig. 5.10
The CA (a) divides into LGA (b), CHA (c), and SpA (d)

Fig. 5.11
The CA (a) divides into LGA (b), CHA (c), and SpA (d) (in autopsy)

Fig. 5.12
The CA (a) divides into LGA (b), CHA (c), and SpA (d)
Adachi’s classification of the CA describes six variant subtypes based on the analysis of 252 cases [18].
Type I: The celiac trunk is formed from the LGA, SpA, and CHA. This is known as the normal type and was present in 222 patients (88.1 %) (Figs. 5.13 and 5.14).
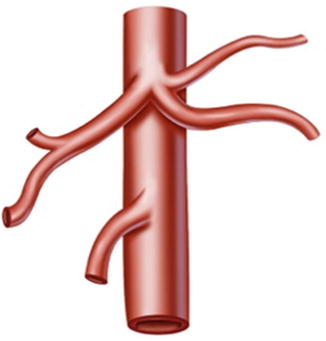
Fig. 5.13
Type I
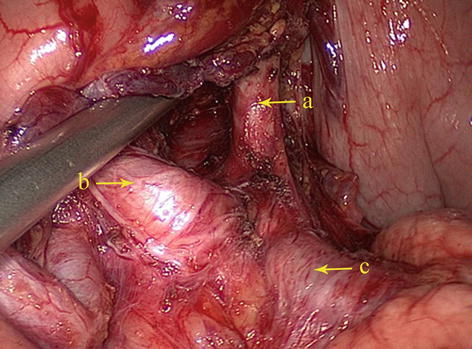
Fig. 5.14
Type I. LGA (a), CHA (b), and SpA (c)
Type II: The LGA originates from the abdominal aorta, while the SpA and CHA issue from the celiac trunk. It is present in 16 patients (6.3 %) (Figs. 5.15, 5.16, and 5.17).
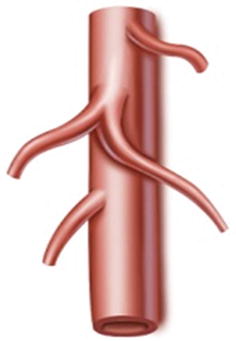
Fig. 5.15
Type II
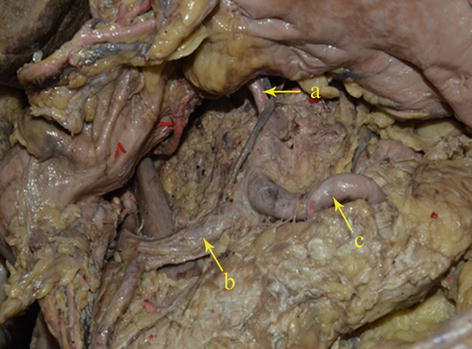
Fig. 5.16
Type II. LGA (a), CHA (b), and SpA (c) (in autopsy)
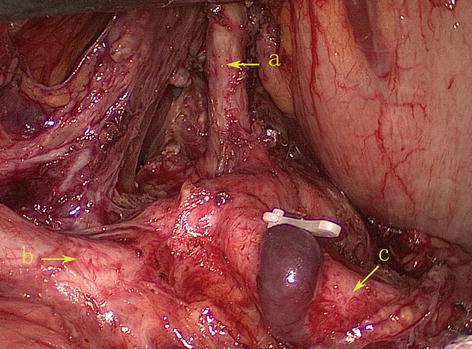
Fig. 5.17
Type II. LGA (a), CHA (b), and SpA (c)
Type III: The LGA arises from the abdominal aorta, while the celiac trunk is formed from the CHA, SpA, and superior mesenteric artery (SMA). It is present in three patients (1.2 %) (Fig. 5.18).
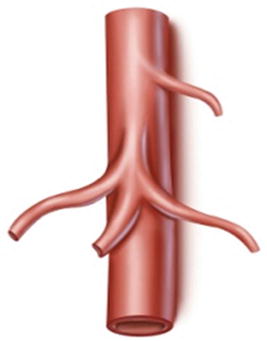
Fig. 5.18
Type III
Type IV: The celiac trunk is formed from the LGA, CHA, SpA, and SMA. It is present in six patients (2.4 %) (Fig. 5.19).
Fig. 5.19
Type IV
Type V: The LGA and SpA originate from a common trunk derived from the abdominal aorta, while the CHA and SMA originate from a separate common trunk derived from the CA. It is present in one patient (0.4 %) (Figs. 5.20 and 5.21).
Fig. 5.20
Type V
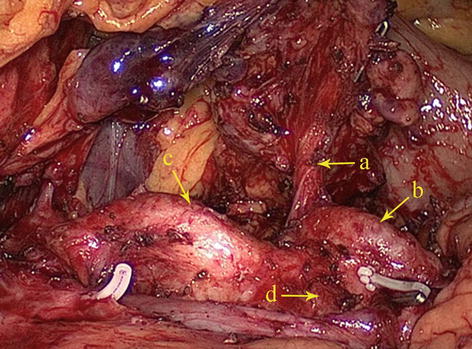
Fig. 5.21
Type V. LGA (a), SpA (b), CHA (c), and SMA (d)
Type VI: The LGA and the SpA originate from the CA, while the CHA is absent and is replaced by a branch of the SMA or abdominal aorta (AA) (for details, see the anatomy of CHA later in this chapter). It is present in five patients (2.0 %) (Figs. 5.22 and 5.23).
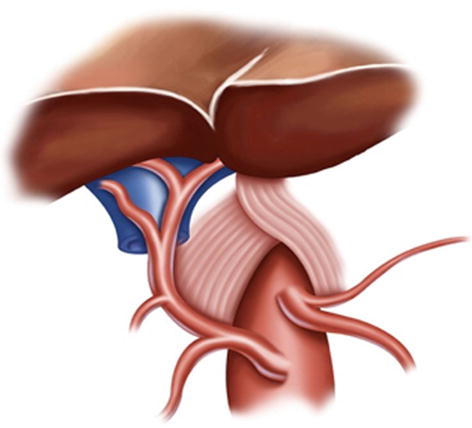
Fig. 5.22
Type VI
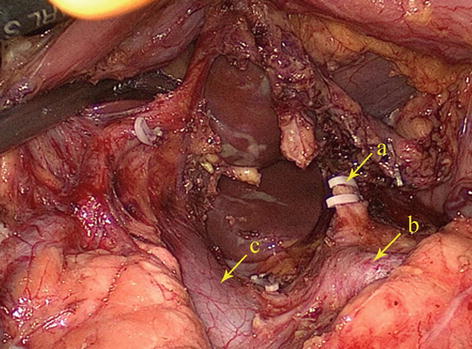
Fig. 5.23
Type VI, the end of LGA (a), SpA (b), and portal vein (PV) (c)
LGA
In most cases, the LGA stems from the CA. In 2.5–7.5 % of cases, it instead arises directly from the right posterior aspect of the abdominal aorta above the origin of the CA. After issuing from the CA, the LGA clings to the posterior abdominal wall and runs toward the upper left side along the deep parietal peritoneum of the omental bursa to the dorsal side of the cardia. Then, it turns right and passes along the lesser curvature of the stomach between the layers of the lesser omentum to join with the right gastric artery (RGA) (Fig. 5.24). In approximately 1.0–23.0 % of cases, an accessory left gastric artery (ALGA) issues from the left or proper hepatic artery (LHA or PHA) (Figs. 5.25, 5.26, and 5.27) and runs along the same course to supply the lesser curvature of the stomach. In addition, in some patients, an accessory LHA (ALHA) originates from the LGA, branching off from this artery at its highest point or at the turning point before it runs down toward the lesser curvature of the stomach. The extrahepatic part of the ALHA is short. Within the HGL, the ALHA extends toward the right or upper right in a straight or slightly tortuous course to enter the hepatic parenchyma through the left sagittal groove, anterior to the caudate lobe. It extends to the left, dividing into several segmental or subsegmental branches to supply the left lateral lobe of the liver. Its diameter is equivalent to the level 2 and 3 hepatic artery.
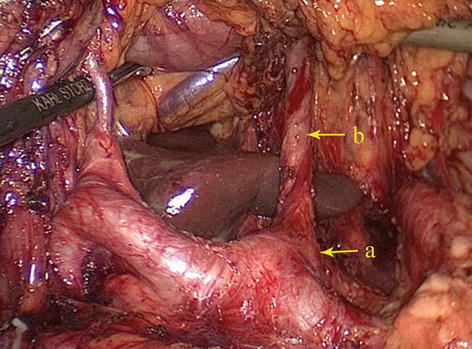
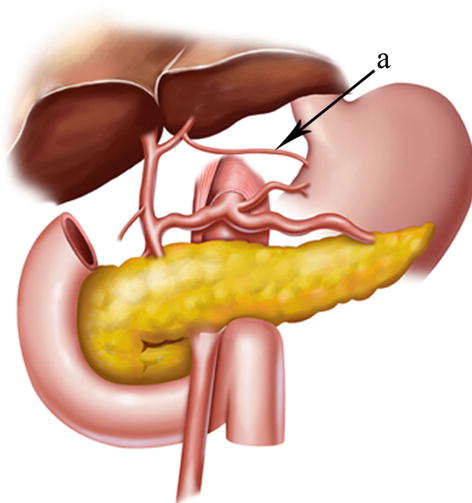
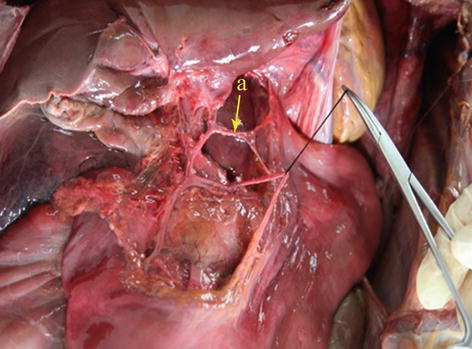
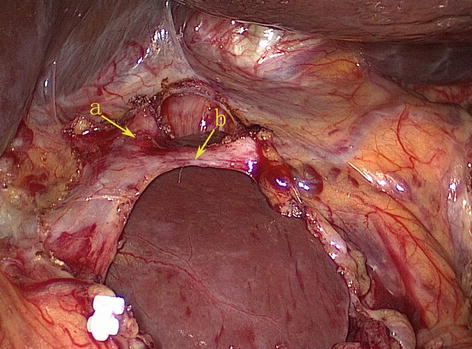

Fig. 5.24
The CA (a) gives off the LGA (b)

Fig. 5.25
ALGA (a)

Fig. 5.26
The LHA gives off the ALGA (a) (in autopsy)

Fig. 5.27
The LHA (a) gives off the ALGA (b)
CHA
The CHA, which arises from the celiac trunk, passes to the right side and runs forward along the upper border of the pancreatic head. It then enters the HDL and gives off the gastroduodenal artery (GDA) and PHA above the duodenum (Figs. 5.30 and 5.31).
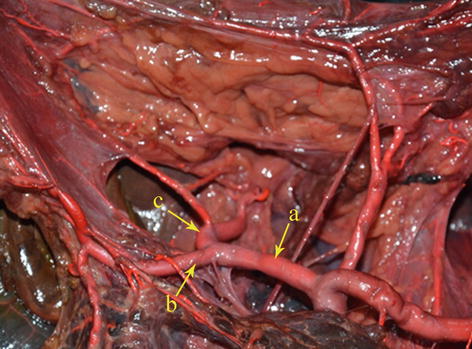
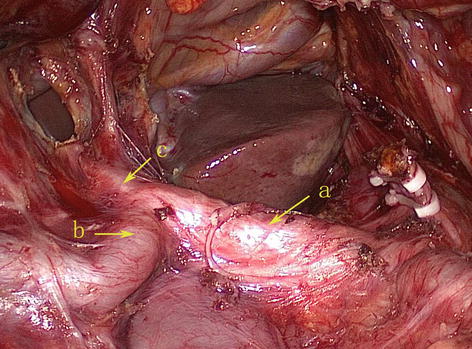

Fig. 5.30
The CHA (a) gives off the GDA (b) and PHA (c) (in autopsy)

Fig. 5.31
The CHA (a) gives off the GDA (b) and PHA (c)
Less variation is associated with the CHA, but occasionally it may be absent (range, 1.4–6.2 % of cases). When the CHA doesn’t arise from the CA as usual, it can be regard as absence according to Gray’s Anatomy [20]. The CHA was found to be absent in 38 cases (1.8 %) based on the analysis of 2,170 patients with gastric cancer by our center. The anatomy of absence of CHA was classified into six types according to the anatomy of the replaced CHA (RCHA) as follows:
Type I: The RCHA arose from SMA and ran across the posterior side of pancreas and was present in 28 cases (1.3 %, 28/2,170). The RCHA branched off GDA when it lay adjacent to posterior wall of duodenal and its distal end is continued with the replaced PHA (RPHA) (Figs. 5.32, 5.33, 5.34, and 5.35).
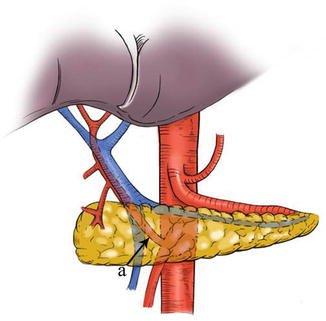
Fig. 5.32
Type I. RCHA (a)
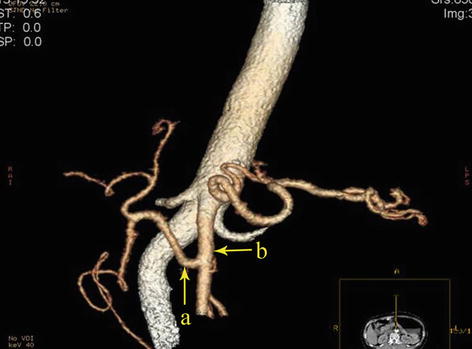
Fig. 5.33
Type I. RCHA (a) arose from SMA (b)
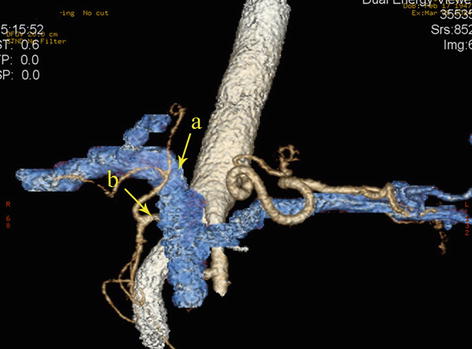
Fig. 5.34
Type I. The PV (a) ran in front of the RCHA (b)
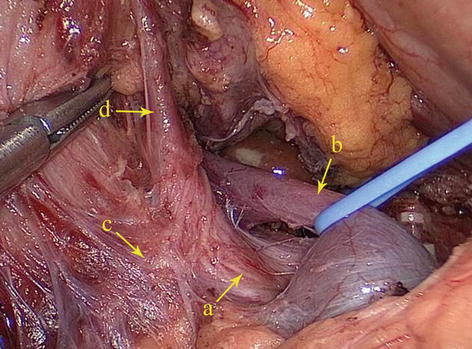
Fig. 5.35
Type I. The RCHA (a) ran behind the PV (b) and branched off the GDA (c) and RPHA (d)
Type II: The RCHA arose from SMA and ran across the anterior side of pancreatic head and was present in one case (0.05 %, 1/2,170). In this type, the RCHA surrounded the pancreatic head like “U” shape and ran toward the direction of upper right. When it reached the junction of superior border of pancreas and medial wall of duodenal, the RCHA branched off the RPHA, RGEA, and SPDA (Figs. 5.36, 5.37, 5.38, 5.39, and 5.40).
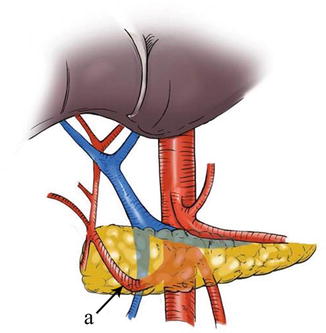
Fig. 5.36
Type II. RCHA (a)
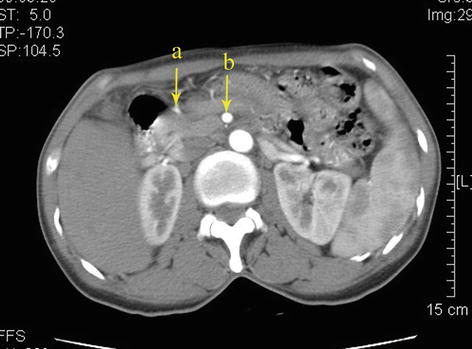
Fig. 5.37
Type II. RCHA (a) arose from SMA (b)
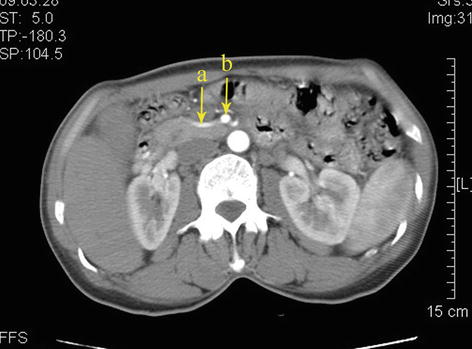
Fig. 5.38
Type II. RCHA (a) arose from SMA (b)
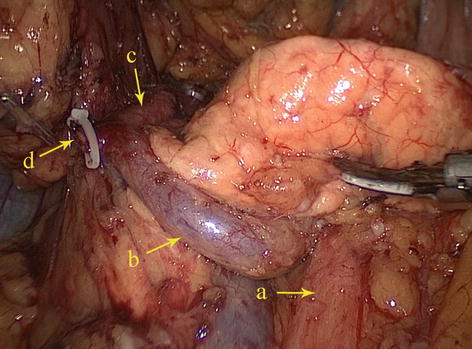
Fig. 5.39
Type II. SMA (a), RCHA (b), RPHA (c), and REGA (d)
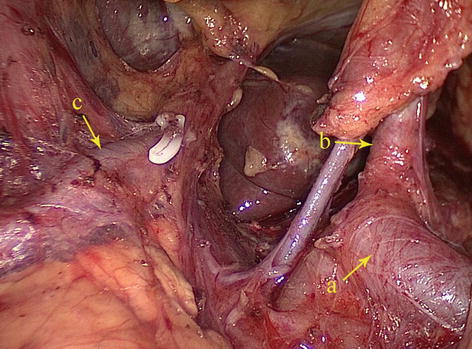
Fig. 5.40
Type II. SpA (a), LGA (b), and RPHA (c)
Type III: The RCHA arose from AA in one case (0.05 %, 1/2,170). The RCHA passed in front of the PV and bifurcated into GDA and RPHA near the posterior wall of duodenal (Figs. 5.41, 5.42, 5.43, and 5.44).
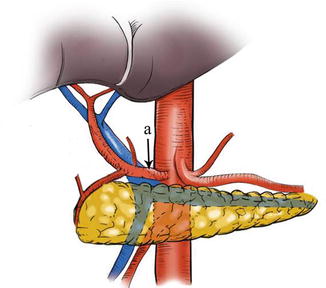
Fig. 5.41
Type III. RCHA (a)
Fig. 5.42
Type III. RCHA (a) and SpA (b)
Fig. 5.43
Type III. RCHA (a) and SpA (b)
Fig. 5.44
Type III. The RCHA (a) bifurcated into the RPHA (b) and GDA (c)
Type IV: The replaced LHA (RLHA) arose from the LGA, and the replaced RHA (RRHA) arose from SMA. It is present in five cases (0.2 %, 5/2,170). In this type, the RLHA lay in the lesser omentum, and the RRHA ran behind the PV and gave off the GDA on the suprapancreatic border (Figs. 5.45, 5.46, 5.47, and 5.48).
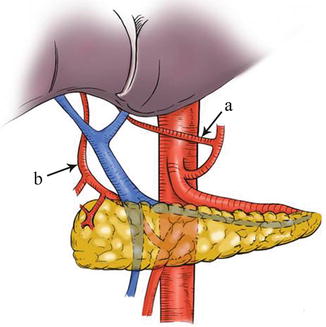
Fig. 5.45
Type IV. RLHA (a) and RRHA (b)
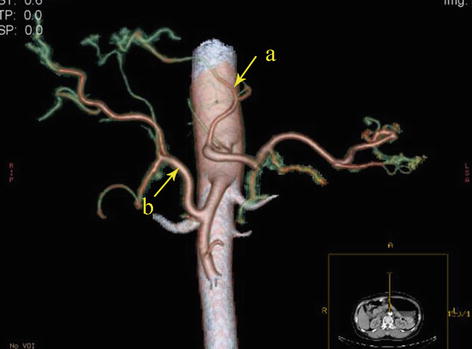
Fig. 5.46
Type IV. RLHA (a) and RRHA (b)
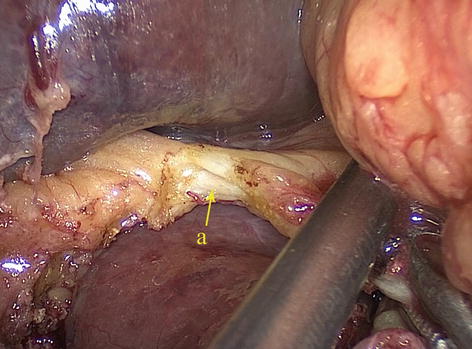
Fig. 5.47
Type IV. RLHA (a)
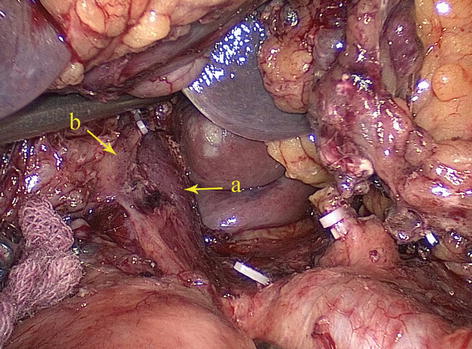
Fig. 5.48
Type IV. PV (a) and RRHA (b)
Type V: The RLHA arose from the LGA, and the RRHA arose from the CA in two cases (0.09 %, 2/2,170). The RLHA lay in the lesser omentum, and the RRHA passed behind the PV. The aberrant GDA arose from the CA (Figs. 5.49, 5.50, and 5.51).
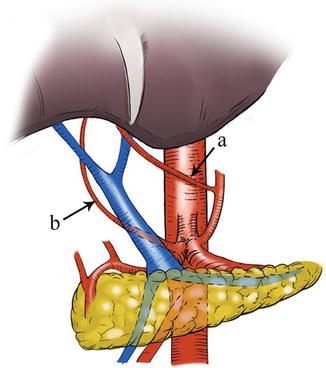
Fig. 5.49
Type V. RLHA (a) and RRHA (b)
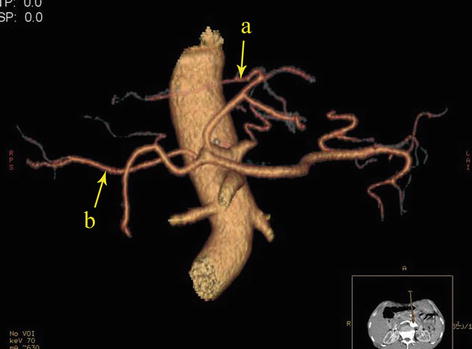
Fig. 5.50
Type V. RLHA (a) and RRHA (b)
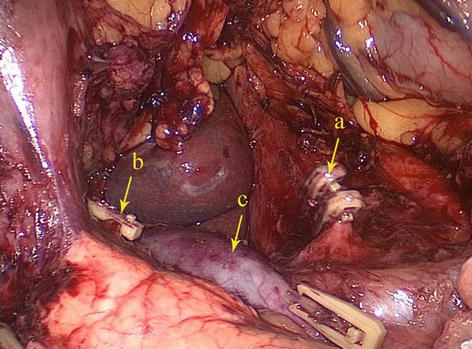
Fig. 5.51
Type V. LGA (a), RGA (b), and PV (c)
Type VI: The RLHA arose from aberrant GDA and RRHA arose from SMA in one case (0.05 %, 1/2,170). The aberrant GDA arose from CA and branched off the RLHA to supply the left liver lobe. The RRHA arose from SMA and passed behind PV, supplying the right liver lobe (Figs. 5.52, 5.53, and 5.54).
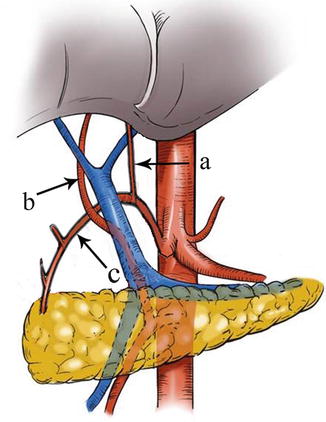
Fig. 5.52
Type VI. RLHA (a), RRHA (b), and aberrant GDA (c)
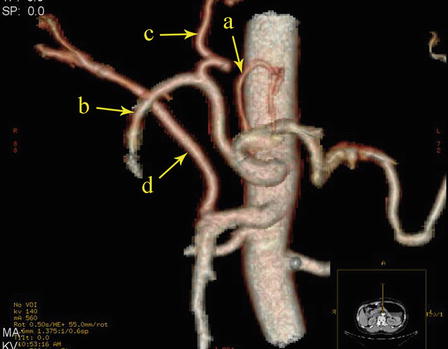
Fig. 5.53
Type VI. LGA (a), aberrant GDA (b), RLHA (c), and RRHA (d)
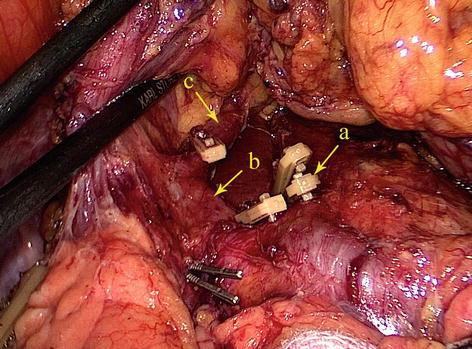
Fig. 5.54
Type VI. LGA (a), aberrant GDA (b), and RLHA (c)
GDA
The GDA stems from the CHA. It descends behind the first part of the duodenum. Upon reaching the lower border of the pylorus, it splits into right gastroepiploic and anterior superior pancreaticoduodenal arteries (Figs. 5.55 and 5.56).
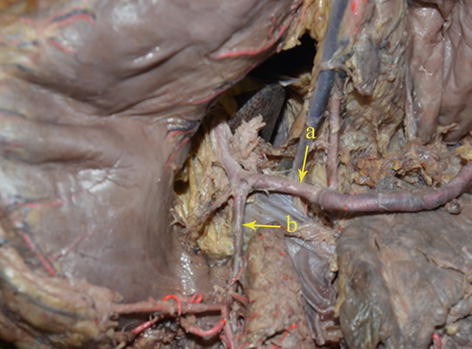
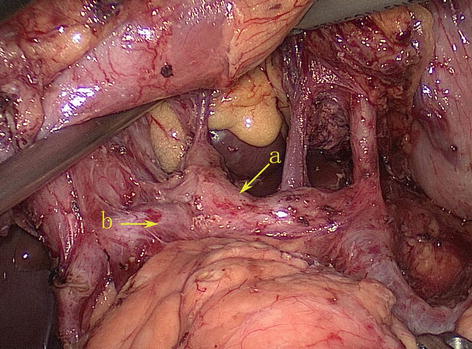

Fig. 5.55
The CHA (a) gives off the GDA (b) (in autopsy)

Fig. 5.56
The CHA (a) gives off the GDA (b)
PHA
The PHA arises directly from the CHA and runs to the upper right within the HDL. It runs to the left and anterior of the PV and on the left side of the common bile duct in the HDL. Then, it terminates by bifurcating into the right and left hepatic arteries near the porta hepatis, sending small branches to the middle of the common bile duct along the way. The PHA is separated from the CHA by the bifurcation of the GDA, called the T-shaped intersection. The PHA is distal to the division, while the CHA is proximal to it (Figs. 5.57 and 5.58).
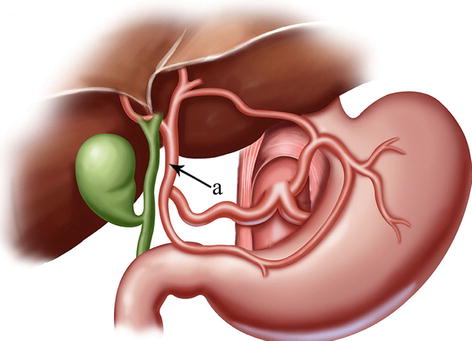
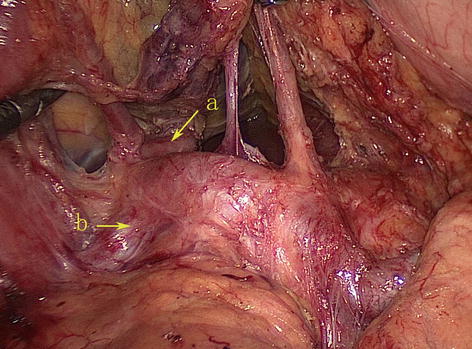

Fig. 5.57
The course of the PHA (a)

Fig. 5.58
The PHA (a) is distal to the bifurcation of the GDA (b)
RGA
The RGA most frequently arises from the PHA above the first part of the duodenum. It runs upward between the two layers of the HDL to the pylorus. It then passes from right to left along the lesser curvature of the stomach, supplying both of its surfaces with branches, and joins with the LGA (Figs. 5.59 and 5.60).
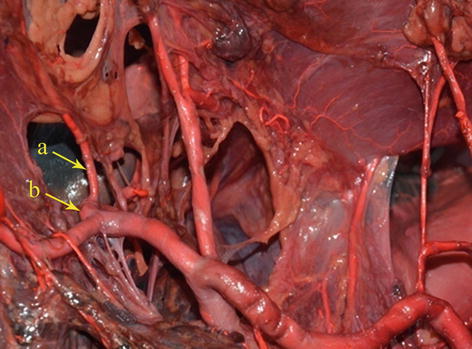
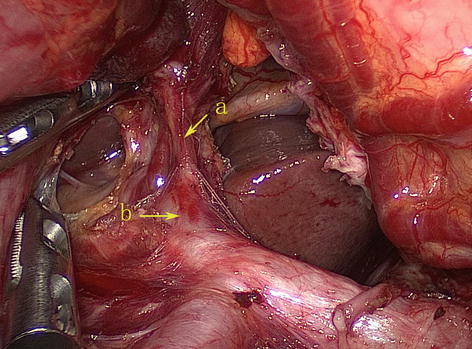

Fig. 5.59
The RGA (a) arises from the PHA (b) (in autopsy)

Fig. 5.60
The RGA (a) arises from the PHA (b)
According to Adachi’s study of the RGA in 191 patients, the origin of this artery varies considerably [18]: The RGA originated from the PHA in 93 patients (48.7 %) (Figs. 5.61 and 5.62); LHA in 38 patients (19.9 %) (Figs. 5.63 and 5.64); GDA in 28 patients (14.7 %) (Figs. 5.65 and 5.66); near the T-shaped intersection of the CHA, PHA, and GDA in 17 patients (8.9 %) (Figs. 5.67 and 5.68); and CHA in 3 patients (1.6 %) (Figs. 5.69 and 5.70).
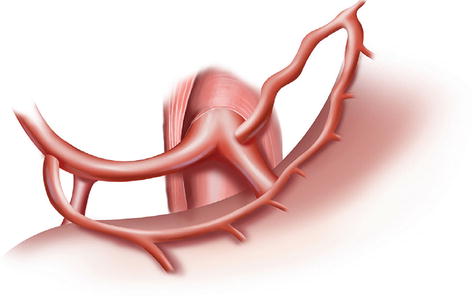
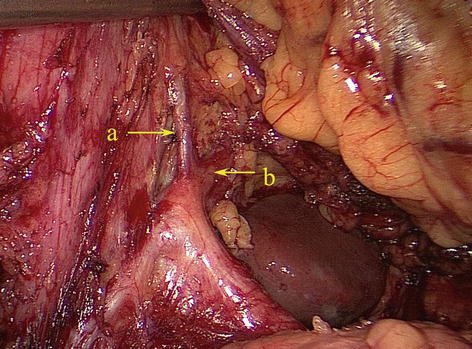
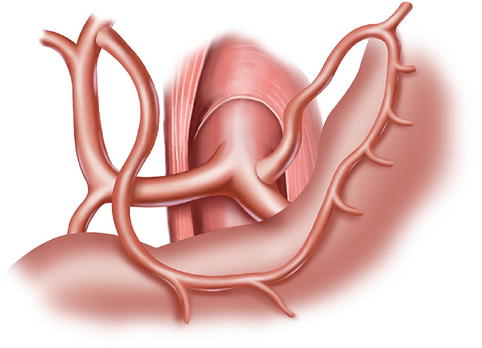
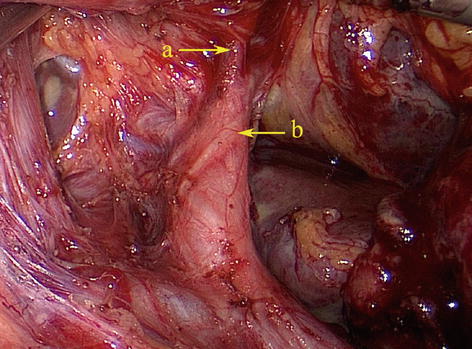
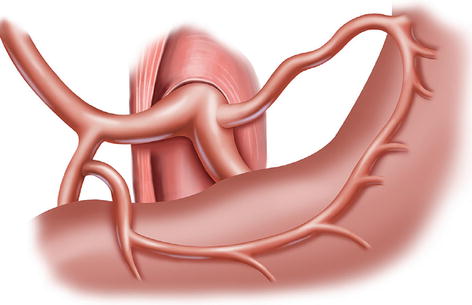
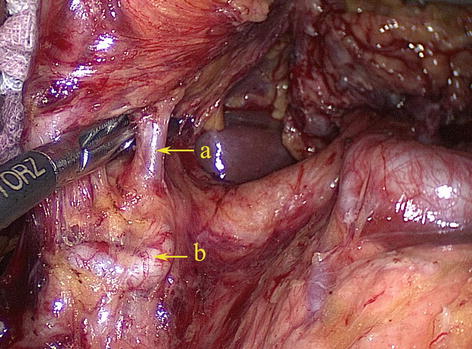
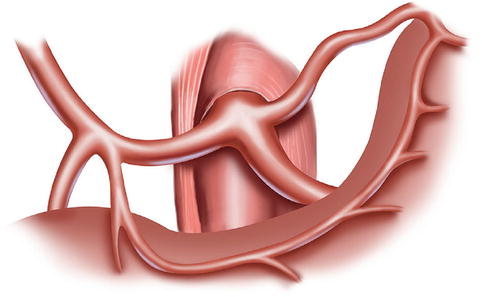
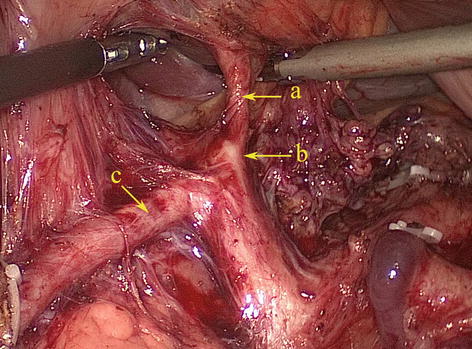
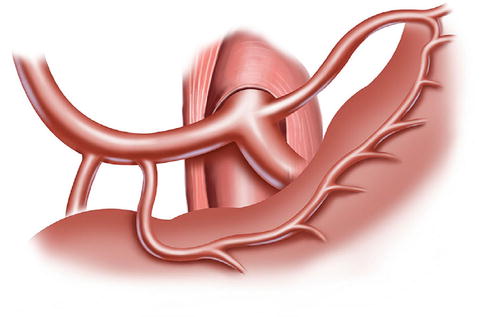
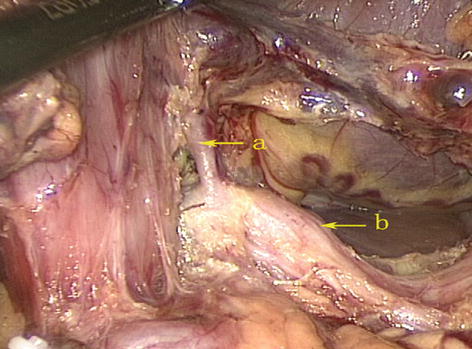

Fig. 5.61
The RGA originated from the PHA

Fig. 5.62
The RGA (a) originated from the PHA (b)

Fig. 5.63
The RGA derived from the LHA

Fig. 5.64
The RGA (a) derived from the LHA (b)

Fig. 5.65
The RGA arose from the GDA

Fig. 5.66
The RGA (a) arose from the GDA (b)

Fig. 5.67
The RGA originated near the bifurcation of the PHA and GDA

Fig. 5.68
The RGA (a) originates near the bifurcation of the PHA (b) and GDA (c)

Fig. 5.69
The RGA originated from the CHA

Fig. 5.70
The RGA (a) originated from the CHA (b)
SpA
The SpA branches from the CA to supply blood to the spleen. It meanders toward the left along the upper border of the pancreas and gives off branches to the stomach and pancreas before reaching the spleen (see the Chap. 6.2 for details).
5.2.2.2 Veins Associated with Lymph Node Dissection in the Suprapancreatic Area
Left Gastric Vein (LGV)
The LGV (or gastric coronary vein) receives venous blood from the branches corresponding to the area of the LGA. It originates from small venous branches coming from the anterior and posterior walls of the stomach and accompanies the artery of the same name to run along the gastric lesser curvature between the two layers of the lesser omentum. It receives the venous blood of branches from the anterior and posterior sides of the gastric lesser curvature, the cardiac region, and the lower half of the esophagus as well as drains one to two small vessels in the posterior abdominal wall. It ascends to the cardia and receives the esophageal vein from the lower half of the esophagus and then turns right. The drainage of the LGV has three possible patterns: the LGV ends in the PV (approximately 60.0 % of cases), it drains into the splenic vein (SpV) (approximately 30.0 %), or it drains into the splenoportal confluence (approximately 10.0 %) (Figs. 5.71, 5.72, 5.73, 5.74, and 5.75). The course of the LGV can also vary. In an analysis of 1,325 patients who underwent laparoscopic radical gastrectomy for gastric cancer by our center [21], we found that the LGV passed to the ventral side of the SpA and the CHA in 56.1 % of cases and to the dorsal side of the CHA in 41.5 % of cases. In approximately 1.6 % of cases, the coronary vein independently passes through the GHL and drains into the PV at the porta hepatis without accompanying the similarly named artery. It is called the intrahepatic LGV in this situation (Figs. 5.76 and 5.77). In about 0.5 % of cases, the LGV is absent, and the right gastric vein (RGV) is enlarged in compensation (Figs. 5.78 and 5.79). Moreover, we have described a rare anatomical variant of the LGV (Fig. 5.80), in which it descends along the GPF, runs across the dorsal side of the SpA, and drains into the SpV [22]. This previously undescribed anatomical variant had an incidence of 0.3 % (six cases) in our study. The mean diameter and length of this variant LGV were 5.10 ± 0.40 mm and 37.40 ± 5.19 mm, respectively, and the mean distance from the end of the LGV to the splenoportal confluence was 13.05 ± 0.86 mm. The closer the LGV and LGA were to the root, the greater the distance between them, with a mean difference of 13.85 ± 1.02 mm between the end of the LGV and the root of the LGA. In addition, there may be double LGVs (Fig. 5.81).
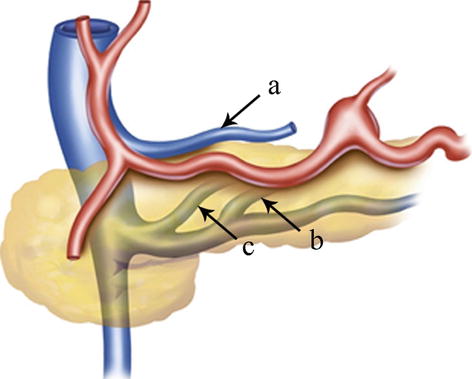





Fig. 5.71
The LGV (a) ends in the PV. The LGV (b) drains into the SpV. The LGV (c) drains into the splenoportal confluence
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree