and Chao-Hui Zheng1
(1)
Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou, China
4.1 Review of Laparoscopic Infrapyloric Area Lymph Node Dissection for Gastric Cancer
Lymph node dissection in the infrapyloric area is an important procedure during laparoscopic radical gastrectomy for gastric cancer, which mainly involves dissection of the No. 6 lymph nodes (LNs) (the infrapyloric LNs) and the No. 14v LNs [LNs located at the root of the superior mesenteric vein (SMV)] (Fig. 4.1).
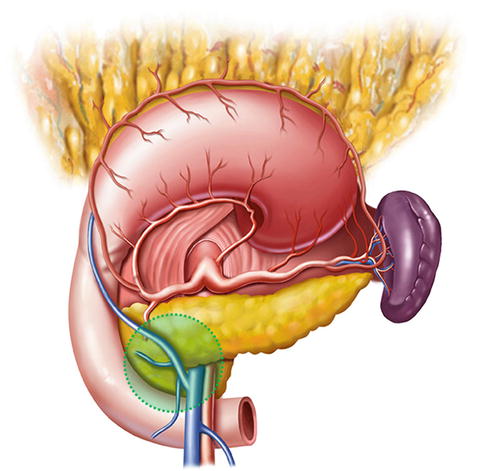

Fig. 4.1
Infrapyloric area
The frequency of No. 6 lymph node metastasis (LNM) varied between 26.0 % and 34.0 % [1, 2], while the 5-year survival rate among patients with No. 6 LNM was 23.0 % [2]. No. 6 LNM was correlated with tumor location, depth of invasion, and other factors [3]. The metastatic rates of No. 6 LNs in early middle and lower gastric cancer were found to be 1.7 % and 7.6 %, respectively [4], while in advanced middle and lower gastric cancer, the incidence of No. 6 LNM varied between 34.2 % and 41.0 % [1, 5], which is the highest incidence of LNM compared with other groups [6, 7]. However, the incidence of No. 6 LNM in early upper gastric cancer was low. Xu et al. [8] reported that No. 6 LNM was not observed in patients with early proximal gastric cancer after total gastrectomy. Ishikawa et al. [9] analyzed the clinical data of 127 cases with proximal gastric cancer and showed that No. 6 LNM was negative in the early stages. However, the frequency of No. 6 LNM was up to 8.6 % in advanced proximal gastric cancer [10]. Consequently, No. 6 LNs should be routinely removed during curative treatment for middle and lower gastric cancer or advanced proximal gastric cancer according to the 3rd edition of the Japanese Gastric Cancer Treatment Guidelines [11]. However, it is unnecessary to dissect No. 6 LNs for early upper gastric cancer during radical proximal subtotal gastrectomy.
Metastasis of the No. 14v LNs acts as an important prognostic factor for patients with gastric cancer. Although the incidence of No. 14v LNM was lower (0.0–1.3 %) in early gastric cancer, it rose to 19.7 % in advanced gastric cancer [1, 8, 12, 13]. The frequency of No. 14v LNM was also associated with various factors including tumor location, tumor size, depth of invasion, N staging, and whether there was invasion in the surrounding organs and tissues [12, 14, 15]. Zhu et al. [1] considered that the No. 14v LNM rate itself was low, while the anatomy here was complicated and it was easy to damage the gastrocolic trunk, middle colonic vein, and SMV, leading to uncontrollable bleeding. These factors increase the difficulty and risk in dissecting No. 14v LNs. In addition, No. 14v LNM is mostly evident in patients with advanced cancer. These patients have deeper depth of invasion and more positive LNs. Thus, patients with positive No. 14v LNs have a poor prognosis even when dissection is performed. Hence, routine dissection of No. 14v LNs is not recommended in lower gastric cancer. Moreover, an analysis of 1,104 patients who underwent gastrectomy including No. 14v lymph node dissection by An et al. [14] showed that the metastatic rate of No. 14v LNs was only 6.6 %, and three-quarters of patients with No. 14v-positive disease suffered stage IV cancer. In patients with stage II and III gastric cancer, the frequency of No. 14v LNM was only 2.4 % and 4.1 %, respectively. Patients with No. 14v-positive gastric cancer had a poor prognosis similar to that of patients with distant metastasis. The 3- and 5-year survival rates of patients with No. 14v-positive disease were demonstrated as 24.0 % and 9.0 %, respectively. Therefore, An et al. [14] suggested that the No. 14v LNs should be regarded as the extra-perigastric LNs, and whether it should be removed during D2 lymphadenectomy for distal gastric cancer was worth consideration. Hence, routine resection of No. 14v LNs is not recommended, which coincides with the stipulation that the No. 14v LNs is excluded from D2 lymphadenectomy according to the 3rd edition of Japanese Gastric Cancer Treatment Guidelines [11].
However, some scholars indicated that there was a tendency of LNM from No. 6 LNs to No. 14v LNs based on the anatomical structure and the lymphatic drainage pathway in the gastric antrum [7, 12]. Moreover, there is more of a propensity for No. 6 LNM in lower gastric cancer, and the status of No. 6 LNs is an independent variable affecting No. 14v LNM. Hence, on the premise that the surgeon is skillful to achieve a radical treatment, it is reasonable to dissect No. 14v LNs for advanced lower gastric cancer when positive No. 6 LNs are suspected. Liang et al. [16] revealed that metastasis of No. 14v was an independent prognostic factor for advanced gastric cancer after D2 lymphadenectomy. Patients with No. 14v LNM after curative surgery demonstrated a significant lower survival rate than those without it (5-year overall survival rate, 8.3 % vs. 37.8 %, P < 0.01). For patients with advanced middle and lower gastric cancer, particularly those with a larger size of tumor, serosal invasion, and the possibility of No. 6 LNM, it was necessary to remove the No. 14v LNs.
4.2 Anatomy Associated with Lymph Node Dissection in the Infrapyloric Area
4.2.1 Fascia and Intrafascial Space in the Infrapyloric Area
4.2.1.1 Greater Omentum
The greater omentum is derived from the downward continuation of the embryonic anterior layer of the dorsal mesogastrium (DM). Its right border extends as far as the commencement of the duodenum, and its left border is continuous with the gastrosplenic ligament (GSL). The greater omentum is a four-layered fold of peritoneum. The two anterior layers, which originate at the greater curvature of the stomach and the commencement of the duodenum, descend a considerable distance and return upward to form the two posterior layers, which reach as far as the transverse colon. The posterior layers then enclose the transverse colon to form the transverse mesocolon (TM), and they eventually attach to the posterior abdominal wall. If the two anterior layers attach to the transverse colon when they pass it, this portion is called the gastrocolic ligament (GCL). In some cases, this attachment is not present and there is no actual GCL (Figs. 4.2, 4.3, and 4.4).
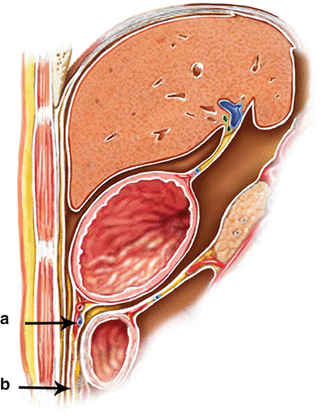
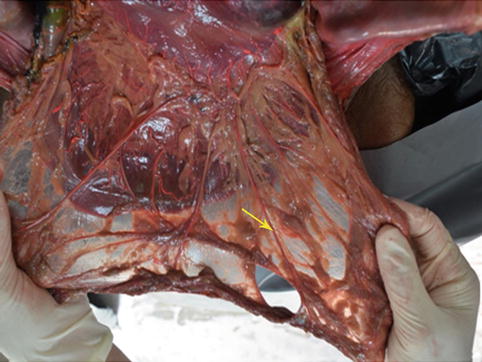
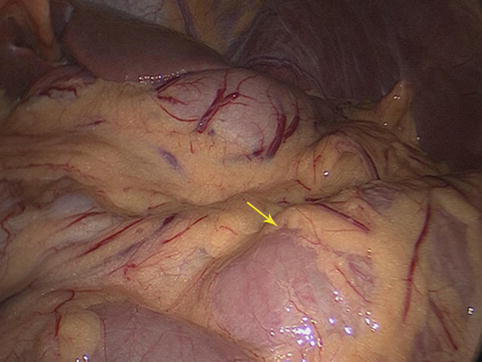

Fig. 4.2
GCL (a) and greater omentum (b)

Fig. 4.3
Greater omentum (in autopsy) (the arrow represents the whole greater omentum)

Fig. 4.4
Intraoperative view of the greater omentum (the arrow represents the whole greater omentum)
4.2.1.2 Gastrocolic Intrafascial Space (GIS)
The two posterior layers of the greater omentum and the TM fuse together near the pylorus to form a latent space called the GIS, which is an avascular zone full of loose connective tissue and some adipose tissue. The right gastroepiploic and infrapyloric vessels can be exposed by opening this space. Thus, the No. 6 LNs can be dissected (Figs. 4.5, 4.6, and 4.7).
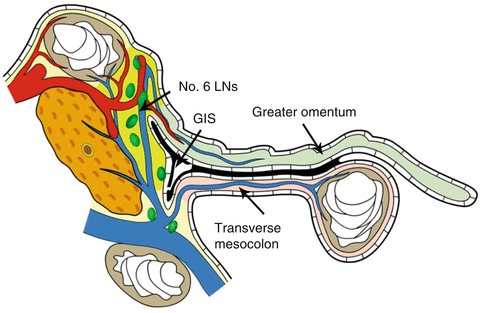
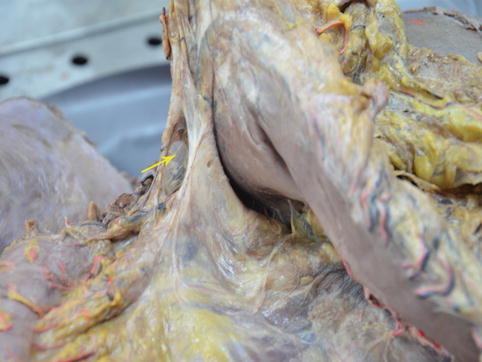
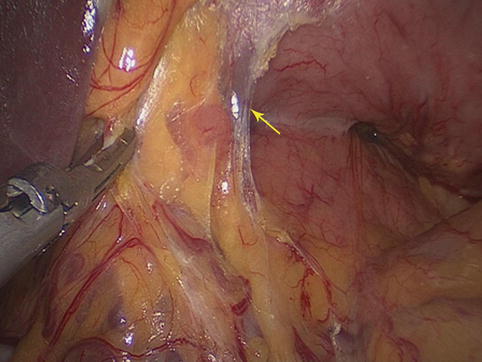

Fig. 4.5
The schematic figure of the GIS

Fig. 4.6
GIS under the pylorus (in autopsy) (the arrow represents the GIS)

Fig. 4.7
GIS (the arrow represents the GIS)
4.2.1.3 TM
The two posterior layers of the greater omentum separate to surround the transverse colon, then continue upward to the posterior wall of the abdomen to form the TM. This is a broad fold of the peritoneum, attaching from the hepatic flexure and extending across the middle of the right kidney, the descending part of the duodenum, the pancreas, and the front of the left kidney to end at the splenic flexure. Both ends of the TM are relatively fixed, while the middle part is longer and more mobile. The TM is composed of anterior and posterior lobes. Between them, there is a fusion space that is easily separated because of the loose connective tissue in it. The vessels, nerves, lymphatics, and LNs of the transverse colon are included in this space (Figs. 4.8, 4.9, and 4.10). The middle colic vessels (MCVs) within the space can be exposed by opening the anterior lobe of the TM. The anterior pancreatic space, which is linked with the TM fusion space, can be entered by opening the anterior pancreatic fascia along the MCVs toward their roots.
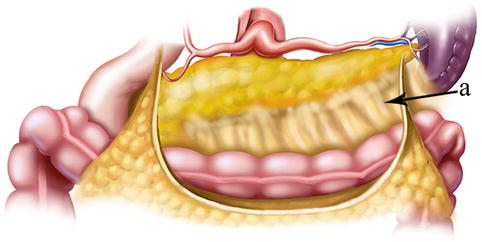
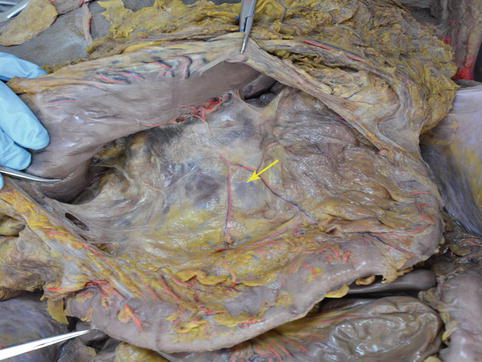
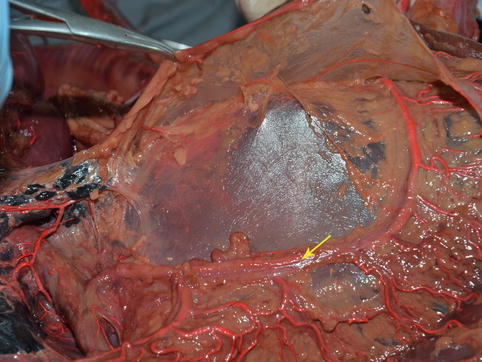

Fig. 4.8
TM (a)

Fig. 4.9
TM (in autopsy) (the arrow represents the TM)

Fig. 4.10
Vessels in the TM fusion space (in autopsy) (the arrow represents vessels)
4.2.1.4 Pancreaticoduodenal Fascia (PDF) and Its Intrafascial Space
The PDF is derived from the posterior layer of the DM. It encloses the pancreatic head and the second part of the duodenum, and it fuses with the ascending mesocolon. The intrafascial space between the posterior PDF and the inherent pancreatic fascia is called the posterior pancreaticoduodenal space (PPDS), which contains the SMV, portal vein (PV), and No. 14v LNs (Figs. 4.11 and 4.12).
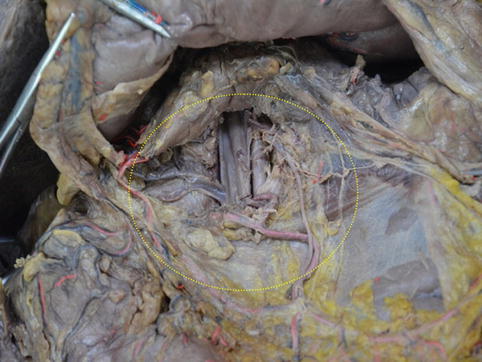
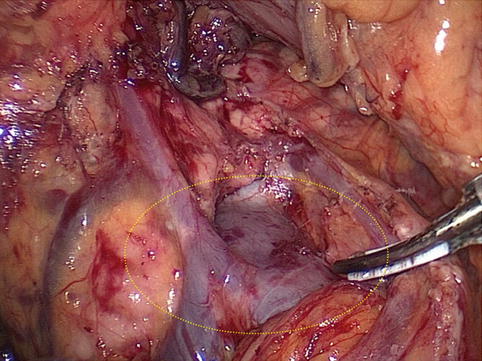

Fig. 4.11
PPDS (in autopsy)

Fig. 4.12
Intraoperative view of the PPDS
4.2.2 Vascular Anatomy Associated with Lymph Node Dissection in the Infrapyloric Area
4.2.2.1 Arteries Associated with Lymph Node Dissection in the Infrapyloric Area
Right Gastroepiploic Artery (REGA)
The RGEA with the larger diameter is the main arterial supply of the gastric antrum area. It is the largest terminal branch of the gastroduodenal artery (GDA) issued under the pylorus. After arising from the GDA, it runs to the left along the greater curvature of the stomach (between the anterior two layers of the greater omentum) and joins the left gastroepiploic artery (LGEA) to form the arterial arch of the gastric greater curvature, supplying the anterior and posterior walls of the stomach and the greater omentum. The RGEA and its branches are distributed within the greater curvature side of the gastric central axis: the upper border is the confluence of the RGEA and LGEA, and the lower border is the pylorus. Anatomical variations of the RGEA are very rare. Its root can generally be identified by confirming and dissecting along the GDA (Figs. 4.13, 4.14, and 4.15).
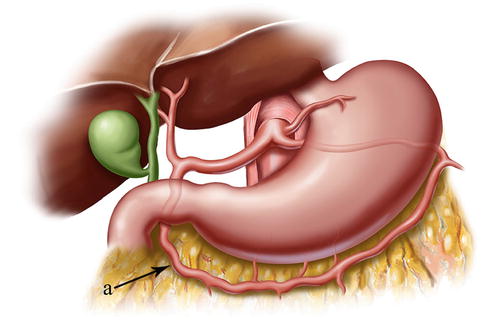
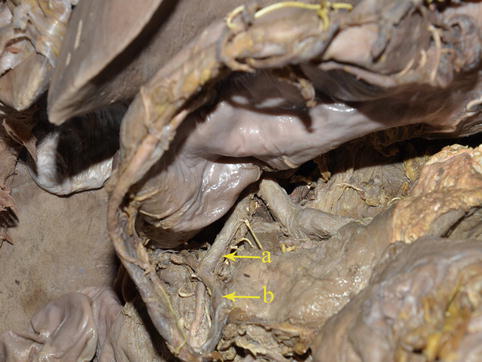
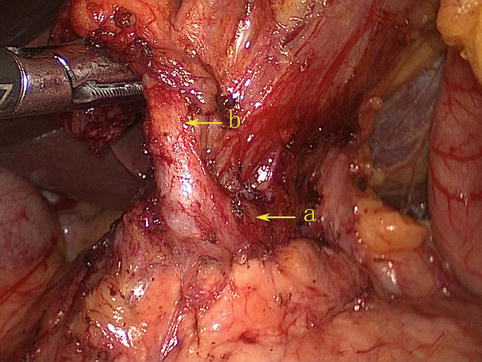

Fig. 4.13
The distributing range of the RGEA (a)

Fig. 4.14
The GDA (a) branches out the RGEA (b) (in autopsy)

Fig. 4.15
The GDA (a) branches out the RGEA (b)
Infrapyloric Artery (IPA)
The IPA arises from the GDA in approximately 85.0 % of cases (Figs. 4.16, 4.17, and 4.18). It branches from the RGEA in approximately 15.0 % of cases (Figs. 4.19 and 4.20). It mainly supplies the pylorus, and it gives off three to four small radial branches near its root. During pylorus-preserving gastrectomy or pylorus-preserving radical gastrectomy for early gastric cancer, the IPA should be left intact. However, if metastasis is judged to be present in the No. 6 LNs by the naked eye, it should be severed.
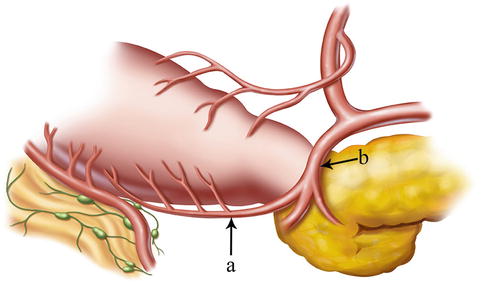
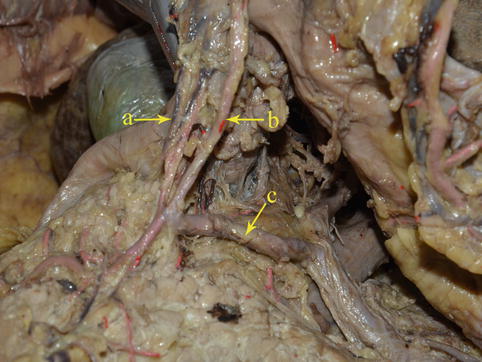
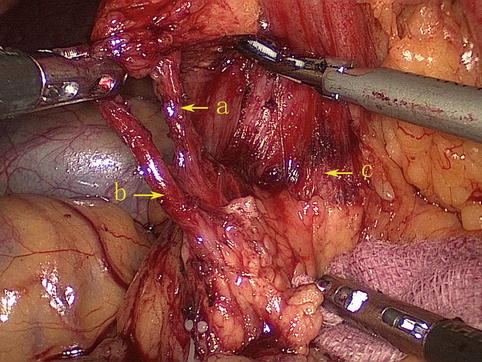
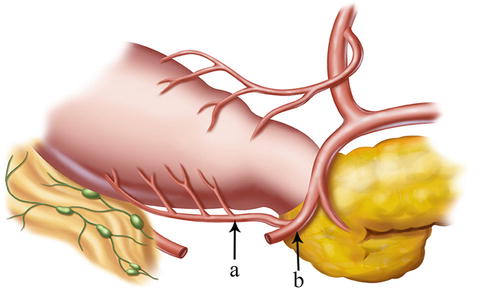
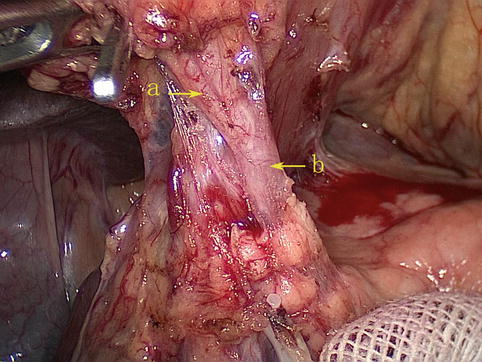

Fig. 4.16
The IPA (a) arises from the GDA (b)

Fig. 4.17
The IPA (a) and the RGEA (b), respectively, arises from the GDA (c) (in autopsy)

Fig. 4.18
The IPA (a) and the RGEA (b), respectively, arises from the GDA (c)

Fig. 4.19
The IPA (a) branches from the RGEA (b)

Fig. 4.20
The IPA (a) branches from the RGEA (b)
Superior Pancreaticoduodenal Artery (SPDA)
At the lower border of the pylorus, two terminal branches, the RGEA and the SPDA, are formed from the GDA (Figs. 4.21 and 4.22). The latter gives off anterior and posterior branches (Fig. 4.23), which pass down the anterior and posterior grooves between the pancreatic head and the duodenum, respectively, and join similar branches of the inferior pancreaticoduodenal artery, a branch of the superior mesenteric artery (SMA). These branches supply blood to the pancreas and duodenum (Figs. 4.24 and 4.25).
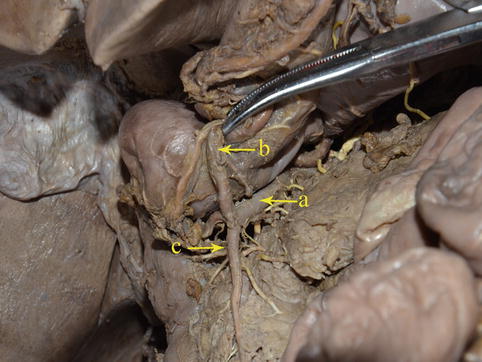
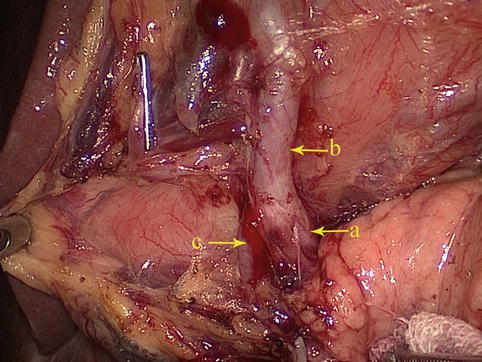
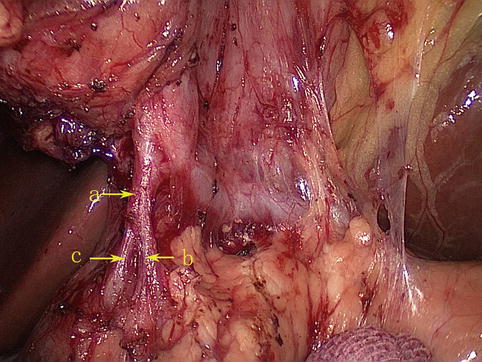
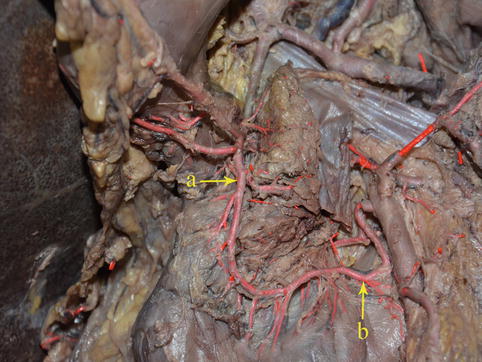
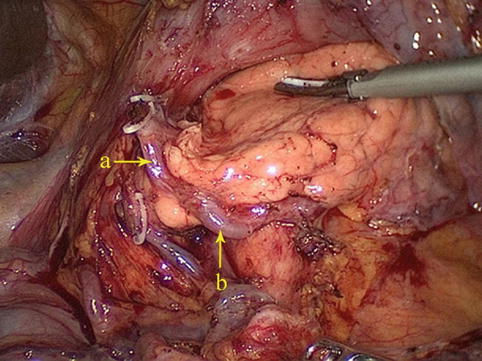

Fig. 4.21
The GDA (a) gives off the RGEA (b) and SPDA (c) (in autopsy)

Fig. 4.22
The GDA (a) gives off the RGEA (b) and SPDA (c)

Fig. 4.23
The SPDA (a) divides into the anterior (b) and posterior (c) branches

Fig. 4.24
The SPDA (a) anastomoses with the IPDA (b) (in autopsy)

Fig. 4.25
The SPDA (a) anastomoses with the IPDA (b)
4.2.2.2 Veins Associated with Lymph Node Dissection in the Infrapyloric Area
Right Gastroepiploic Vein (RGEV)
The RGEV runs parallel with the corresponding artery but diverges under the pylorus. At this point, the vein turns downward over the front of the pancreatic head to join the anterior superior pancreaticoduodenal vein (ASPDV) and form the gastroduodenal vein (GDV). The GDV forms Henle’s trunk together with the right or accessory right colic vein (RCV or ARCV), flows into the SMV, and finally drains into the portal system (Fig. 4.26).
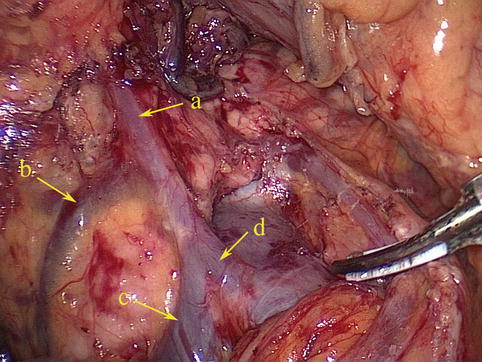

Fig. 4.26
The RGEV (a) joins the ASPDV (b) to form Henle’s trunk (d) together with the RCV or ARCV (c)
SMV
The SMV lies to the right of the corresponding artery within the mesentery. At its termination behind the neck of the pancreas, the vein combines with the splenic vein (SpV) to form the PV. It receives venous blood from the tributaries that correspond to the branches of the SMA and the RGEA. These main tributaries are the RGEV, ASPDV, RCV or ARCV, and the MCV (Figs. 4.27 and 4.28).
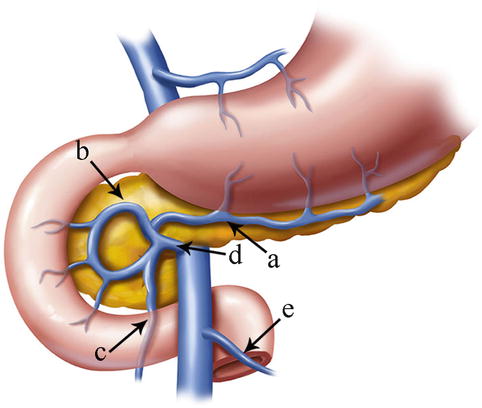
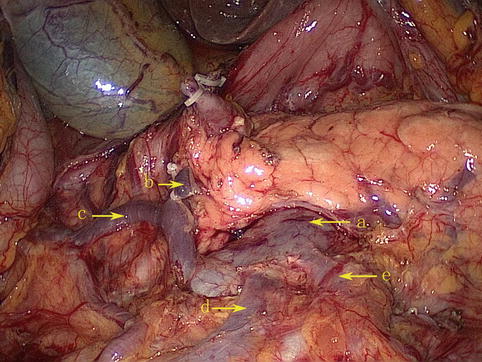

Fig. 4.27
The tributaries of the SMA are the RGEV (a), ASPDV (b), RCV or ARCV (c), gastrocolic trunk (d), and MCV (e)

Fig. 4.28
The tributaries of the SMV (a) are the RGEV (b), ASPDV (c), RCV (d), and MCV (e)
There are six branching patterns of the SMV:
1.
The RGEV combines with the ASPDV to form the SMV (Fig. 4.29).
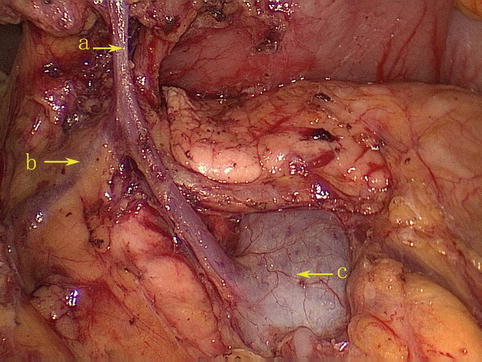

Fig. 4.29
The RGEV (a) combines with the ASPDV (b) to form the SMV (c)
2.
The RGEV directly drains into the SMV (Fig. 4.30).
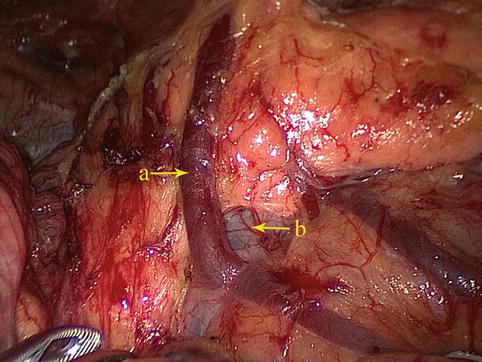

Fig. 4.30
The RGEV (a) directly drains into the SMV (b)
3.
The RGEV joins the ASPDV to form Henle’s trunk together with the RCV or ARCV, which finally flows into the SMV (Fig. 4.31).
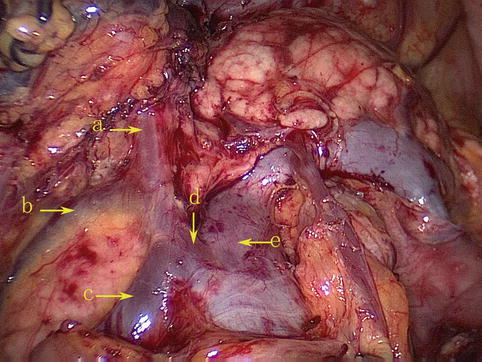

Fig. 4.31
The RGEV (a) joins the ASPDV (b) to form Henle’s trunk (d) together with the RCV or ARCV (c), which finally flows into the SMV (e)
4.
The RGEV joins the SMV around the confluence of the ASPDV and RCV (Fig. 4.32).
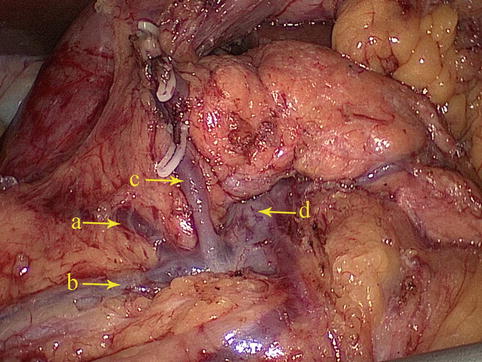

Fig. 4.32
The confluence of the ASPDV (a) and RCV (b) joins the RGEV (c) to drain into the SMV (d)
5.
The RGEV combines with the RCV to flow into the SMV (Fig. 4.33).
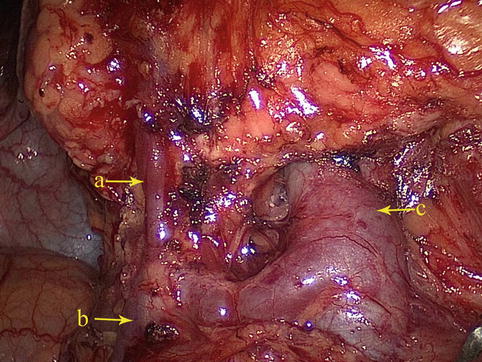

Fig. 4.33
The RGEV (a) combines with the RCV (b) to flow into the SMV (c)
6.
The confluence of the RGEV and ASPDV forms Henle’s trunk together with the RCV and the ARCV, which finally flows into the SMV (Fig. 4.34).
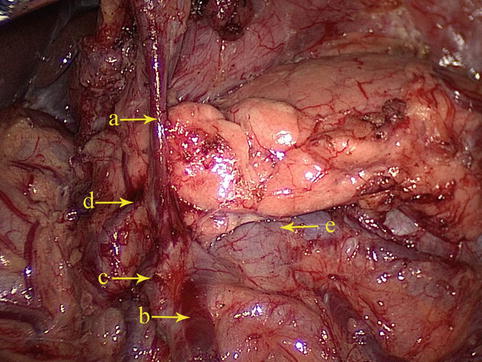

Fig. 4.34
The confluence of the RGEV (a) and ASPDV (b) forms Henle’s trunk with the RCV (c) and the ARCV (d), which finally flows into the SMV (e)
MCV
The MCV follows the path of its corresponding artery. It is an important anatomic landmark for finding the SMV during the operation. There are four variations of the convergence:
1.
The MCV directly drains into the SMV (Fig. 4.35).
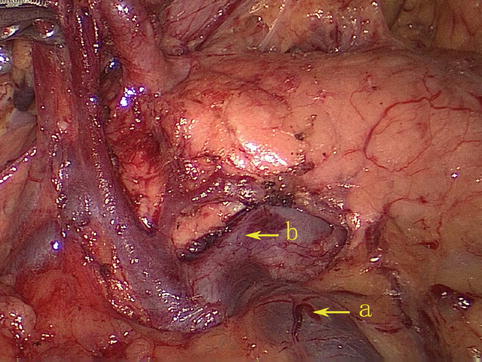

Fig. 4.35
The MCV (a) directly drains into the SMV (b)
2.
The RCV unites with the MCV to drain into the SMV (Fig. 4.36).
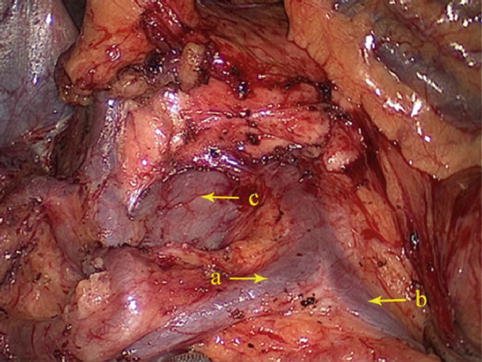

Fig. 4.36
The RCV (a) unites with the MCV (b) to drain into the SMV (c)
3.
The MCV directly drains into the SpV (Fig. 4.37).
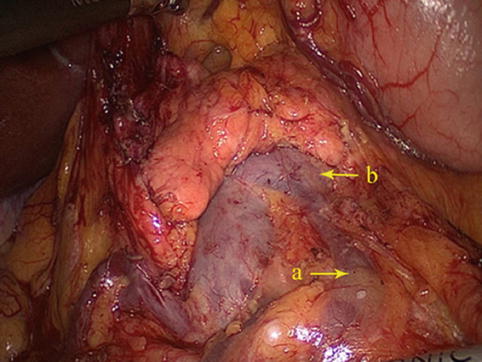

Fig. 4.37
The MCV (a) directly drains into the SpV (b)
4.
The MCV joins Henle’s trunk to flow into the SMV (Fig. 4.38).
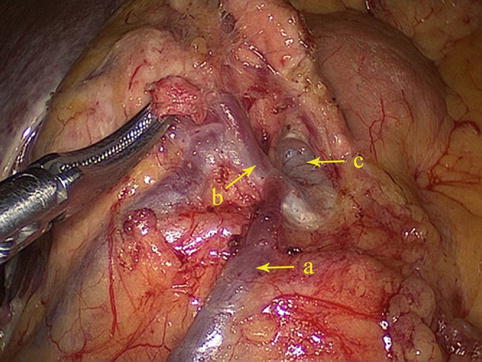

Fig. 4.38
The MCV (a) joins Henle’s trunk (b) to flow into the SMV (c)
4.2.3 Lymph Node Anatomy of the Infrapyloric Area
4.2.3.1 The No. 6 LNs (Infrapyloric LNs)
Definition of the No. 6 LNs
The No. 6 LNs are included in the two layers of the mesogastrium along the greater curvature of the stomach under the pylorus. This group includes the LNs along the infrapyloric vessels (the infrapyloric and retropyloric LNs) and those located at the confluence of the RGEV and ASPDV (Figs. 4.39, 4.40, and 4.41).
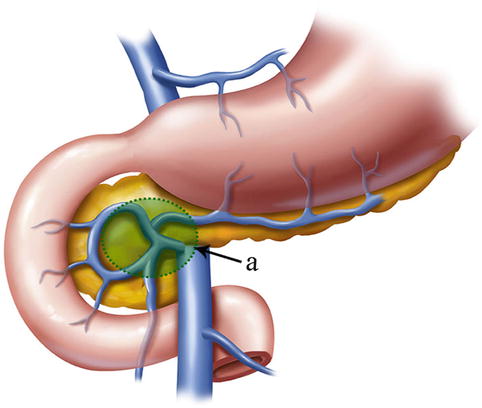
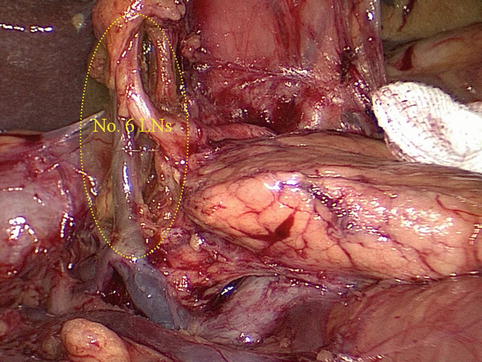
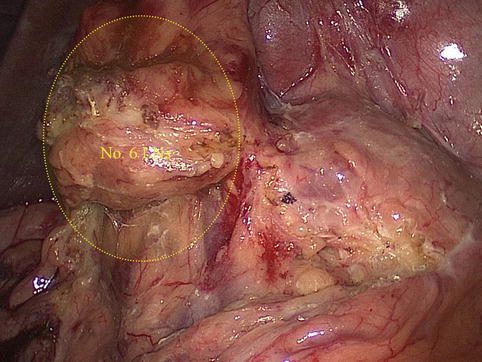

Fig. 4.39
The scope of the No. 6 LNs (a)

Fig. 4.40
The scope of the No. 6 LNs

Fig. 4.41
No. 6 LNs
The No. 6 LNs are separated from the No. 4d LNs by the first branch of the RGEA that feeds the gastric walls. The No. 6 LNs are on the right side of the artery and the No. 4d LNs are on the left. During the dissection of the No. 6 LNs and the exposure of the RGEV, the united veins should be confirmed, and the RGEV should be ligated and transected above the confluence of the ASPDV.
Cases of No. 6 LNM
Case one: Figs. 4.42 and 4.43
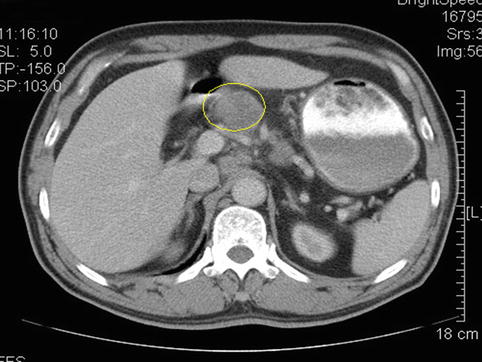
Fig. 4.42
Computed tomography (CT) scan showing No. 6 LNM (the circle represents the swollen No. 6 LNs)
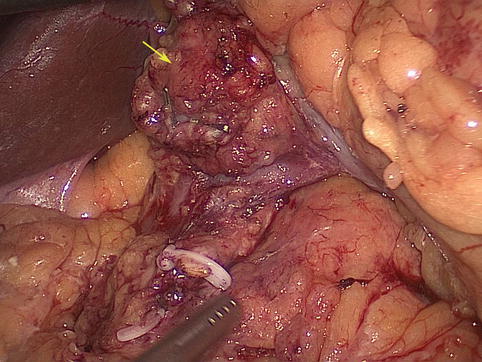
Fig. 4.43
Intraoperative view of No. 6 LNM (the arrow represents the swollen No. 6 LNs)
Case two: Views of the No. 6 LNs before and after neoadjuvant chemotherapy (Figs. 4.44, 4.45, 4.46, and 4.47)
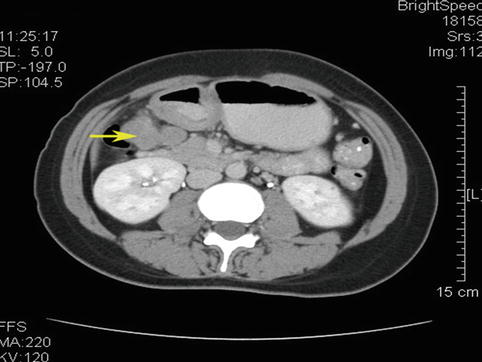
Fig. 4.44
CT scan image of No. 6 LNM before neoadjuvant chemotherapy (the arrow represents the swollen No. 6 LNs)
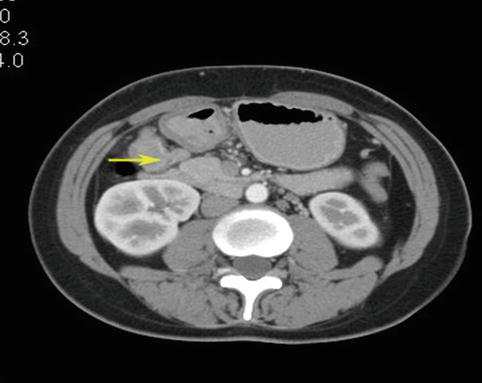
Fig. 4.45
CT scan image of No. 6 LNM after neoadjuvant chemotherapy (the arrow represents the No. 6 LNs)
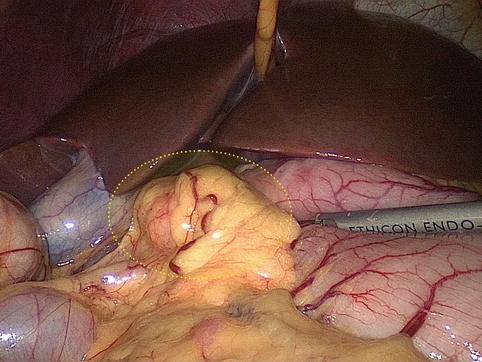
Fig. 4.46
Intraoperative view of the swollen No. 6 LNs< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






