Chapter 23 Laparoscopic Management of Acute Small Bowel Obstruction
Operative indications
Contraindications to laparoscopic adhesiolysis for SBO are listed in Table 23-1. These generally concern the severity or nature of the disease and the surgeon’s ability to deal with these conditions. In general, an ideal candidate for minimally invasive release of SBO would (1) be hemodynamically stable; (2) not have generalized peritonitis; (3) have minimal small bowel dilatation; (4) not have a “frozen” abdomen (i.e., a peritoneal cavity obliterated with adhesions); and (5) not have multiple previous laparotomies. The risk for enterotomy (both recognized and delayed) appears to be higher in patients with multiple previous laparotomies. Successful completion of laparoscopic adhesiolysis for SBO would depend on patient selection as guided by Table 23-1.
Table 23-1 Contraindications to Laparoscopic Adhesiolysis
| Absolute Contraindications | Relative Contraindications |
|---|---|
| Small bowel dilatation (>4 cm) on a plain abdominal film | Number of previous laparotomies >2 |
| Peritonitis on physical examination | Multiple adhesions |
| Severe cardiovascular, respiratory, or hemostatic disease | |
| Hemodynamic instability | |
| Surgeon inexperience |
Preoperative evaluation, testing, and preparation
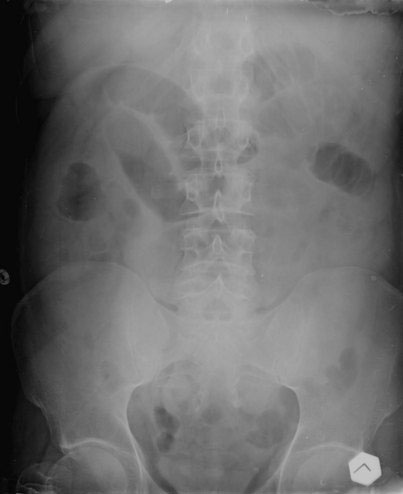
The plain abdominal radiograph (Fig. 23-1) remains the primary radiologic test performed in suspected SBO by virtue of low cost, wide availability, and utility. Identification of level of obstruction (small bowel vs. colon) on the plain film may be possible, although grossly distended small bowel can be mistaken for large bowel, and a colonic volvulus can be mistaken for small bowel. In addition, an obstructing colon cancer can cause an SBO in up to 15% of cases. On the other end of the spectrum, a normal abdominal radiograph does not exclude the diagnosis of SBO.
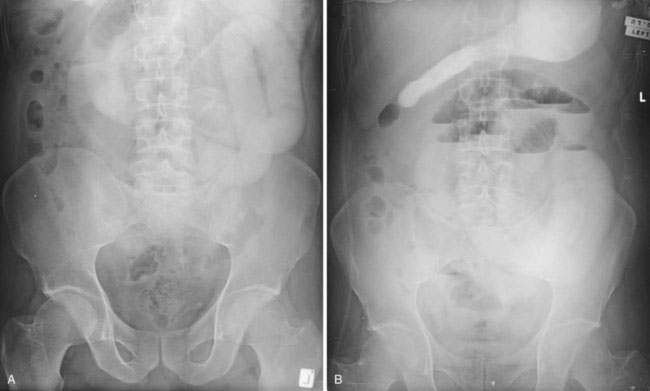
The oral ingestion of water-soluble contrast agents has become a common diagnostic and therapeutic procedure for SBO. Gastrografin (a mixture of sodium and meglumine diatrizoates) is a widely used contrast agent. Ingestion of this high-osmolarity substance draws water into the small bowel lumen, which is thought to decrease wall edema and stimulate motility. The therapeutic role of Gastrografin probably is limited to speeding resolution of an obstruction that would have resolved without an operation. Typically, 60 to 100 mL of Gastrografin is administered through the nasogastric tube after the diagnosis of SBO has been made and when there is no concern for strangulation or ischemia. Abdominal radiographs should be repeated 3 to 4 hours after Gastrografin administration (Fig. 23-2). If the contrast has reached the cecum and the patient is clinically well, then the patient may be started on liquids. The diet is advanced as tolerated, and early discharge should be possible. If the contrast does not reach the cecum within 24 hours, then surgical intervention may be required.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree