Laparoscopic Subtotal Gastrectomy and D2 Lymphadenectomy for Gastric Carcinoma
Vivian E. Strong
The only known curative treatment for gastric adenocarcinoma involves surgical resection. With recent advances in neoadjuvant and adjuvant treatment in combination with surgery, the curative potential as measured by overall survival has improved, although overall cure rates are still low with 58% 1-year survival and 28% 5-year survival according to national U.S. statistics. This is partly due to the late stage at which most gastric adenocarcinomas are identified. Nevertheless, in recent years more early-stage gastric cancers have been identified, allowing for earlier treatment and better outcomes. Indications for gastrectomy for adenocarcinoma include patients with pathologically proven adenocarcinoma and staging work-up that demonstrates nonmetastatic disease. In addition, those patients with very early-stage (T1a) disease who met specific criteria may be considered for endoscopic mucosal resection. Indications for gastrectomy include gastric cardia tumors and gastroesophageal junction adenocarcinomas that meet criteria of Siewert type III and in some cases Siewert type II cancers. For these tumors a total gastrectomy with D2 lymphadenectomy is required.
Indications for a laparoscopic approach depend largely on surgeon skill and training with both advanced laparoscopic techniques and with appropriate oncologic principles for resection of gastric cancer. As few centers see more than a dozen such cases per year, it may be advisable to refer such patients to high-volume gastric cancer centers. Contraindications are relative to the specific patient; however, relative contraindications may include patients with multiple prior laparotomies, those with locally invasive or very large tumors, or those with a high body mass index rendering safe and timely resection more difficult. Additionally, patients with neoadjuvant treatment sometimes have intense peritumoral fibrosis that prompts an open approach. In cases that are borderline for the laparoscopic approach, starting minimally invasively in order to assess the patient is reasonable as long as the operating surgeon has a low threshold for conversion. The primary goal of resection is an oncologically safe and complete resection. Minimally invasive removal is the secondary goal, and this should be emphasized to the patient prior to resection.
All patients with gastric cancer who are being considered for operative resection need a complete staging work-up. This includes an upper endoscopy with biopsy, computed tomography of the chest, abdomen, and pelvis, and for locally advanced tumors, a positron emission tomography scan. Patients require endoscopic ultrasound as an important aspect of staging. Patients who are found to have ultrasonic penetration up to and into the muscularis propria (T1 and T2) are potential candidates for upfront surgical resection. Those patients with endoscopic ultrasound findings of penetration into the subserosal connective tissue (T3 or higher) or N+ (node positive) disease require additional staging with diagnostic laparoscopy and cytologic washings. This same-day operative procedure requires general anesthesia and staging includes inspection of the surface and undersurface of the liver, the peritoneum and remainder of the abdominal cavity (and ovaries for female patients), and in addition, peritoneal fluid cytology is obtained. If results are positive for cancer cells in biopsies of distant tumor deposits or in the collected fluid samples, these patients are staged as metastatic or stage IV gastric cancer and are not candidates for gastric resection. Patients with washings that are negative and with no other intra-abdominal deposits are staged as locally advanced and usually undergo a treatment with neoadjuvant chemotherapy prior to operation. Postoperative treatment for patients varies depending on final pathology from the operation and may include chemotherapy (MAGIC trial) or chemotherapy and radiation treatment (MacDonald trial).
Laparoscopic Subtotal Gastrectomy with D2 Lymphadenectomy and Roux-en-Y Reconstruction
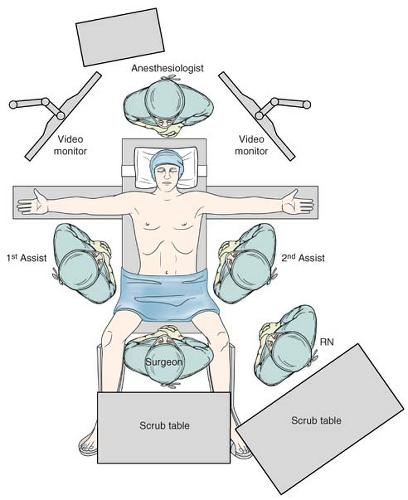
Patients are admitted the morning of surgery after being on clear liquids prior to surgery and being NPO after midnight the day of the operation. In select cases, where a conversion to an open approach is considered likely, an epidural catheter may be placed pre-operatively. When in the operating room, patients have sequential compression devices placed on their lower extremities and undergo general anesthesia. Unless other medical conditions exist to prompt more extensive monitoring, large bore intravenous catheters are placed in addition to a Foley catheter. The operative set-up and steps are as follows;
Instrumentation and Equipment
In addition to the typical laparoscopic set-up, special instruments include
Nathanson liver retractor with Elmed or other anchoring device for the retractor
Ultrasonic scalpel or sonosurg device (Ligasure may be used as well)
Universal Endo-GIA stapler with stapling loads for vessels, bowel, and stomach
5-mm argon beam coagulator (optional)
laparoscopic needle holder
split-leg bed with foot pads
gastroscopy set-up with a separate tower for intraoperative tumor localization and marking.
Positioning and Trocar Placement
Place the patient in the supine position in the split leg position with spreader bars and foot pads (Fig. 18.1).
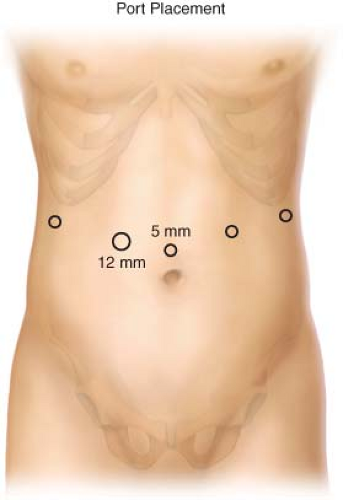
Place five trocars into the lower abdomen (Fig. 18.2), typically four 5-mm ports and one 12-mm port (in the right periumbilical position). Carbon dioxide insufflation is maintained at a pressure of 15 mm Hg.
The Nathanson liver retractor is placed to retract the left lobe of the liver via a 3-mm skin incision made in the midline at the subxiphoid position, and the retractor is then held into place with a stationary retractor. The patient is placed in steep reverse Trendelenburg position.
Operative Technique
Part I: Tumor Localization and Entry into the Lesser Sac
Prior to beginning resection, visualize the tumor and the intended extent of resection. If the tumor cannot be visualized from outside the stomach, then begin with intraoperative endoscopy to mark the exact tumor site with a stitch.
Lift the greater omentum in the cephalad direction to visualize the transverse colon.
Enter the lesser sac via the top of the transverse colon. Carefully dissect under the omentum with care to preserve the transverse mesocolon, until the posterior wall of the stomach is visualized (Fig. 18.3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree