and Chao-Hui Zheng1
(1)
Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou, China
6.1 Review of Laparoscopic Splenic Hilar Area Lymph Node Dissection for Gastric Cancer
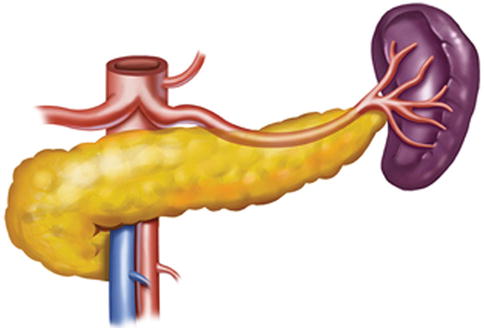
Generally, the lymph nodes (LNs) in the splenic hilar region include the No. 4sa, No. 4sb, No. 10, and No. 11d LNs. This is an important segment in gastrectomy regarding D2 lymph node dissection in gastric cancer, particularly cancer located in the middle and upper third of the stomach (Fig. 6.1).
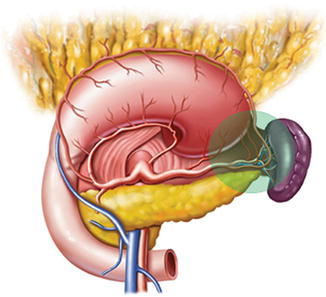

Fig. 6.1
Splenic hilar area
With respect to the relationship between the metastasis of the No. 4sa and No. 4sb LNs and the location of the tumor, it has been reported [1] that the rate of No. 4sa LNs metastasis (LNM) is 4.0–12.0 % for gastric body cancer and 19.0–36.0 % for the gastric fundus and cardial cancer. However, the incidence is relatively low for distal gastric cancer and is commonly <5.0 %. The metastatic rate of No. 4sb LNs is 18.0 %, 19.0 %, and 26.0 % in cardial, gastric body, and antrum cancer, respectively. Therefore, routine resection of the No. 4sa and No. 4sb LNs for advanced upper or middle gastric cancer and routine resection of the No. 4sb LNs for advanced lower gastric cancer are no longer controversial during D2 lymphadenectomy.
The ratio of No. 10 and No. 11 LNM has been reported to be 9.8–27.9 % [2–4] and 13.7–20.0 % [5, 6], respectively. A 346 case analysis for laparoscopic spleen-preserving No. 10 lymph node dissection for proximal gastric cancer by our center indicated that the incidence of No. 10 LNM is 10.1 % in advanced proximal gastric cancer [7]. No. 10 LNM is mainly associated with tumor location, depth of invasion, and Borrmann type [8]. The rate of gastric cancer in the upper and middle third of the stomach is significantly higher, particularly for advanced cancer located on the side with the greater curvature. No metastatic No. 10 and No. 11 LNs have been observed in early gastric cancer [9]. Our results [7] revealed that tumor location, depth of invasion, and metastases to No. 7 and No. 11 LNs were independent risk factors for No. 10 LNM. The status of No. 10 and No. 11 LNs is a significant prognostic factor for gastric cancer. It has been demonstrated that the 5-year survival rate of patients without metastases is 51.6 %, while that of patients with LNM is 11.0 % [10]. Therefore, dissection of the No. 10 and No. 11 LNs is necessary in advanced upper and middle gastric cancer. This approach is also suggested in the 3rd edition of Japanese Gastric Cancer Treatment Guidelines [11]. However, for advanced proximal gastric cancer located in the lesser curvature, without serosal invasion or No.7 and No. 11 LNM, whether there is a need to undergo No. 10 lymph node dissection demands further research [7]. In the 1990s, pancreaticosplenectomy was performed to entirely excise the No. 10 and No. 11 LNs. Prophylactic dissection of splenic hilar LNs by conducting such surgery increases the risk of complications associated with distal pancreatectomy such as pancreatitis, pancreatic fistula, intra-abdominal infection or abscess, and postoperative diabetes. It also does not significantly improve the 5-year survival rate (35.6 % vs. 42.2 %; P = 0.622) [12, 13]. Hence, some investigators have suggested that pancreaticosplenectomy cannot be used as a routine surgical method. In addition, the spleen and pancreas tail are only resected when obvious tumor invasion has been observed [14]. To achieve radical excision and avoid the complications caused by distal pancreatectomy as mentioned above, some investigators have advocated that it should be appropriate to exclusively perform pancreas-preserving splenic hilar lymph node dissection using splenectomy to cure upper and middle third gastric cancer. In recent years, researchers have gradually concentrated on the immunological aspects of the spleen, such as antitumor and anti-infection functions, which are also important in maintaining the health of patients. It is reported by Schwarz et al. [14] that, with the development of surgical techniques, spleen-preserving splenic hilar lymph node dissection has been demonstrated to be technically feasible, with a curative effect similar to splenectomy. And no survival benefits have been observed after splenectomy (48.3 % vs. 54.8 %; P = 0.503), while patient morbidity and mortality were significantly increased. In contrast, pancreas- and spleen-preserving lymph node dissection (D2) has achieved improved prognosis. Therefore, whether splenectomy should be performed requires careful consideration. The concept of spleen-preserving splenic hilar lymph node dissection is being accepted by an increasing number of clinicians.
In the clinic, the areas adjacent to the splenic hilar are complex and located in a narrow, but very deep, operating space. The spleen often adheres to the omentum or peritoneum, making it susceptible to injury. The vessels in the splenic hilar are particularly intricate and variable; thus, in open surgery, the spleen and distal pancreas must be pulled out of the abdomen, and the No. 10 LNs can then be dissected thoroughly. However, this manipulation is traumatic and time-consuming; moreover, spleen floating or twisting might occur after the operation. If the spleen and distal pancreas are not mobilized before splenic hilar region dissection, it can be difficult to radically remove the LNs because of the lack of adequate exposure. Laparoscopic amplification and the superior effects of ultrasonic scalpel for cutting and hemostasis allow the surgeon to clearly visualize the perigastric fascia, intrafascial space, vasculature, nerves, and other structures. The splenic vessels and their branches can thereby be comfortably exposed, and the meticulous procedure involved in No. 10 and No. 11 lymphadenectomy can be smoothly and efficiently completed. Therefore, the application of laparoscopy may have advantages in spleen-preserving splenic hilar lymph node dissection.
The first report of laparoscopic spleen-preserving splenic hilar lymph node dissection, regarding the treatment of gastric cancer in the upper and middle third of the stomach, was published by Hyung et al. [15] in 2008. A mean number of 2.7 (1–5) LNs per patient was found in the splenic hilar, and the effect of dissection was the same as that in open surgery. In addition, laparoscopic surgery provides obvious advantages including smaller incision, minimal invasiveness, and shorter operating time because of maintenance of the spleen in situ. In addition, it has been found that laparoscopic spleen-preserving splenic hilar lymphadenectomy is safe and feasible. In our study, the average number of retrieved LNs under laparoscopic surgery was 3.6 per patient, and no patients required open conversion resulting from injury to the spleen or its vessels; no complications associated with dissection of the splenic hilar region, such as hemorrhage, splenic ischemia, and splenic necrosis, have been observed postoperatively indicating favorable short-term outcomes [16]. It can be predicted that, with the improvement of standardized training and the development of laparoscopic techniques, laparoscopic spleen-preserving splenic hilar lymphadenectomy will become one of the standard treatments for advanced upper and middle gastric cancer.
6.2 Anatomy Associated with Lymph Node Dissection in the Splenic Hilar Area
6.2.1 Fascia and Intrafascial Space in the Splenic Hilar Area
6.2.1.1 Gastrosplenic and Splenorenal Ligaments
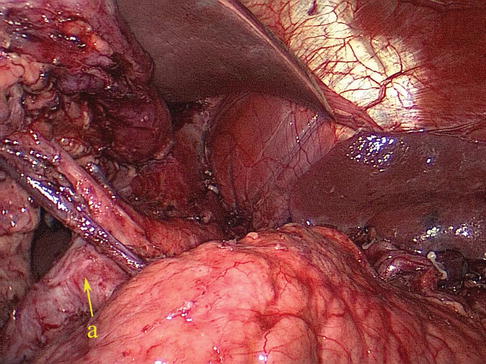
The gastrosplenic ligament (GSL) connects the greater curvature of the stomach with the splenic hilar above the pancreas (Figs. 6.2, 6.3, and 6.4), while the splenorenal ligament (SRL) extends from the spleen to the left kidney at the lateral abdominal wall (Figs. 6.5 and 6.6). The GSL consists of two layers of peritoneum, and the short gastric and left gastroepiploic vessels lie between them. The posterior layer is continuous with the peritoneum, which covers the splenic hilar and the posterior wall of the stomach. The anterior layer is formed by peritoneal reflection which leaves the spleen in the gastric notch of its upper pole. It then becomes continuous with the peritoneum covering the anterior wall of the stomach.
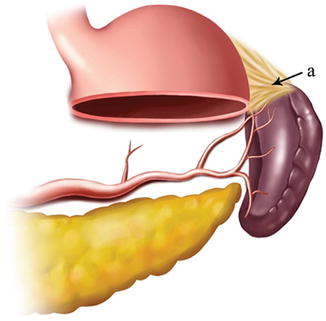
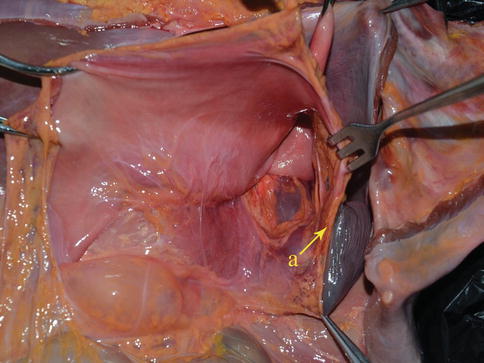
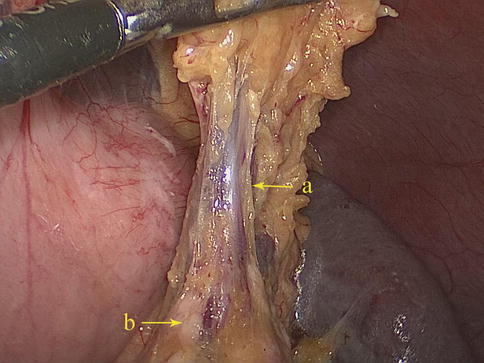
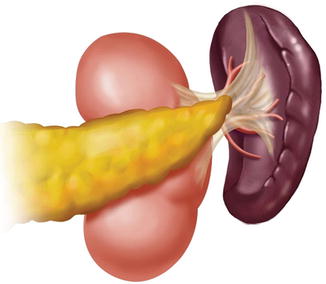
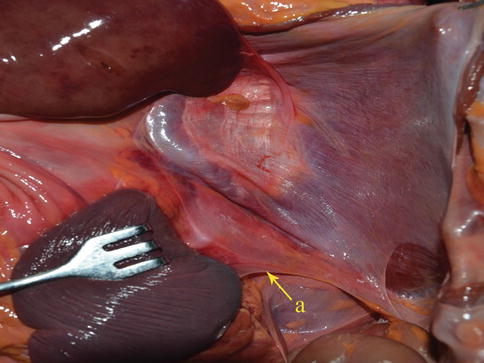

Fig. 6.2
GSL (a)

Fig. 6.3
GSL (a) (in autopsy)

Fig. 6.4
GSL (a). Pancreas tail (b)

Fig. 6.5
The schematic figure of the SRL

Fig. 6.6
SRL (a) (in autopsy)
The SRL also consists of two layers of peritoneum, with the following structures located between them: the splenic artery (SpA) and all of its branches, nerves, and lymph vessels; the tail of the pancreas is located at the lower part of the SRL. The anterior layer of the SRL inwardly joins the peritoneum of the posterior wall of the omental bursa above the left kidney, and it extends upward to the splenic hilar, where it connects with the posterior layer of the GSL. The posterior layer of the SRL passes outward to join the peritoneum below the diaphragm and then runs to the surface of the spleen above the splenic incisura. There is a mutually linked potential space within the GSL and the SRL. This space, which is linked with the space at the upper pancreatic border, provides a pathway for the splenic vessels and their branches. The front of the GSL is continuous with the anterior pancreatic fascia (APF) at the pancreatic tail. Thus, the space within the GSL can be entered by opening the APF from the pancreatic tail along the space at the upper border of the pancreas. The ligaments around the splenic hilar can be dissected to expose the terminal branches of the SpA and the origin of the left gastroepiploic artery (LGEA) (Fig. 6.7).
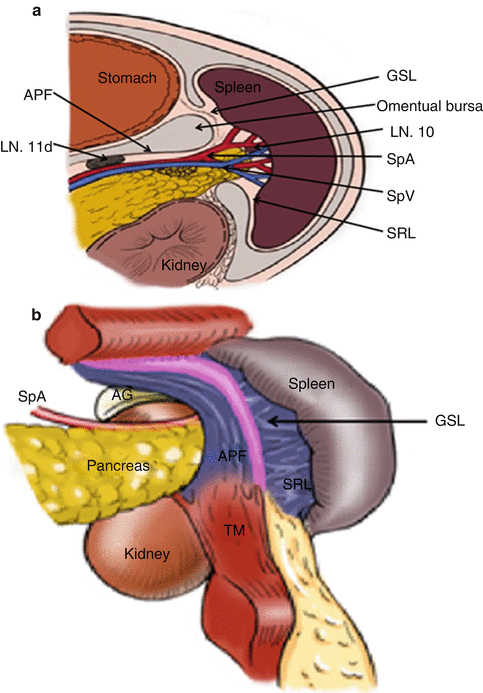

Fig. 6.7
(a) The GSL and the SRL are mutually linked. (b) The GSL and the SRL are mutually linked
6.2.1.2 Toldt’s Space (TS) and Gerota’s Fascia
TS is a widely distributed avascular plane that forms a complete boundary between the retropancreatic fascia and Gerota’s fascia. Gerota’s fascia, which encloses the renal vessels, the left kidney, and the adrenal gland, constitutes the rear of TS. The front consists of the back of the pancreatic body and tail. The transverse colonic intrafascial space is linked with the anteroinferior side of TS (Fig. 6.8).
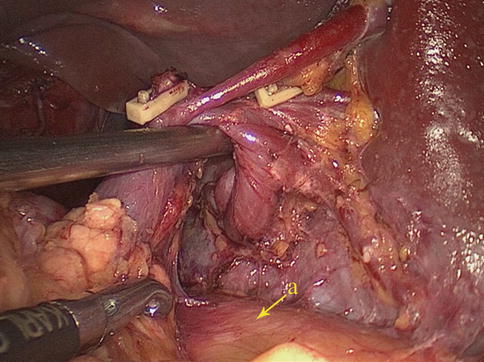

Fig. 6.8
Gerota’s fascia (a)
6.2.2 Vascular Anatomy Associated with Lymph Node Dissection in the Splenic Hilar Area
6.2.2.1 Arterial Anatomy Associated with Lymph Node Dissection in the Splenic Hilar Area
SpA
The SpA branches from the celiac artery (CA). It meanders toward the left along the upper border of the pancreas, and it gives off the great pancreatic artery, caudal pancreatic artery, and some small arteries to the pancreas along the way. It also gives rise to the posterior gastric, short gastric, and left gastroepiploic arteries, which supply the posterior wall and the greater curvature of the stomach (Figs. 6.9, 6.10, and 6.11).
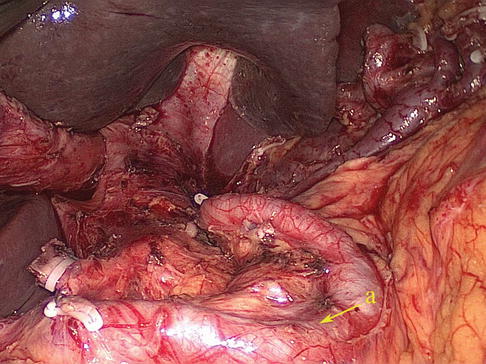
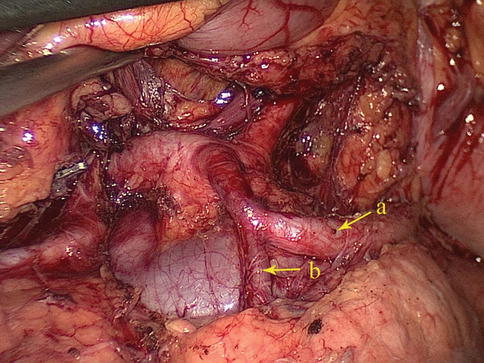
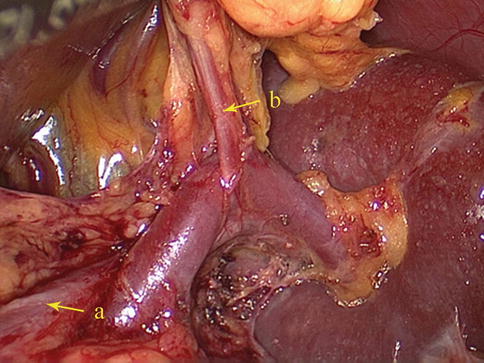

Fig. 6.9
SpA (a)

Fig. 6.10
The SpA (a) gives off the caudal pancreatic artery (b)

Fig. 6.11
The SpA (a) gives rise to the LGEA (b) before reaching the spleen
Relation Between the Course of the SpA and the Pancreas
The course of the SpA is intimately associated with the pancreas. The anatomical data of 319 patients with advanced proximal gastric cancer who underwent laparoscopic spleen-preserving hilar lymph node dissection in our center were retrospectively analyzed. The course of the SpA was divided into four variations based on the results.
Type I: In the majority of cases, the SpA follows the suprapancreatic course to the splenic hilar after arising from the CA. Present in 87 cases (27.2 %) (Figs. 6.12, 6.13, and 6.14).
Fig. 6.12
Type I. SpA
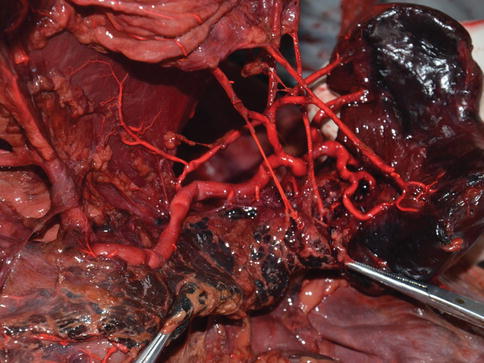
Fig. 6.13
Type I. SPA (in autopsy)
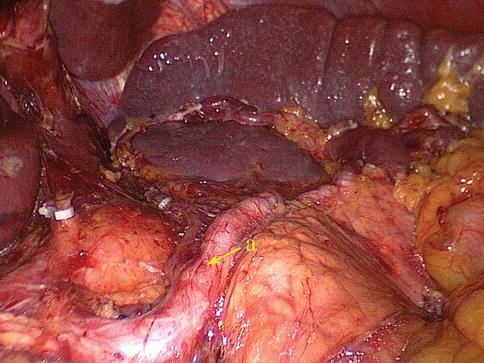
Fig. 6.14
Type I. SpA (a)
Type II: The middle one-half of the SPA has either a retro- or intrapancreatic course in 213 cases (66.8 %) (Figs. 6.15 and 6.16).
Fig. 6.15
Type II. SpA
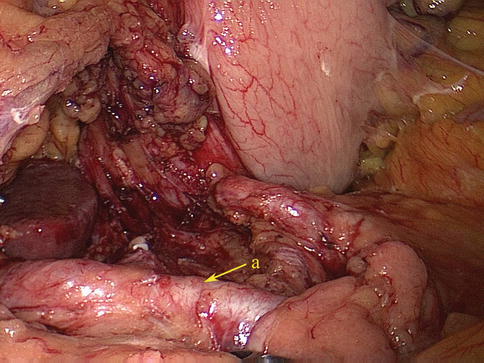
Fig. 6.16
Type II. SpA (a)
Type III: The distal one-half of the SPA follows either a retro- or intrapancreatic course in 13 cases (4.1 %) (Figs. 6.17 and 6.18).
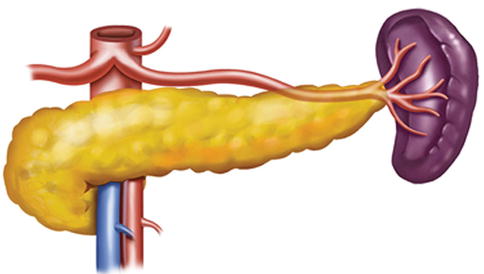
Fig. 6.17
Type III. SpA
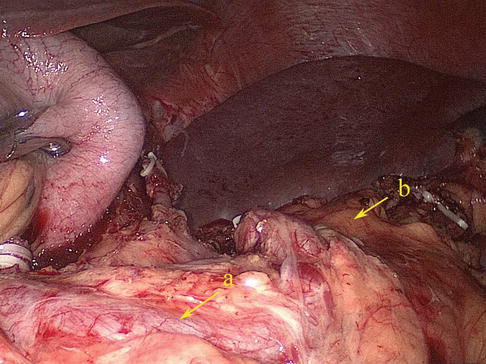
Fig. 6.18
Type III. SpA (a) and pancreatic tail (b)
Branches of the SpA
Splenic Lobar Artery (SLA)
The SLA refers to the terminal branch of the SpA at the splenic hilar, and there are four types of anatomical variation according to the anatomical data of 319 cases in our center:
One-branched type: The SpA passes tortuously through the splenic hilar without dividing into terminal branches. This type is relatively rare, observed in 22 cases (6.9 %) (Figs. 6.21 and 6.22).
Fig. 6.21
One-branched type
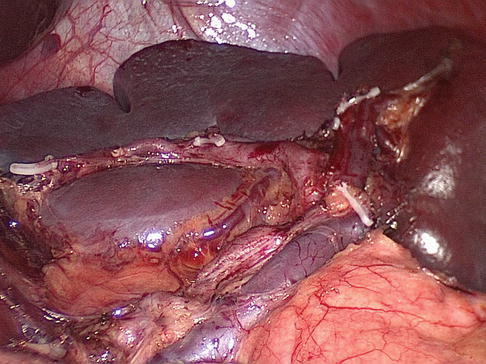
Fig. 6.22
One-branched type
Two-branched type: The SpA gives off the superior and inferior lobar arteries of the spleen. This type is common, observed in 252 cases (79.0 %) (Figs. 6.23, 6.24, and 6.25).
Fig. 6.23
Two-branched type
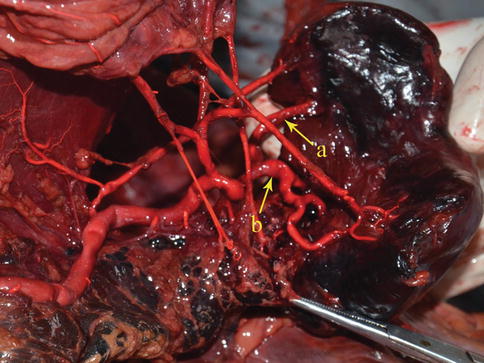
Fig. 6.24
Two-branched type. The superior lobar artery (a) and inferior lobar artery of the spleen (b) (in autopsy)
Fig. 6.25
Two-branched type
Three-branched type: The SpA divides into the superior, middle, and inferior lobar arteries of the spleen. Present in 43 cases (13.5 %) (Figs. 6.26 and 6.27).
Fig. 6.26
Three-branched type
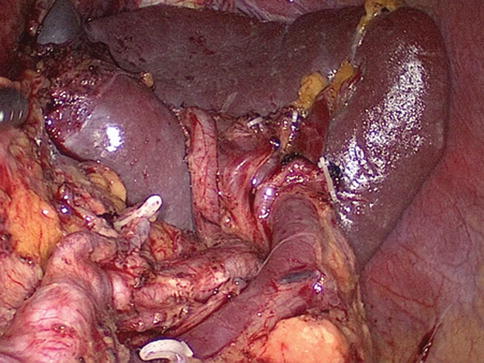
Fig. 6.27
Three-branched type
Splenic Pole Artery (SPoA)
The SPoA refers to an artery that directly enters the upper and/or lower pole of the spleen without passing through the splenic hilar. The splenic upper-pole artery (SUPA) mostly arises from the SpA trunk (SpAT), but in rare cases it arises from the SLA. The splenic lower pole artery (SLPA) generally issues from the LGEA or inferior lobar artery of the spleen (ISLA) and infrequently originates from the SpAT. Our results show that the incidence of the SUPA is 16.6 % (53/319), and the incidence of the SLPA is 5.0 % (16/319) (Figs. 6.30, 6.31, 6.32, and 6.33).
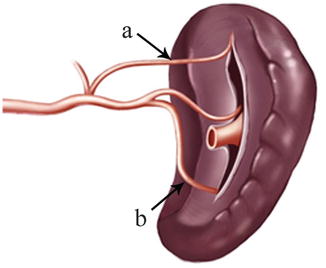
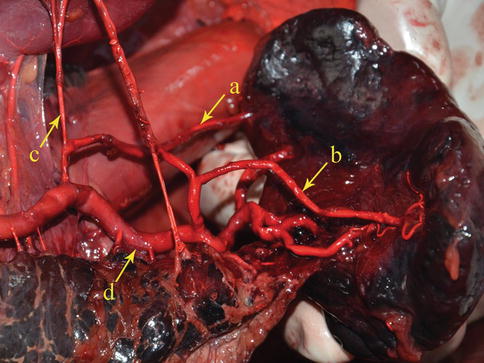
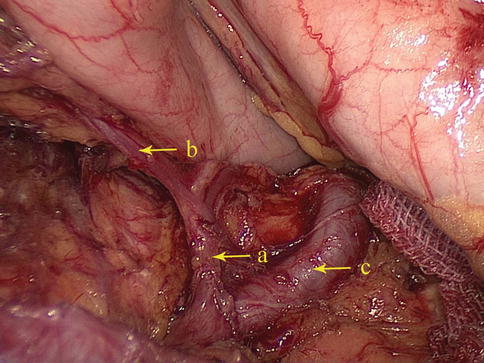
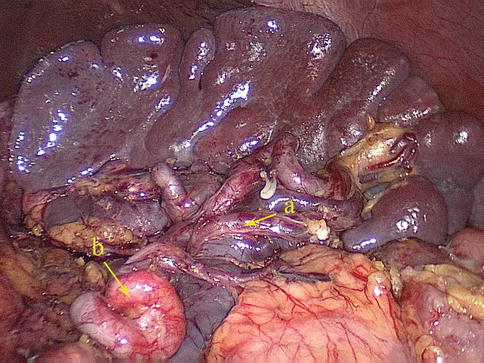

Fig. 6.30
SUPA (a) and SLPA (b)

Fig. 6.31
SUPA (a), SLPA (b), PGA (c), and SpA (d) (in autopsy)

Fig. 6.32
SUPA (a), PGA (b), and SpA (c)

Fig. 6.33
The SLPA (a) arises from the SpA (b)
LGEA
The LGEA arises as a branch of the SpA, ISLA, or SLPA. It then runs from left to right along the greater curvature of the stomach between the two anterior layers of the greater omentum in the GSL. The LGEA gives off several branches to both surfaces of the stomach and to the greater omentum before it joins the right gastroepiploic artery (RGEA) to form the gastroepiploic arterial arch (Figs. 6.34, 6.35, 6.36, and 6.37).
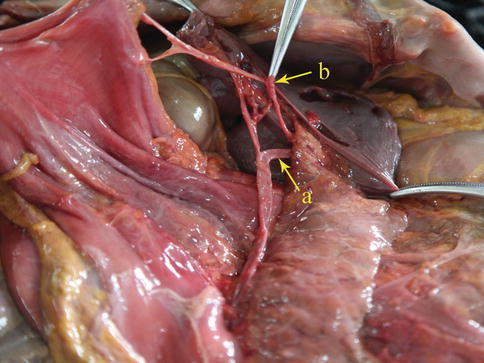
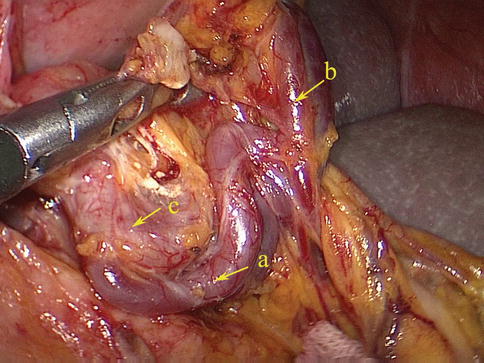
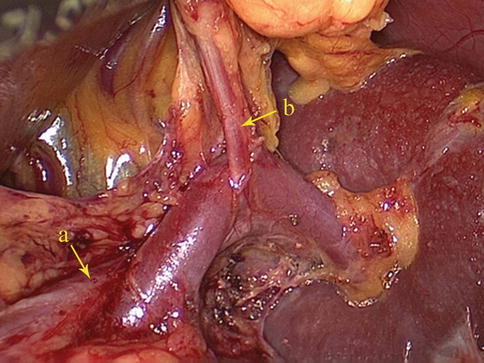
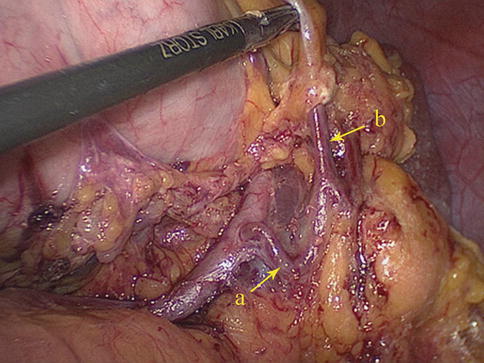

Fig. 6.34
The SLPA (a) gives off the LGEA (b) (in autopsy)

Fig. 6.35
The SLPA (a) gives off the LGEA (b) and the SpA (c)

Fig. 6.36
The SpA (a) gives off the LGEA (b)

Fig. 6.37
The ISLA (a) branches out the LGEA (b)
Short Gastric Artery (SGA)
The SGA, which has four to six small branches, arises from the SpAT or its terminal divisions. Individual SGAs occasionally issue from the LGEA (Figs. 6.38, 6.39, 6.40, and 6.41). They all pass between the layers of the GSL and are distributed outside of the gastric fundus. During total gastrectomy, it should be noted that the lengths of the short gastric vessels (SGVs) are shorter, the closer they are to the superior pole region of the spleen (Fig. 6.42).
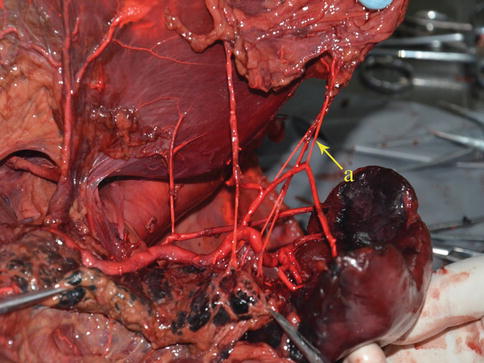
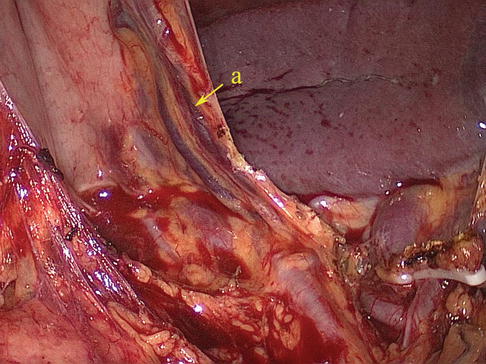
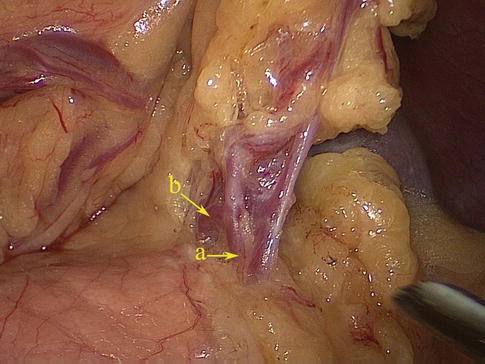
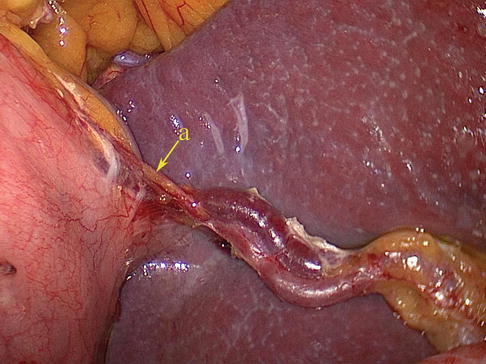

Fig. 6.38
SGA (a)

Fig. 6.39
SGA (a) (in autopsy)

Fig. 6.40
The SGAs (a) issue from the SpA or its branches

Fig. 6.41
The left gastroepiploic vessels (a) give off the SGVs (b)

Fig. 6.42
The lengths of the SGVs (a) are shorter, the closer they are to the superior pole region of the spleen, which is closely against the fundus
Posterior Gastric Artery (PGA)
The PGA arises from the SpAT and its branches on the posterior wall of the stomach. It most frequently arises from the trunk of the SpA (Figs. 6.43 and 6.44), while it may arise from the SUPA in a few cases (Figs. 6.45, 6.46, and 6.47). The PGA is present in 60.0–80.0 % of cases and ascends in the rear of the omental bursa accompanying the vein of the same name.
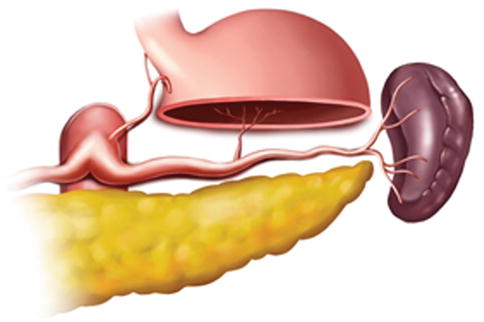
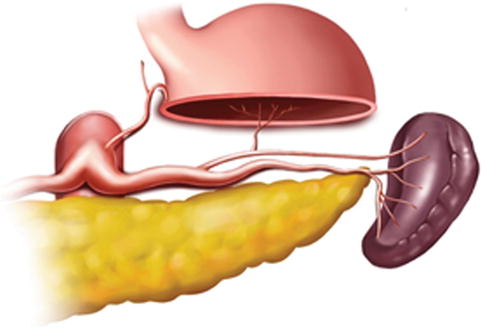
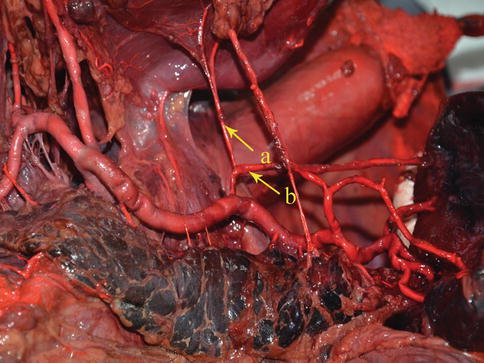
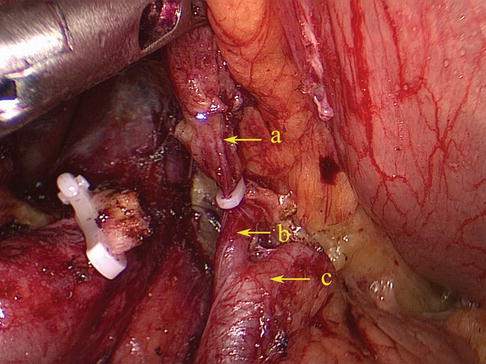

Fig. 6.43
The PGA arises from the trunk of SpA

Fig. 6.44
The PGA (a) arises from the trunk of SpA (b)

Fig. 6.45
The PGA issues from the SUPA

Fig. 6.46
The PGA (a) issues from the SUPA (b) (in autopsy)

Fig. 6.47
The PGA (a) issues from the SUPA (b). SpA (c)
Classification of the Terminal Branches of the SpA
We usually define the different terminal branches by the distance between the artery’s furcation to the splenic hilar region, including the distributed type and concentrated type of SpA. The concentrated-type SpA always divides into its terminal branches within 2 cm from the splenic hilum. And the SpAT is long while the SLAs are short and centralized (Figs. 6.48 and 6.49). On the contrary, the distributed-type SpA generally divides into its terminal branches more than 2 cm from the splenic hilum. And the branches are long and of smaller caliber, always accompanying the SPoA (Figs. 6.50 and 6.51). The anatomical data of 319 patients in our center showed 64.3 % of the patients were the concentrated type (205 cases) and 35.7 % were the distributed type (114 cases).
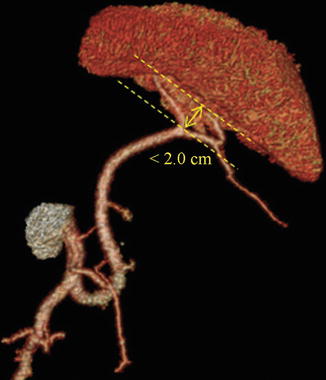
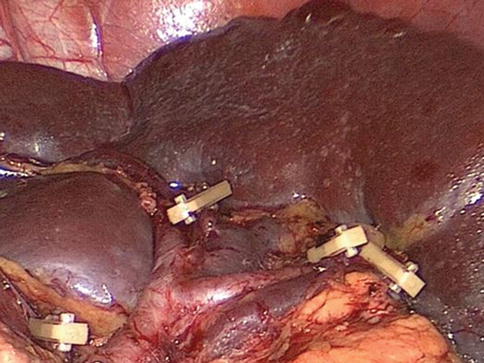
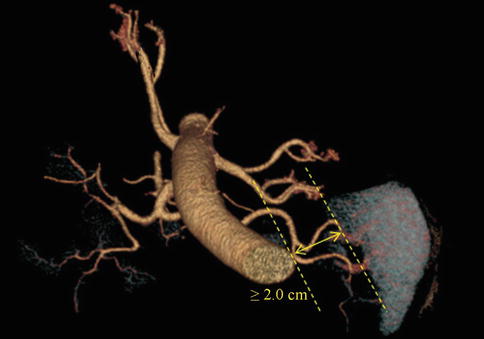
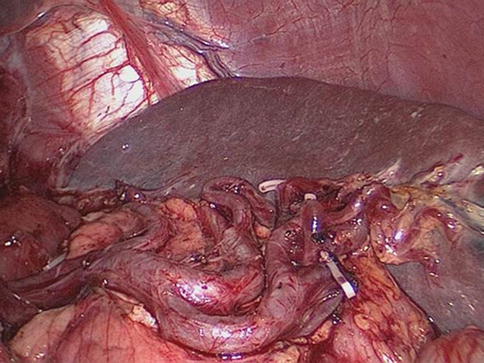

Fig. 6.48
Concentrated type (3D CT)

Fig. 6.49
Concentrated type

Fig. 6.50
Distributed type (3D CT)

Fig. 6.51
Distributed type
6.2.2.2 Veins Associated with Lymph Node Dissection in the Splenic Hilar Area
Left Gastroepiploic Vein (LGEV)
The LGEV runs parallel with the artery of the same name and ends in the splenic vein (SpV) (Fig. 6.52).
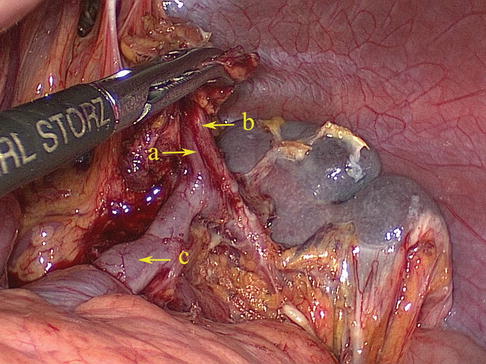

Fig. 6.52
The LGEV (a) runs parallel with the artery of the same name (b) and ends in the SpV (c)
SpV
The SpV is formed by the union of splenic lobar veins that issue from the splenic hilar. It also receives the blood of the splenic pole vein (SPoV), branches of the pancreatic vein, the short gastric vein, the LGEV, and the inferior mesenteric vein (IMV). The SpV generally accompanies the SpA, but it is less tortuous than the artery (Figs. 6.53 and 6.54).
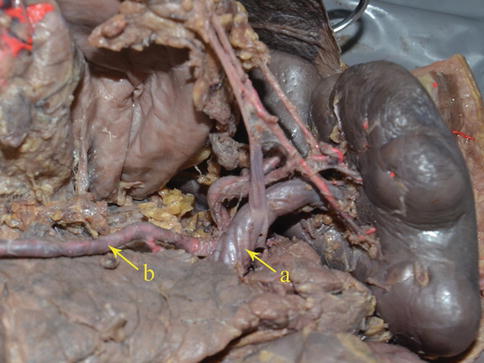
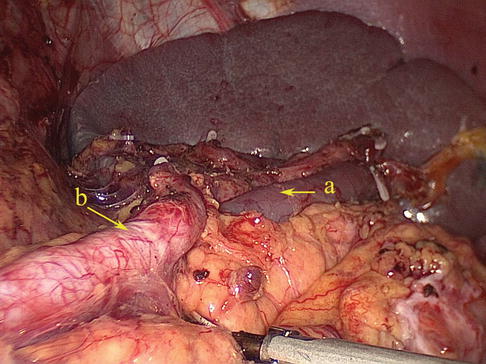

Fig. 6.53
The SpV (a) accompanies with the SpA (b) (in autopsy)

Fig. 6.54
The SpV (a) accompanies with the SpA (b)
6.2.3 Lymph Node Anatomy Associated with Lymph Node Dissection in the Splenic Hilar Area
6.2.3.1 No. 4 LNs (LNs Around the Greater Curvature of the Stomach)
The No. 4 LNs are divided into the following three substations according to the arteries they accompany.
No. 4d LNs
Definition of the No. 4d LNs
The No. 4d LNs are included in the two layers of the mesogastrium attached to the greater curvature of the stomach along the RGEA. They are separated from the No. 6 LNs by the first branch of the RGEA feeding the gastric wall. The No. 6 LNs are on the right side of the branch, and the No. 4d LNs are on the left side (Figs. 6.55 and 6.56).
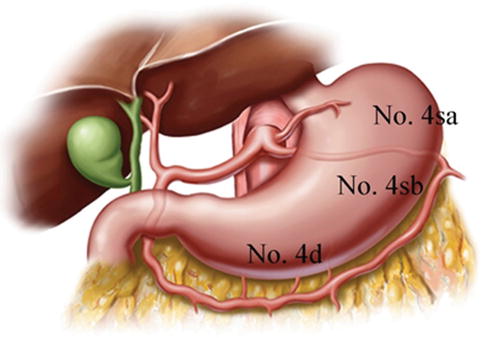
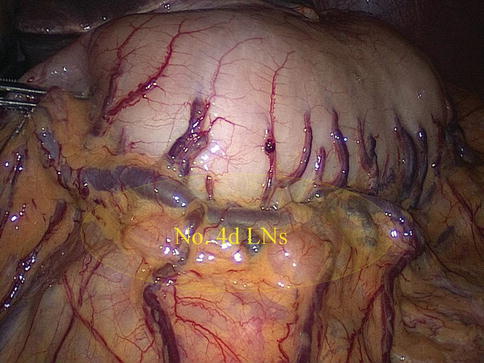

Fig. 6.55
Distribution of the LNs around the greater curvature of stomach

Fig. 6.56
The scope of the No. 4d LNs
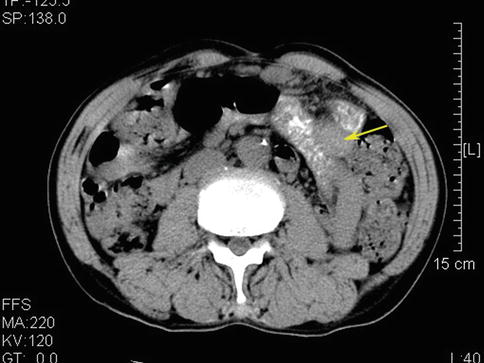
No. 4d LNM (Figs. 6.57 and 6.58)
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree